Hospitalisation Is Prognostic of Survival in Chronic Thromboembolic Pulmonary Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design and Outcome Measures
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Landmark Analysis: Operable vs. Inoperable
3.3. Landmark Analysis: Operated vs. Not-Operated
3.4. Landmark Analysis of Operable Subgroups: Already-Operated vs. Not-Currently-Operated
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.; Torbicki, A.; Gopalan, D.; Sitbon, O.; Klok, F.A.; Lang, I.; Jenkins, D.; Kim, N.H.; Humbert, M.; Jais, X.; et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2020, 59, 2102006. [Google Scholar] [CrossRef]
- Leber, L.; Beaudet, A.; Muller, A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Identification of the most accurate estimates from a systematic literature review. Pulm Circ. 2021, 11, 2045894020977300. [Google Scholar] [CrossRef]
- Delcroix, M.; Lang, I.; Pepke-Zaba, J.; Jansa, P.; D’Armini, A.M.; Snijder, R.; Bresser, P.; Torbicki, A.; Mellemkjaer, S.; Lewczuk, J.; et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. Circulation 2016, 133, 859–871. [Google Scholar] [CrossRef]
- Quadery, S.R.; Swift, A.J.; Billings, C.G.; Thompson, A.A.; Elliot, C.A.; Hurdman, J.; Charalampopoulos, A.; Sabroe, I.; Armstrong, I.J.; Hamilton, N.; et al. The impact of patient choice on survival in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2018, 52, 1800589. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Delcroix, M.; Jais, X.; Madani, M.M.; Matsubara, H.; Mayer, E.; Ogo, T.; Tapson, V.F.; Ghofrani, H.A.; Jenkins, D.P. Chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1465–1472. [Google Scholar] [CrossRef]
- Gall, H.; Hoeper, M.M.; Richter, M.J.; Cacheris, W.; Hinzmann, B.; Mayer, E. An epidemiological analysis of the burden of chronic thromboembolic pulmonary hypertension in the USA, Europe and Japan. Eur. Respir. Rev. 2017, 26, 160121. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur. Heart J. 2022, 43, ehac237. [Google Scholar] [CrossRef]
- McLaughlin, V.V.; Hoeper, M.M.; Channick, R.N.; Chin, K.M.; Delcroix, M.; Gaine, S.; Ghofrani, H.A.; Jansa, P.; Lang, I.M.; Mehta, S.; et al. Pulmonary arterial hypertension-related morbidity is prognostic for mortality. J. Am. Coll. Cardiol. 2018, 71, 752–763. [Google Scholar] [CrossRef]
- Dafni, U. Landmark analysis at the 25-year landmark point. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 363–371. [Google Scholar] [CrossRef]
- Anderson, J.R.; Cain, K.C.; Gelber, R.D. Analysis of survival by tumor response. J. Clin. Oncol. 1983, 1, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Jansa, P.; Ambrož, D.; Kuhn, M.; Dytrych, V.; Aschermann, M.; Černý, V.; Gressin, V.; Heller, S.; Kunstýř, J.; Širanec, M.; et al. Epidemiology of chronic thromboembolic pulmonary hypertension (CTEPH) in the Czech Republic. Pulm. Circ. 2022, 12, e12038. [Google Scholar] [CrossRef] [PubMed]
- IHIS CR. Institute of Health Information and Statistics. Available online: https://www.uzis.cz/index-en.php (accessed on 19 July 2022).
- Pocock, S.J.; Clayton, T.C.; Altman, D.G. Survival plots of time-to-event outcomes in clinical trials: Good practice and pitfalls. Lancet 2002, 359, 1686–1689. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Kramer, T.; Pan, Z.; Eichstaedt, C.A.; Spiesshoefer, J.; Benjamin, N.; Olsson, K.M.; Meyer, K.; Vizza, C.D.; Vonk-Noordegraaf, A.; et al. Mortality in pulmonary arterial hypertension: Prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur. Respir. J. 2017, 50, 1700740. [Google Scholar] [CrossRef] [PubMed]
- Mathai, S.C.; Mathew, S. Breathing (and coding?) a bit easier: Changes to international classification of disease coding for pulmonary hypertension. Chest 2018, 154, 207–218. [Google Scholar] [CrossRef]
- Peacock, A.; Keogh, A.; Humbert, M. Endpoints in pulmonary arterial hypertension: The role of clinical worsening. Curr. Opin. Pulm. Med. 2010, 16 (Suppl. S1), S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.A.; D’Armini, A.M.; Grimminger, F.; Hoeper, M.M.; Jansa, P.; Kim, N.H.; Mayer, E.; Simonneau, G.; Wilkins, M.R.; Fritsch, A.; et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N. Engl. J. Med. 2013, 369, 319–329. [Google Scholar] [CrossRef]
- Jaïs, X.; D’Armini, A.M.; Jansa, P.; Torbicki, A.; Delcroix, M.; Ghofrani, H.A.; Hoeper, M.M.; Lang, I.M.; Mayer, E.; Pepke-Zaba, J.; et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J. Am. Coll. Cardiol. 2008, 52, 2127–2134. [Google Scholar] [CrossRef]
- Sadushi-Kolici, R.; Jansa, P.; Kopec, G.; Torbicki, A.; Skoro-Sajer, N.; Campean, I.-A.; Halank, M.; Simkova, I.; Karlocai, K.; Steringer-Mascherbauer, R.; et al. Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPH): A double-blind, phase 3, randomised controlled trial. Lancet Respir. Med. 2019, 7, 239–248. [Google Scholar] [CrossRef]
- Sitbon, O.; Channick, R.; Chin, K.M.; Frey, A.; Gaine, S.; Galiè, N.; Ghofrani, H.-A.; Hoeper, M.M.; Lang, I.M.; Preiss, R.; et al. Selexipag for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2015, 373, 2522–2533. [Google Scholar] [CrossRef]
- Ghofrani, H.-A.; Simonneau, G.; D’Armini, A.M.; Fedullo, P.; Howard, L.S.; Jaïs, X.; Jenkins, D.P.; Jing, Z.-C.; Madani, M.M.; Martin, N.; et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): Results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir. Med. 2017, 5, 785–794. [Google Scholar] [CrossRef]
- CT.gov. NCT04271475. A Study to Evaluate Efficacy and Safety of Macitentan 75 mg in Inoperable or Persistent/Recurrent Chronic Thromboembolic Pulmonary Hypertension (MACiTEPH). Available online: https://clinicaltrials.gov/ct2/show/NCT04271475 (accessed on 19 July 2022).
- Burger, C.D.; Long, P.K.; Shah, M.R.; McGoon, M.D.; Miller, D.P.; Romero, A.J.; Benton, W.W.; Safford, R.E. Characterization of first-time hospitalizations in patients with newly diagnosed pulmonary arterial hypertension in the REVEAL registry. Chest 2014, 146, 1263–1273. [Google Scholar] [CrossRef] [PubMed]

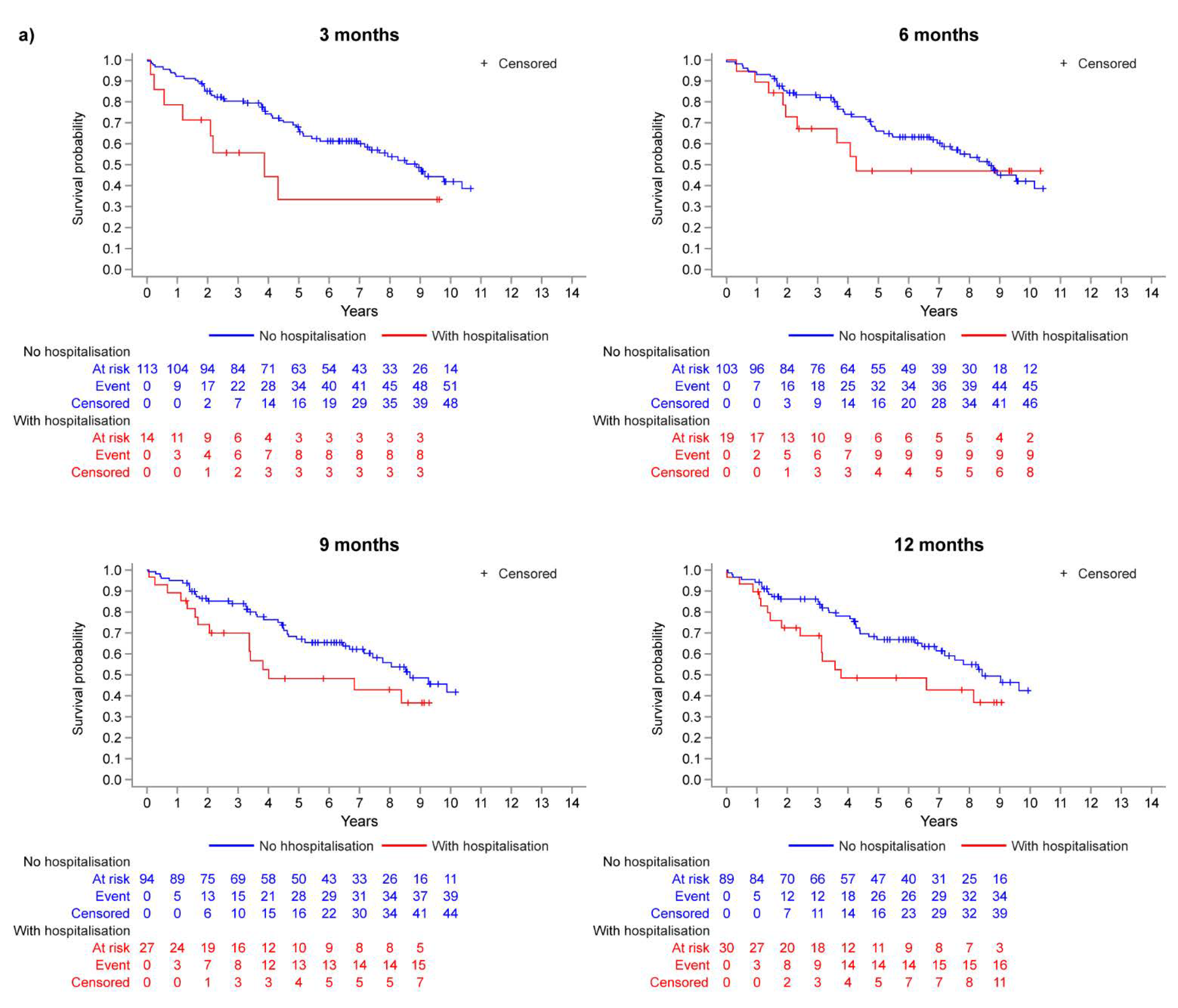
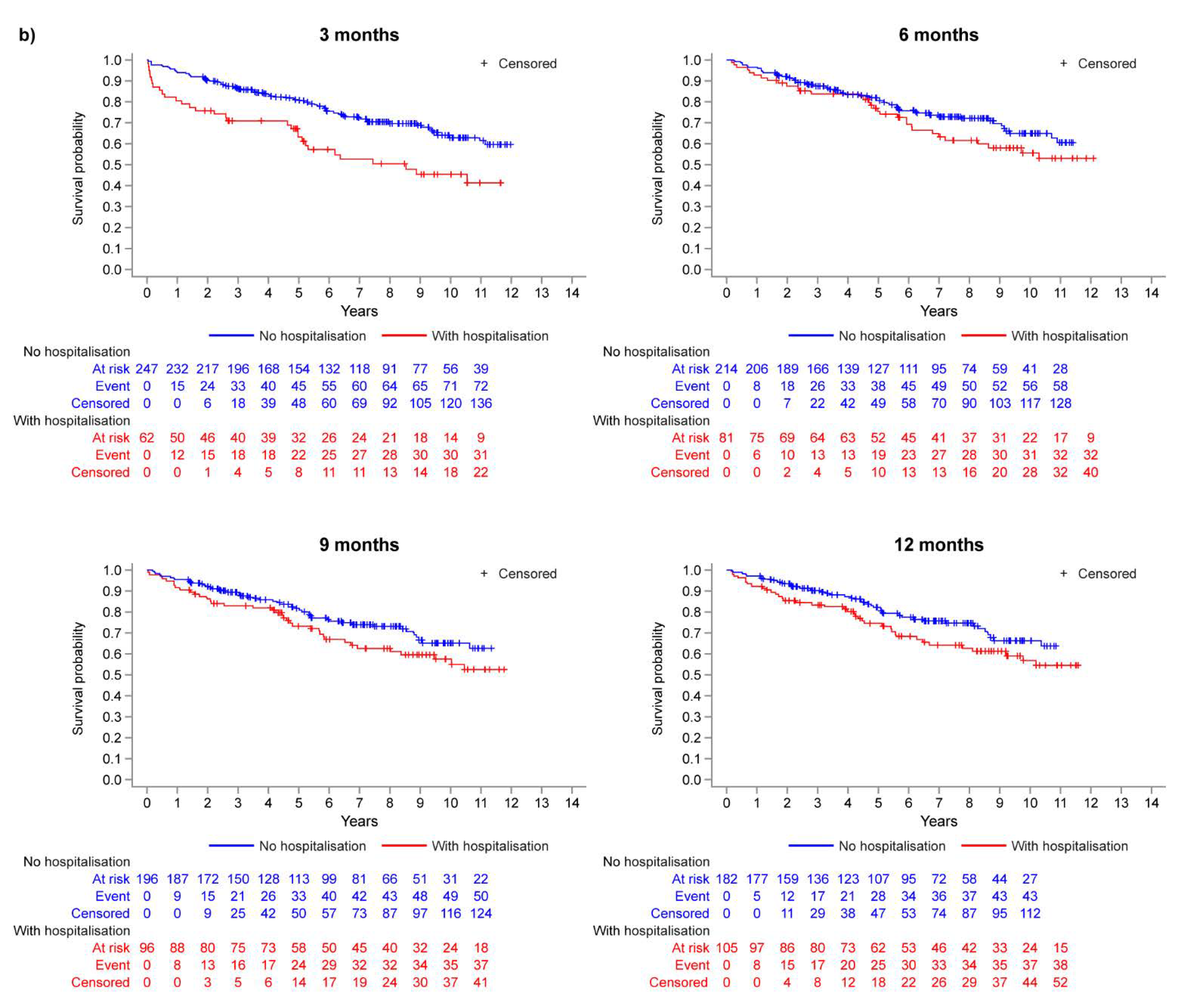
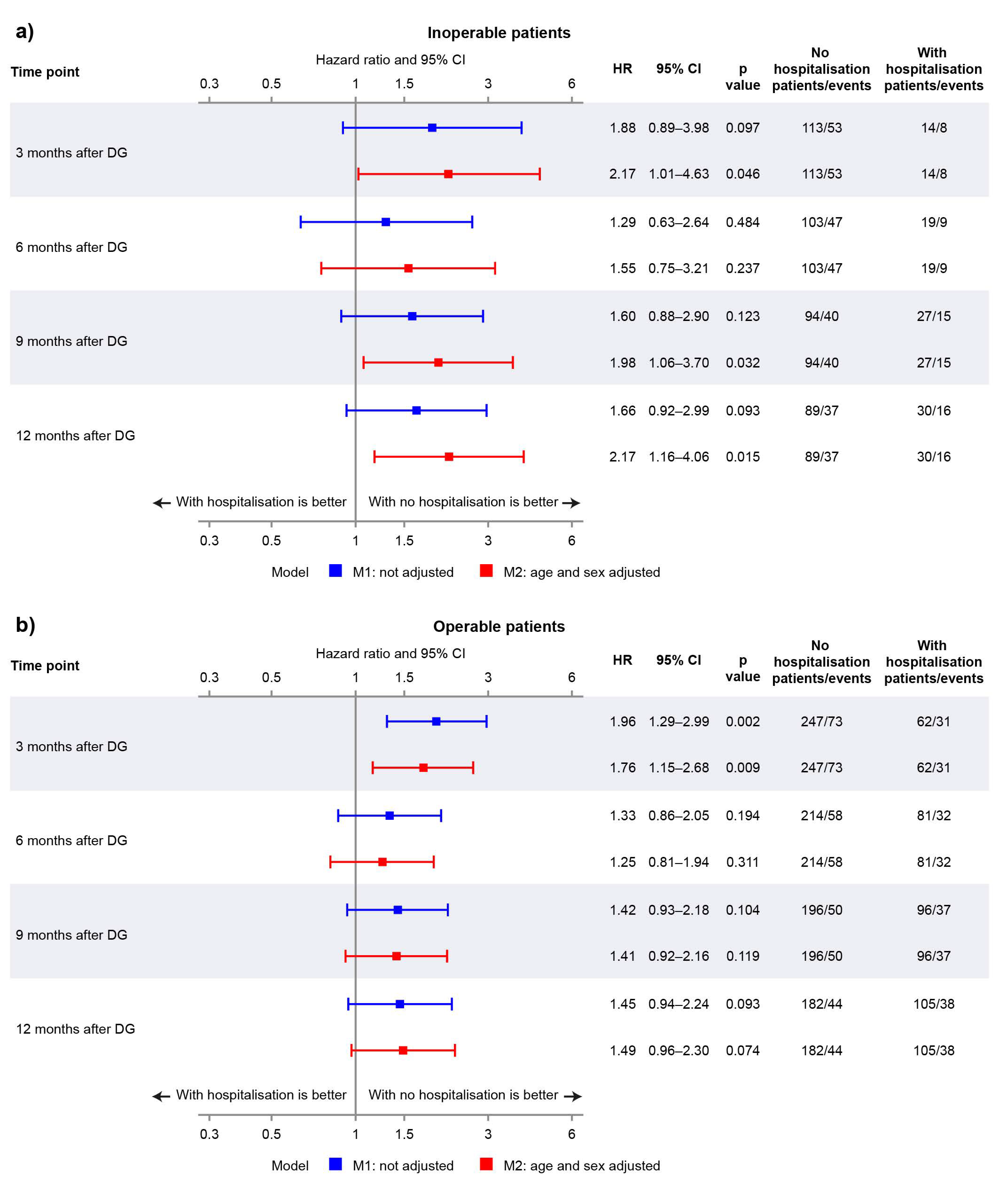
| Month 3 Landmark | Month 6 Landmark | Month 9 Landmark | Month 12 Landmark | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Prior Hospitalisation Event (n = 14) | No Prior Hospitalisation Event (n = 113) | Prior Hospitalisation Event (n = 19) | No Prior Hospitalisation Event (n = 103) | Prior Hospitalisation Event (n = 27) | No Prior Hospitalisation Event (n = 94) | Prior Hospitalisation Event (n = 30) | No Prior Hospitalisation Event (n = 89) |
| Age, mean (SD), years | 65.7 (10.04) | 66.7 (13.05) | 64.6 (13.11) | 66.8 (12.89) | 64.2 (12.53) | 67.1 (13.06) | 64.1 (12.80) | 67.2 (13.12) |
| Sex, n (%), female | 9 (64.3%) | 61 (54.0%) | 12 (63.2%) | 56 (54.4%) | 13 (48.1%) | 55 (58.5%) | 16 (53.3%) | 51 (57.3%) |
| BMI, mean (SD), kg/m2 [n] | 28.1 (5.67) [13] | 28.3 (5.08) [105] | 28.7 (5.11) [17] | 28.4 (5.08) [97] | 28.9 (4.49) [25] | 28.4 (5.20) [88] | 29.7 (4.03) [28] | 28.2 (5.24) [84] |
| DVT history, n (%) | 3 (21.4%) | 40 (36.0%) | 5 (27.8%) | 37 (36.3%) | 7 (26.9%) | 35 (37.6%) | 9 (31.0%) | 32 (36.4%) |
| PE history, n (%) | 10 (71.4%) | 82 (72.6%) | 11 (57.9%) | 77 (74.8%) | 18 (66.7%) | 69 (73.4%) | 21 (70.0%) | 66 (74.2%) |
| Time from first PE to diagnosis, mean (SD), years | 5.4 (8.22) | 5.2 (7.03) | 6.0 (7.55) | 5.1 (7.20) | 6.7 (7.76) | 4.9 (7.09) | 6.5 (7.63) | 4.9 (7.10) |
| NYHA FC, n (%) [n] | ||||||||
| FC I/II | – | 4 (4.0%) [101] | – | 4 (4.3%) [92] | – | 4 (4.8%) [84] | – | 4 (5.1%) [79] |
| FC III/IV | 12 (100%) [12] | 97 (96.0%) [101] | 16 (100%) [16] | 88 (95.7%) [92] | 24 (100%) [24] | 80 (95.2%) [84] | 27 (100%) [27] | 75 (94.9%) [79] |
| 6MWT, mean (SD), metres [n] | 269.7 (104.9) [11] | 337.8 (112.4) [82] | 279.8 (133.5) [14] | 343.8 (107.7) [75] | 309.6 (127.7) [21] | 341.1 (109.0) [68] | 308.8 (121.7) [24] | 348.0 (107.9) [63] |
| RHC | ||||||||
| mPAP, mean (SD), mmHg [n] | 50.1 (12.60) [13] | 43.2 (11.69) [108] | 51.3 (14.65) [18] | 42.5 (11.03) [99] | 50.2 (12.89) [25] | 42.0 (11.29) [91] | 50.1 (13.03) [28] | 41.5 (11.09) [86] |
| PVR, mean (SD), dyn s/cm5 [n] | 905.1 (381.1) [12] | 629.2 (325.5) [107] | 822.6 (430.6) [17] | 613.0 (311.6) [98] | 765.3 (389.7) [24] | 604.7 (311.8) [90] | 716.4 (365.3) [27] | 601.5 (314.6) [85] |
| CI, mean (SD), L/min/m2 [n] | 2.0 (0.40) [12] | 2.3 (0.58) [106] | 2.3 (0.64) [17] | 2.3 (0.56) [97] | 2.3 (0.60) [24] | 2.3 (0.56) [89] | 2.4 (0.64) [27] | 2.3 (0.54) [84] |
| BNP, mean (SD), pg/mL [n] | 745.0 (475.7) [6] | 284.4 (322.9) [49] | 445.5 (312.1) [8] | 289.4 (332.2) [46] | 350.0 (276.1) [13] | 300.6 (349.1) [41] | 264.8 (251.4) [15] | 294.0 (323.9) [37] |
| Anticoagulation, n (%) | ||||||||
| NOAC | 1 (7.1%) | 4 (3.5%) | 1 (5.3%) | 4 (3.9%) | 1 (3.7%) | 4 (4.3%) | 1 (3.3%) | 4 (4.5%) |
| Vitamin K antagonist | 11 (78.6%) | 99 (87.6%) | 15 (78.9%) | 91 (88.3%) | 23 (85.2%) | 82 (87.2%) | 26 (86.7%) | 78 (87.6%) |
| Other anticoagulants | 2 (14.3%) | 10 (8.8%) | 3 (15.8%) | 8 (7.8%) | 3 (11.1%) | 8 (8.5%) | 3 (10.0%) | 7 (7.9%) |
| Time to diagnosis from study start 1, mean (SD), years | 8.91 (3.631) | 7.66 (3.103) | 8.02 (3.372) | 7.78 (3.143) | 7.69 (2.871) | 7.79 (3.218) | 7.98 (2.887) | 7.68 (3.253) |
| Month 3 Landmark | Month 6 Landmark | Month 9 Landmark | Month 12 Landmark | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Prior Hospitalisation Event (n = 62) | No Prior Hospitalisation Event (n = 247) | Prior Hospitalisation Event (n = 81) | No Prior Hospitalisation Event (n = 214) | Prior Hospitalisation Event (n = 96) | No Prior Hospitalisation Event (n = 196) | Prior Hospitalisation Event (n = 105) | No Prior Hospitalisation Event (n = 182) |
| Age, mean (SD), years | 64.4 (10.59) | 60.7 (12.65) | 62.8 (11.54) | 60.7 (12.84) | 61.3 (12.08) | 61.3 (12.72) | 60.8 (13.06) | 61.5 (12.28) |
| Sex, n (%), female | 28 (45.2%) | 100 (40.5%) | 39 (48.1%) | 84 (39.3%) | 46 (47.9%) | 76 (38.8%) | 51 (48.6%) | 70 (38.5%) |
| BMI, mean (SD), kg/m2 [n] | 28.5 (6.08) [53] | 28.4 (5.54) [208] | 28.1 (5.12) [66] | 28.5 (5.65) [184] | 28.5 (5.27) [79] | 28.3 (5.65) [168] | 28.4 (5.30) [89] | 28.4 (5.59) [154] |
| DVT history, n (%) | 27 (45.8%) | 106 (43.1%) | 35 (44.3%) | 93 (43.7%) | 40 (42.6%) | 85 (43.6%) | 45 (43.7%) | 79 (43.6%) |
| PE history, n (%) | 50 (80.6%) | 196 (79.4%) | 66 (81.5%) | 170 (79.4%) | 75 (78.1%) | 158 (80.6%) | 84 (80.0%) | 146 (80.2%) |
| Time from first PE to diagnosis, mean (SD), years | 7.1 (8.70) | 4.9 (7.19) | 5.5 (6.54) | 5.0 (7.49) | 5.8 (7.77) | 4.7 (6.94) | 5.7 (7.57) | 4.8 (7.07) |
| NYHA FC, n (%) [n] | ||||||||
| FC I/II | 2 (3.2%) [62] | 30 (12.2%) [246] | 5 (6.2%) [81] | 26 (12.2%) [213] | 5 (5.2%) [96] | 25 (12.8%) [195] | 6 (5.8%) [104] | 24 (13.2%) [182] |
| FC III/IV | 60 (96.8%) [62] | 216 (87.8%) [246] | 76 (93.8%) [81] | 187 (87.8%) [213] | 91 (94.8%) [96] | 170 (87.2%) [195] | 98 (94.2%) [104] | 158 (86.8%) [182] |
| 6MWT, mean (SD), metres [n] | 305.6 (102.3) [49] | 354.0 (107.4) [217] | 309.5 (94.79) [67] | 361.4 (108.4) [186] | 309.0 (98.60) [83] | 365.9 (106.5) [169] | 316.7 (102.4) [90] | 366.5 (106.0) [157] |
| RHC | ||||||||
| mPAP, mean (SD), mmHg [n] | 52.0 (12.97) [62] | 47.8 (12.58) [240] | 50.7 (12.50) [79] | 47.5 (12.63) [209] | 50.8 (12.30) [94] | 47.1 (12.71) [191] | 50.4 (12.45) [102] | 47.0 (12.63) [178] |
| PVR, mean (SD), dyn s/cm5 [n] | 802.2 (319.2) [62] | 730.2 (347.3) [239] | 807.3 (344.8) [79] | 714.4 (333.0) [208] | 783.9 (324.7) [94] | 714.3 (341.4) [190] | 780.4 (336.4) [101] | 704.5 (319.7) [179] |
| CI, mean (SD), L/min/m2 [n] | 2.2 (0.45) [61] | 2.3 (0.53) [234] | 2.2 (0.46) [78] | 2.3 (0.52) [203] | 2.2 (0.46) [93] | 2.3 (0.52) [185] | 2.2 (0.50) [101] | 2.3 (0.50) [172] |
| BNP, mean (SD), pg/mL [n] | 313.5 (251.7) [18] | 392.9 (597.1) [113] | 267.0 (234.1) [25] | 397.8 (601.8) [102] | 311.4 (341.4) [34] | 395.8 (613.0) [92] | 316.6 (349.4) [36] | 373.8 (605.5) [88] |
| Anticoagulation, n (%) [n] | ||||||||
| NOAC | – | 7 (2.9%) [244] | – | 7 (3.3%) [214] | – | 7 (3.6%) [196] | – | 7 (3.8%) [182] |
| Vitamin K antagonist | 47 (75.8%) [62] | 208 (85.2%) [244] | 68 (84.0%) [81] | 179 (83.6%) [214] | 81 (84.4%) [96] | 164 (83.7%) [196] | 90 (85.7%) [105] | 150 (82.4%) [182] |
| Other anticoagulants | 15 (24.2%) [62] | 29 (11.9%) [244] | 13 (16.0%) [81] | 28 (13.1%) [214] | 15 (15.6%) [96] | 25 (12.8%) [196] | 15 (14.3%) [105] | 25 (13.7%) [182] |
| Time to diagnosis from study start 1, mean (SD), years | 6.60 (3.253) | 7.58 (3.434) | 6.21 (3.179) | 7.87 (3.395) | 6.41 (3.273) | 7.88 (3.393) | 6.51 (3.472) | 7.90 (3.333) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansa, P.; Ambrož, D.; Aschermann, M.; Černý, V.; Dytrych, V.; Heller, S.; Kunstýř, J.; Lindner, J.; Linhart, A.; Nižnanský, M.; et al. Hospitalisation Is Prognostic of Survival in Chronic Thromboembolic Pulmonary Hypertension. J. Clin. Med. 2022, 11, 6189. https://doi.org/10.3390/jcm11206189
Jansa P, Ambrož D, Aschermann M, Černý V, Dytrych V, Heller S, Kunstýř J, Lindner J, Linhart A, Nižnanský M, et al. Hospitalisation Is Prognostic of Survival in Chronic Thromboembolic Pulmonary Hypertension. Journal of Clinical Medicine. 2022; 11(20):6189. https://doi.org/10.3390/jcm11206189
Chicago/Turabian StyleJansa, Pavel, David Ambrož, Michael Aschermann, Vladimír Černý, Vladimír Dytrych, Samuel Heller, Jan Kunstýř, Jaroslav Lindner, Aleš Linhart, Matúš Nižnanský, and et al. 2022. "Hospitalisation Is Prognostic of Survival in Chronic Thromboembolic Pulmonary Hypertension" Journal of Clinical Medicine 11, no. 20: 6189. https://doi.org/10.3390/jcm11206189
APA StyleJansa, P., Ambrož, D., Aschermann, M., Černý, V., Dytrych, V., Heller, S., Kunstýř, J., Lindner, J., Linhart, A., Nižnanský, M., Paďour, M., Prskavec, T., Širanec, M., Edwards, S., Gressin, V., Kuhn, M., & Di Scala, L. (2022). Hospitalisation Is Prognostic of Survival in Chronic Thromboembolic Pulmonary Hypertension. Journal of Clinical Medicine, 11(20), 6189. https://doi.org/10.3390/jcm11206189







