Advances in Noninvasive Carotid Wall Imaging with Ultrasound: A Narrative Review
Abstract
1. Introduction
2. Plaque Echogenicity
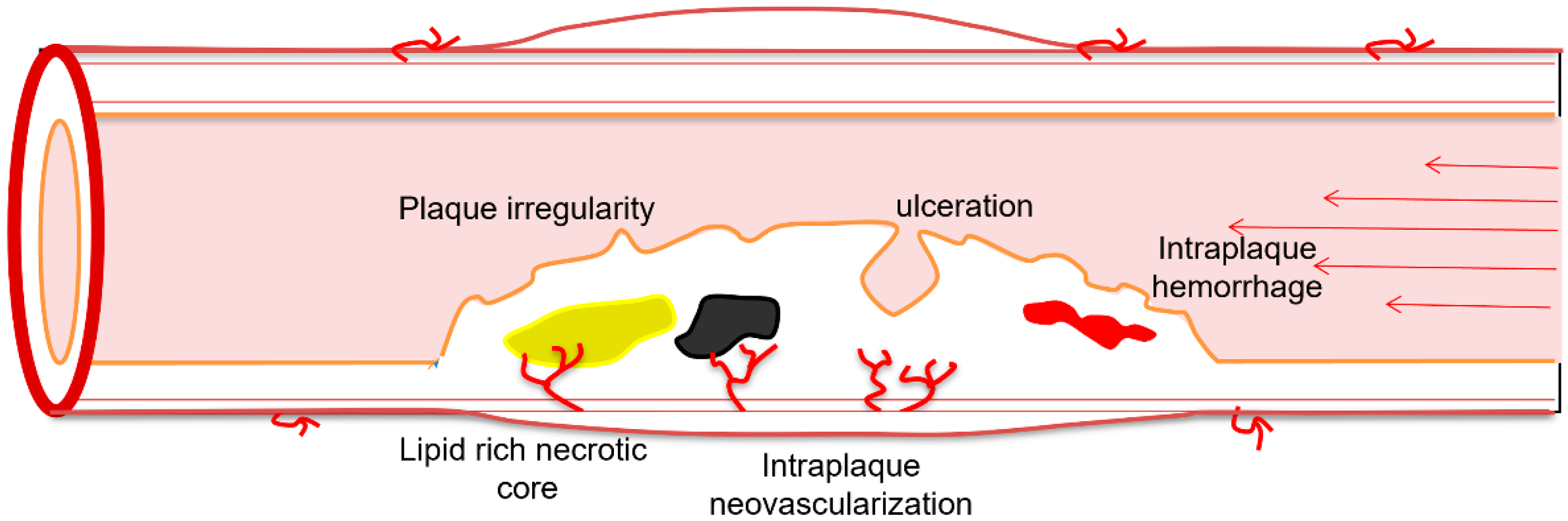
3. Surface Morphology
4. Contrast-Enhanced Ultrasound (CEUS)
4.1. CEUS and Histopathology
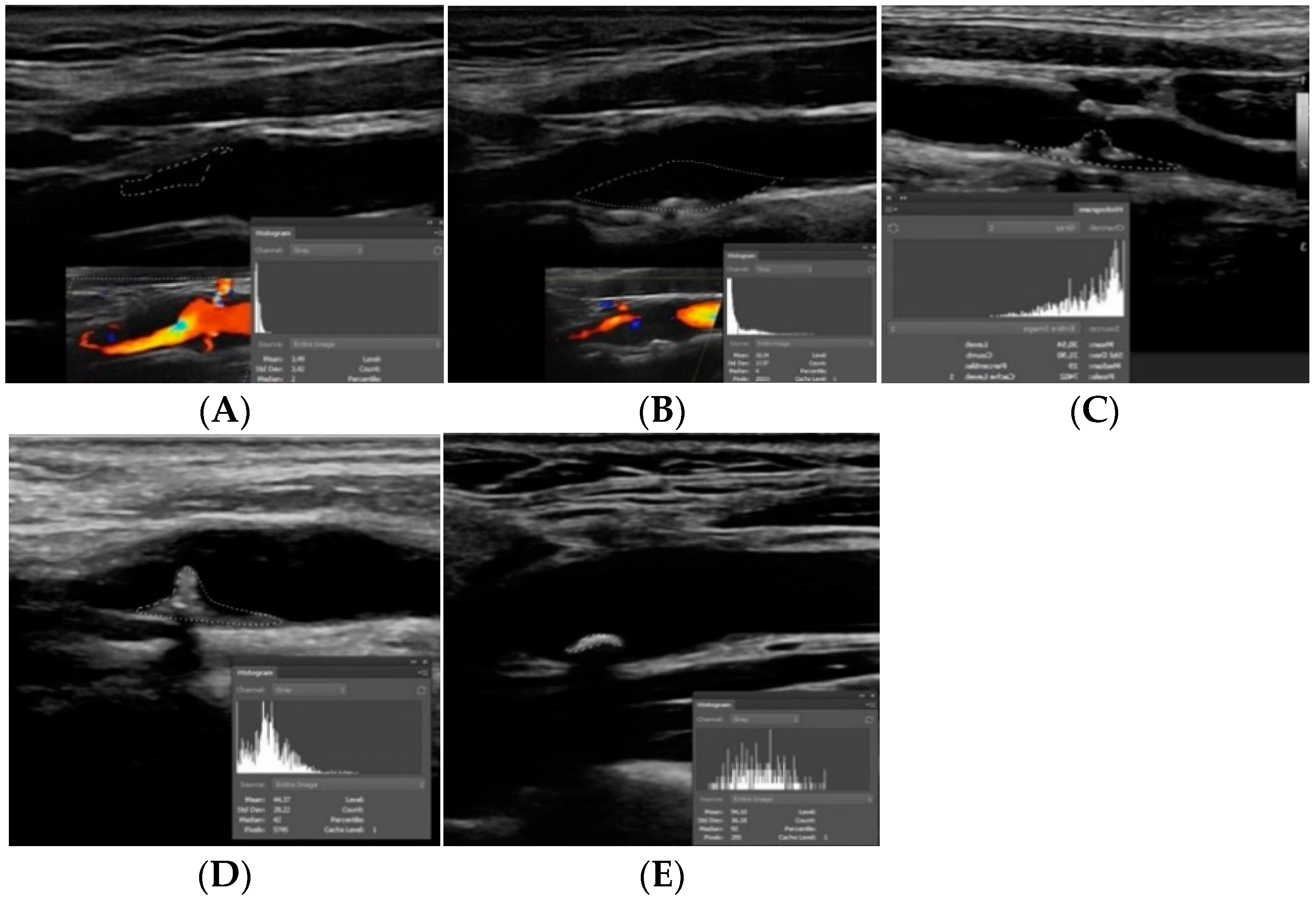
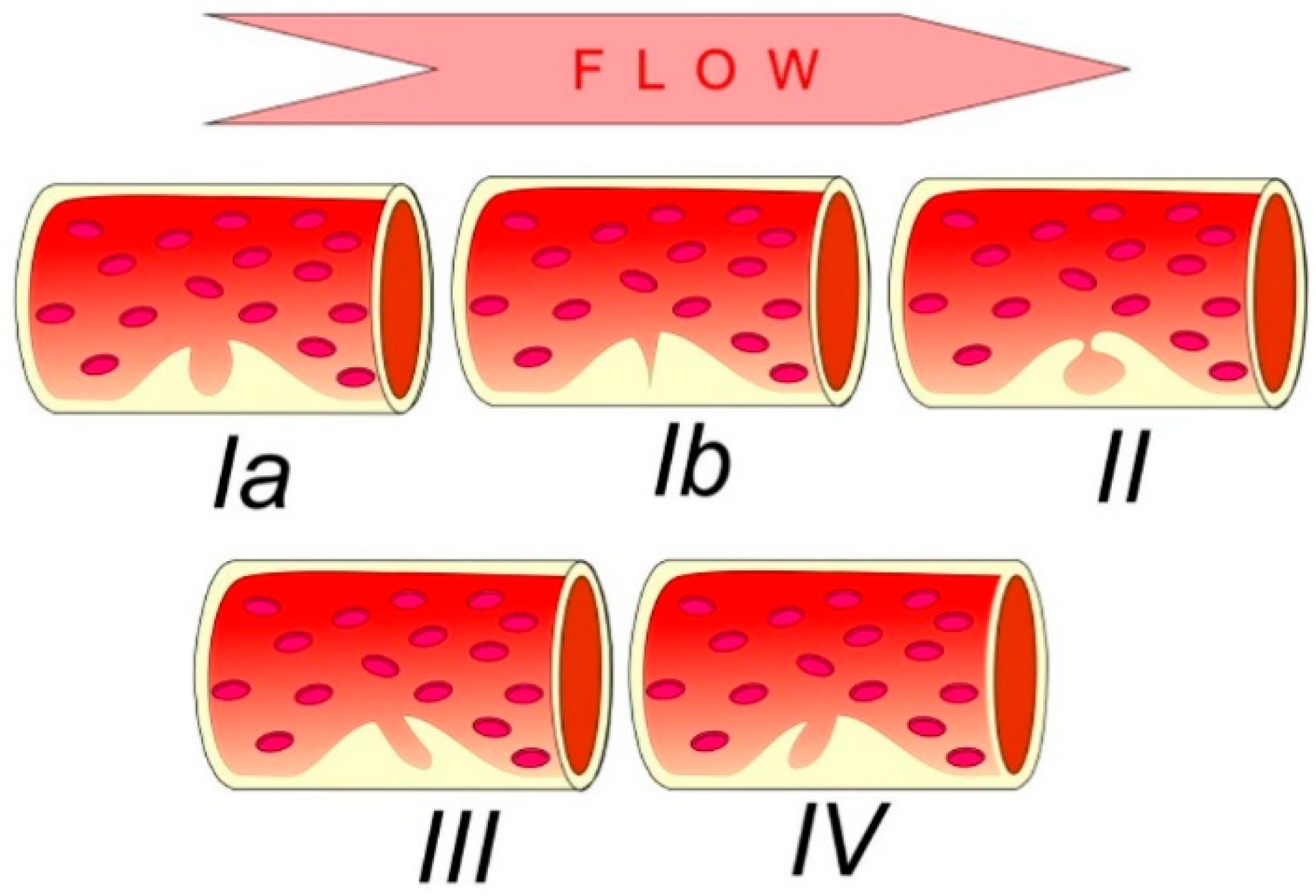
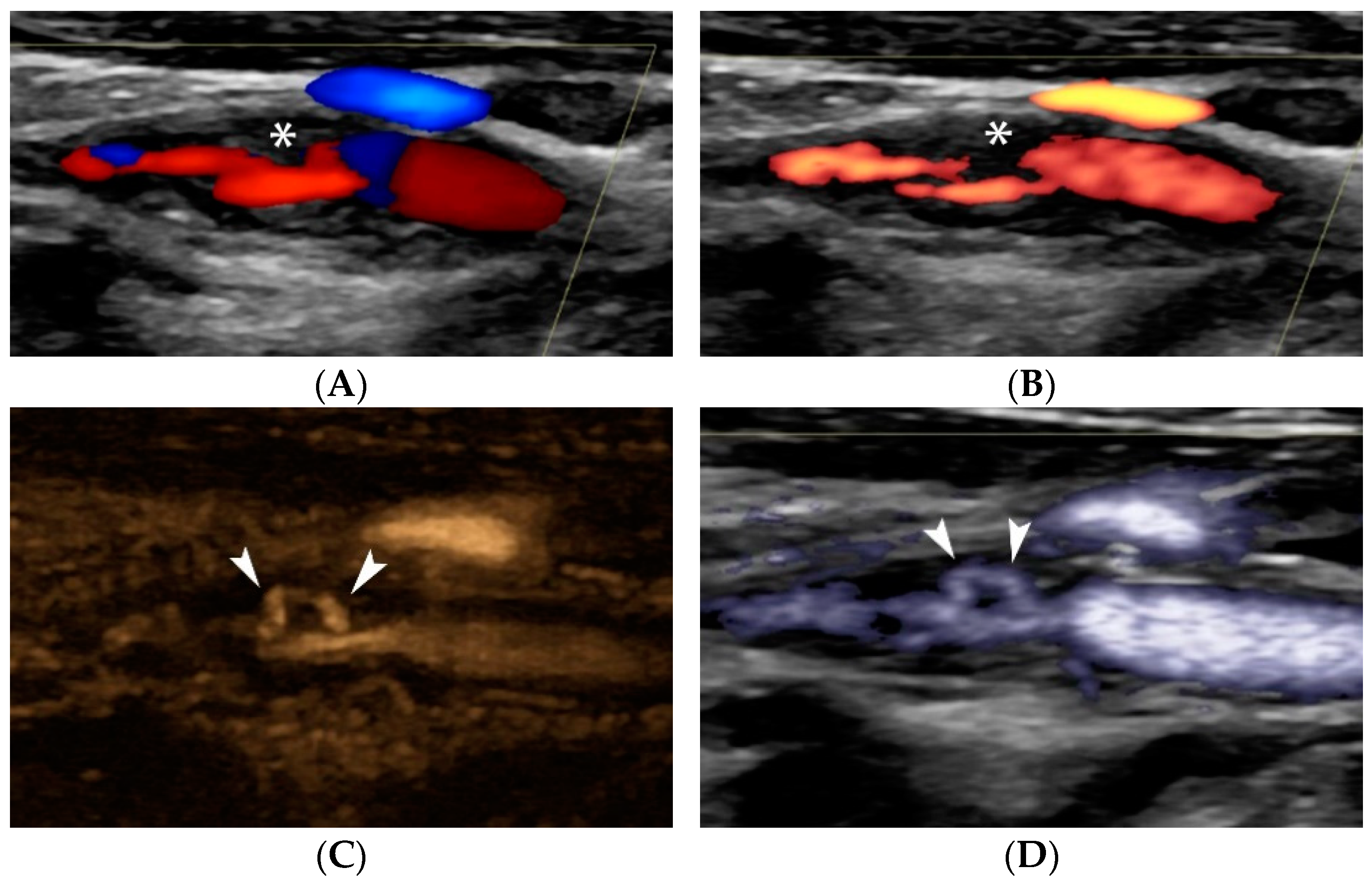
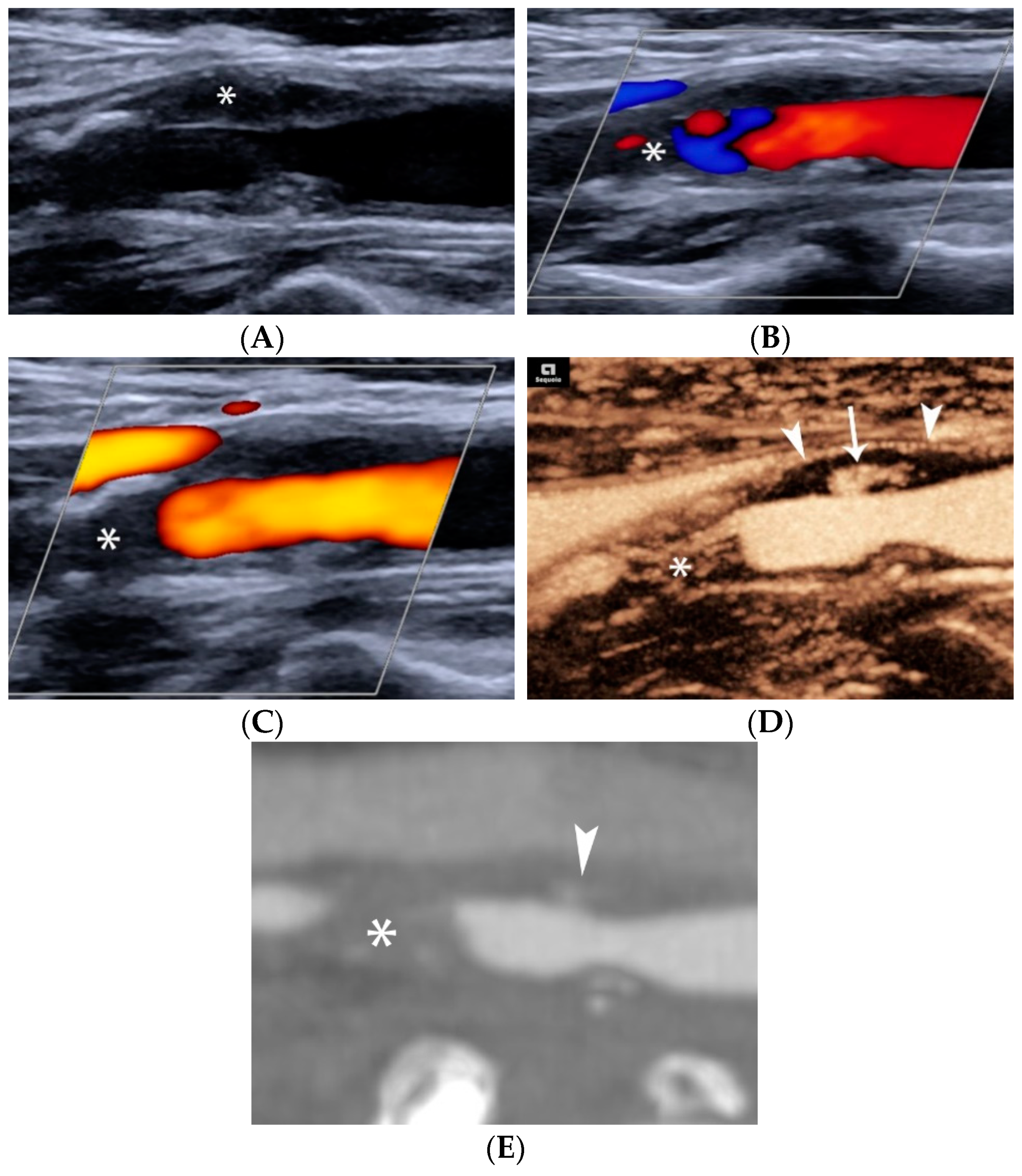
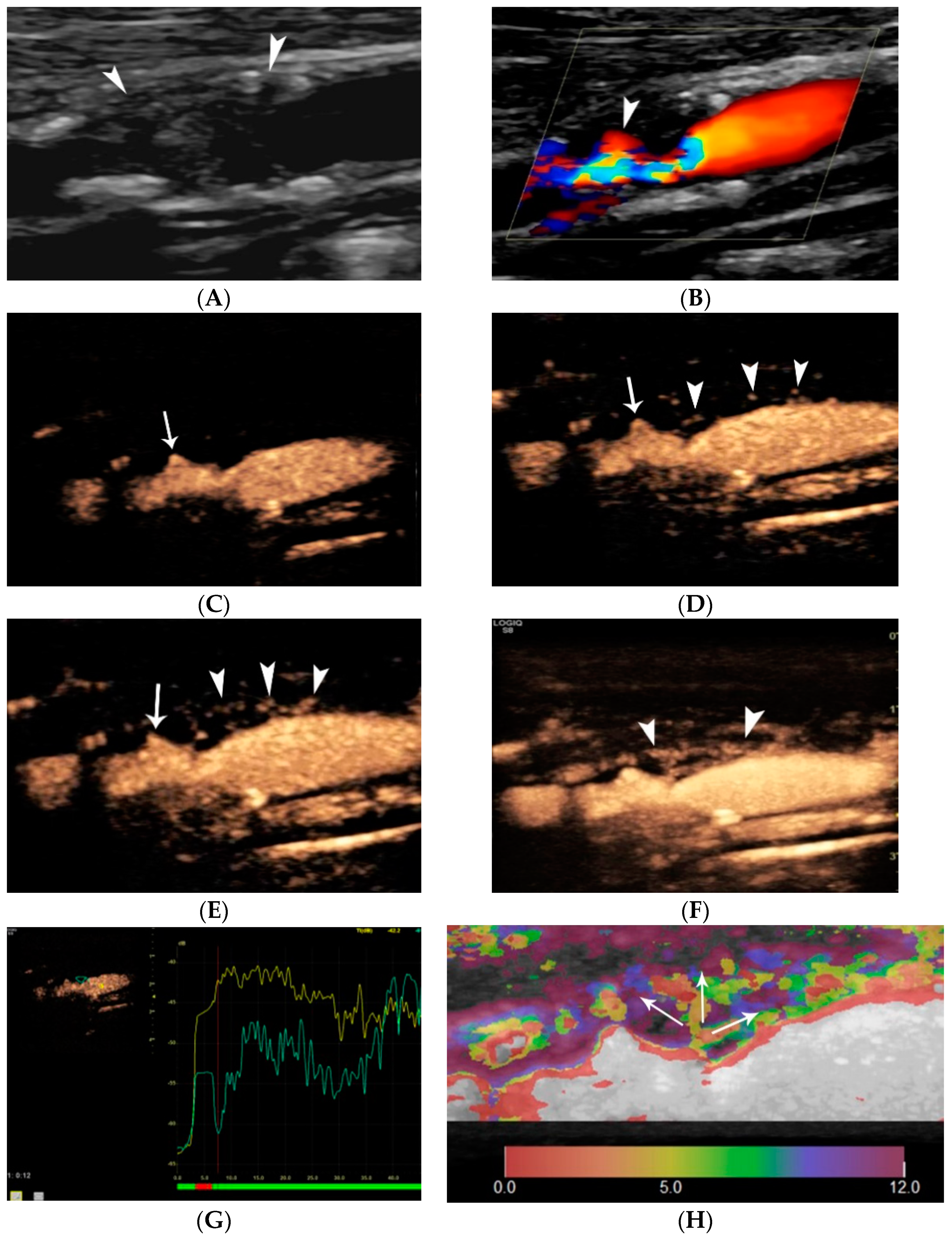
4.1.1. Intraplaque Neovascularization
4.1.2. Intraplaque Inflammation
4.1.3. Vulnerable Plaques
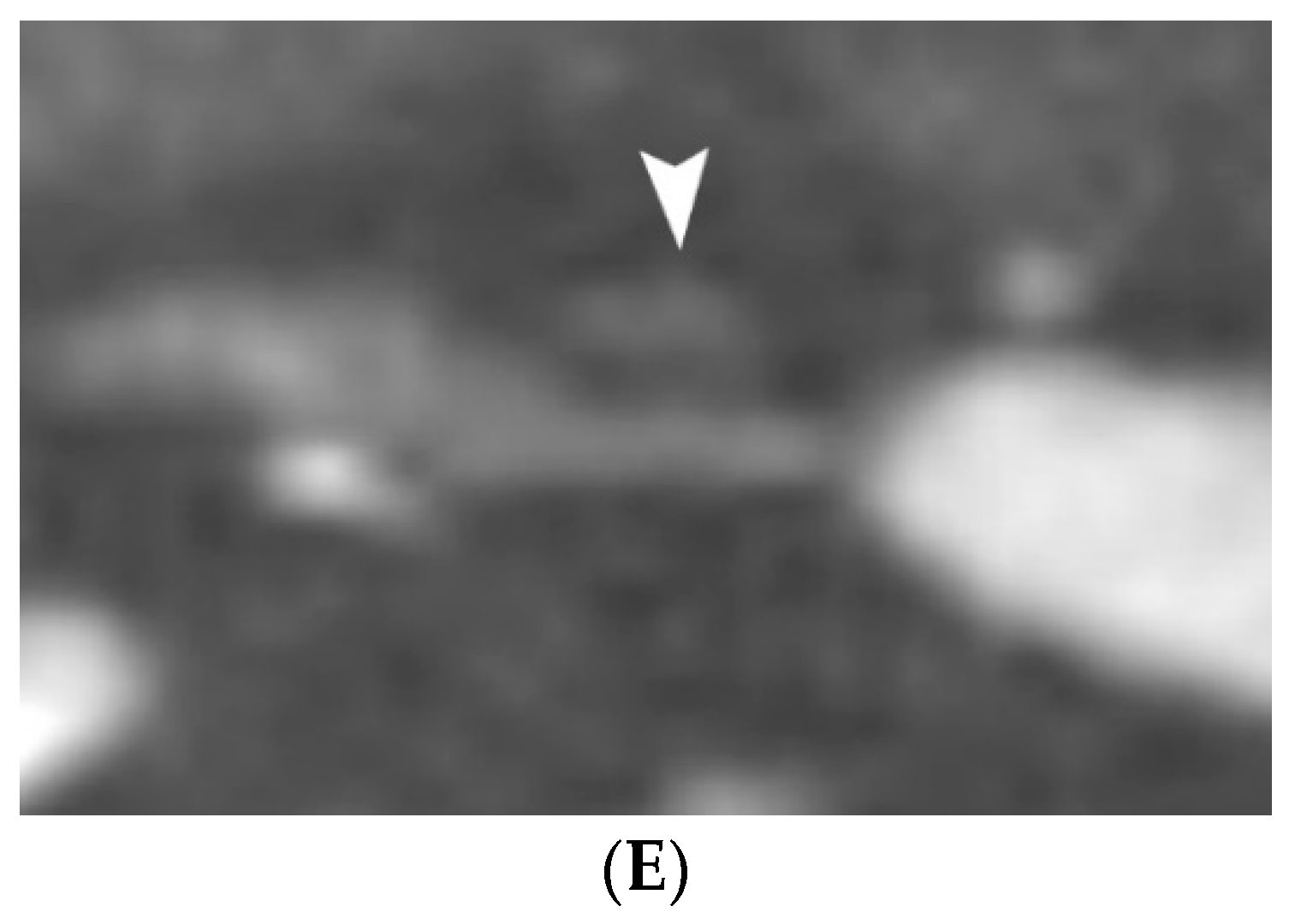
4.1.4. Plaque Ulceration
4.2. CEUS and Intraplaque Inflammation
4.2.1. Serum Inflammatory Markers
4.2.2. PET Imaging
4.3. CEUS and Clinical Events
4.3.1. Prior Cardiovascular/Cerebrovascular Events
4.3.2. Future Cardiovascular/Cerebrovascular Events
5. Elastography of Carotid Atherosclerotic Disease
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saba, L.; Yuan, C.; Hatsukami, T.S.; Balu, N.; Qiao, Y.; DeMarco, J.K.; Saam, T.; Moody, A.R.; Li, D.; Matouk, C.C.; et al. Carotid Artery Wall Imaging: Perspective and Guidelines from the ASNR Vessel Wall Imaging Study Group and Expert Consensus Recommendations of the American Society of Neuroradiology. Am. J. Neuroradiol. 2018, 39, E9–E31. [Google Scholar] [CrossRef]
- Rafailidis, V.; Li, X.; Sidhu, P.S.; Partovi, S.; Staub, D. Contrast imaging ultrasound for the detection and characterization of carotid vulnerable plaque. Cardiovasc. Diagn. Ther. 2020, 10, 965–981. [Google Scholar] [CrossRef]
- Sidhu, P.S. Multiparametric Ultrasound (MPUS) Imaging: Terminology Describing the Many Aspects of Ultrasonography. Ultraschall Med. 2015, 36, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, H.; Gupta, A.; Anzai, Y.; Mushlin, A.I.; Kamel, H.; Pandya, A. Cost Effectiveness of Assessing Ultrasound Plaque Characteristics to Risk Stratify Asymptomatic Patients With Carotid Stenosis. J. Am. Heart Assoc. 2019, 8, e012739. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Rabinstein, A.A.; Lanzino, G.; Murad, M.H.; Williamson, E.E.; DeMarco, J.K.; Huston, J., 3rd. Ultrasound Characteristics of Symptomatic Carotid Plaques: A Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2015, 40, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Katsanos, A.H.; Köhrmann, M.; Caso, V.; Lemmens, R.; Tsioufis, K.; Paraskevas, G.P.; Bornstein, N.M.; Schellinger, P.D.; Alexandrov, A.V.; et al. Embolic strokes of undetermined source: Theoretical construct or useful clinical tool? Ther. Adv. Neurol. Disord. 2019, 12, 1756286419851381. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Iguchi, Y.; Arai, A.; Sakuta, K.; Sakai, K.; Terasawa, Y.; Mitsumura, H.; Matsushima, M. Large but Nonstenotic Carotid Artery Plaque in Patients With a History of Embolic Stroke of Undetermined Source. Stroke 2018, 49, 3054–3056. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, J.M.; Derkatch, S.; Potvin, A.R.; Tomlinson, G.; Kiehl, T.R.; Silver, F.L.; Mandell, D.M. Nonstenotic carotid plaque on CT angiography in patients with cryptogenic stroke. Neurology 2016, 87, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Moody, A.R.; Panzov, V.; Gladstone, D.J. Carotid Intraplaque Hemorrhage in Patients with Embolic Stroke of Unde termined Source. J. Stroke Cerebrovasc. Dis. 2018, 27, 1956–1959. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, S.; Zhang, J.; Wang, F.; Liu, X.; He, W. Advance ultrasound techniques for the assessment of plaque vulnerability in symptomatic and asymptomatic carotid stenosis: A multimodal ultrasound study. Cardiovasc. Diagn. Ther. 2021, 11, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Saam, T.; Habs, M.; Buchholz, M.; Schindler, A.; Bayer-Karpinska, A.; Cyran, C.C.; Yuan, C.; Reiser, M.; Helck, A. Expansive arterial remodeling of the carotid arteries and its effect on atherosclerotic plaque composition and vulnerability: An in-vivo black-blood 3T CMR study in symptomatic stroke patients. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2016, 18, 11. [Google Scholar] [CrossRef]
- Gupta, A.; Kesavabhotla, K.; Baradaran, H.; Kamel, H.; Pandya, A.; Giambrone, A.E.; Wright, D.; Pain, K.J.; Mtui, E.E.; Suri, J.S.; et al. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: A systematic review and meta-analysis. Stroke 2015, 46, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Marko, M.; Ospel, J.M.; Goyal, M.; Almekhlafi, M. The Risk of Stroke and TIA in Nonstenotic Carotid Plaques: A Systematic Review and Meta-Analysis. Am. J. Neuroradiol. 2020, 41, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Kamtchum-Tatuene, J.; Noubiap, J.J.; Wilman, A.H.; Saqqur, M.; Shuaib, A.; Jickling, G.C. Prevalence of High-risk Plaques and Risk of Stroke in Patients With Asymptomatic Carotid Stenosis: A Meta-analysis. JAMA Neurol. 2020, 77, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Molecular bases of the acute coronary syndromes. Circulation 1995, 91, 2844–2850. [Google Scholar] [CrossRef]
- Donners, S.J.A.; Toorop, R.J.; de Kleijn, D.P.V.; de Borst, G.J. A narrative review of plaque and brain imaging biomarkers for stroke risk stratification in patients with atherosclerotic carotid artery disease. Ann. Transl. Med. 2021, 9, 1260. [Google Scholar] [CrossRef]
- Steffen, C.M.; Gray-Weale, A.C.; Byrne, K.E.; Lusby, R.J. Carotid artery atheroma: Ultrasound appearance in symptomatic and asymptomatic vessels. Aust. N. Z. J. Surg. 1989, 59, 529–534. [Google Scholar] [CrossRef]
- Geroulakos, G.; Ramaswami, G.; Nicolaides, A.; James, K.; Labropoulos, N.; Belcaro, G.; Holloway, M. Characterization of symptomatic and asymptomatic carotid plaques using high-resolution real-time ultrasonography. Br. J. Surg. 1993, 80, 1274–1277. [Google Scholar] [CrossRef]
- Brinjikji, W.; Huston, J., 3rd; Rabinstein, A.A.; Kim, G.M.; Lerman, A.; Lanzino, G. Contemporary carotid imaging: From degree of stenosis to plaque vulnerability. J. Neurosurg. 2016, 124, 27–42. [Google Scholar] [CrossRef]
- Jashari, F.; Ibrahimi, P.; Bajraktari, G.; Grönlund, C.; Wester, P.; Henein, M.Y. Carotid plaque echogenicity predicts cerebrovascular symptoms: A systematic review and meta-analysis. Eur. J. Neurol. 2016, 23, 1241–1247. [Google Scholar] [CrossRef]
- Poredos, P.; Gregoric, I.D.; Jezovnik, M.K. Inflammation of carotid plaques and risk of cerebrovascular events. Ann. Transl. Med. 2020, 8, 1281. [Google Scholar] [CrossRef] [PubMed]
- Kamtchum-Tatuene, J.; Nomani, A.Z.; Falcione, S.; Munsterman, D.; Sykes, G.; Joy, T.; Spronk, E.; Vargas, M.I.; Jickling, G.C. Non-stenotic Carotid Plaques in Embolic Stroke of Unknown Source. Front. Neurol. 2021, 12, 719329. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, H.; Gupta, A. Brain imaging biomarkers of carotid artery disease. Ann. Transl. Med. 2020, 8, 1277. [Google Scholar] [CrossRef] [PubMed]
- Gensicke, H.; van der Worp, H.B.; Nederkoorn, P.J.; Macdonald, S.; Gaines, P.A.; van der Lugt, A.; Mali, W.P.; Lyrer, P.A.; Peters, N.; Featherstone, R.L.; et al. Ischemic brain lesions after carotid artery stenting increase future cerebrovascular risk. J. Am. Coll. Cardiol. 2015, 65, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Hua, Y.; Ji, X.; Jia, L.; Zhang, K.; Li, Q.; Li, Q.; Yang, J.; Li, J.; Jiao, L. Ultrasound-Based Carotid Plaque Characteristics Help Predict New Cerebral Ischemic Lesions after Endarterectomy. Ultrasound Med. Biol. 2021, 47, 244–251. [Google Scholar] [CrossRef]
- Sabetai, M.M.; Tegos, T.J.; Nicolaides, A.N.; Dhanjil, S.; Pare, G.J.; Stevens, J.M. Reproducibility of computer-quantified carotid plaque echogenicity: Can we overcome the subjectivity? Stroke 2000, 31, 2189–2196. [Google Scholar] [CrossRef]
- Wohlin, M.; Sundström, J.; Andrén, B.; Larsson, A.; Lind, L. An echolucent carotid artery intima-media complex is a new and independent predictor of mortality in an elderly male cohort. Atherosclerosis 2009, 205, 486–491. [Google Scholar] [CrossRef]
- Sabetai, M.M.; Tegos, T.J.; Clifford, C.; Dhanjil, S.; Belcaro, G.; Kakkos, S.; Kalodiki, E.; Stevens, J.M.; Nicolaides, A.N. Carotid plaque echogenicity and types of silent CT-brain infarcts. Is there an association in patients with asymptomatic carotid stenosis? Int. Angiol. J. Int. Union Angiol. 2001, 20, 51–57. [Google Scholar]
- Kakkos, S.K.; Griffin, M.B.; Nicolaides, A.N.; Kyriacou, E.; Sabetai, M.M.; Tegos, T.; Makris, G.C.; Thomas, D.J.; Geroulakos, G. The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J. Vasc. Surg. 2013, 57, 609–618.e601; discussion 617–608. [Google Scholar] [CrossRef]
- Bassiouny, H.S.; Sakaguchi, Y.; Mikucki, S.A.; McKinsey, J.F.; Piano, G.; Gewertz, B.L.; Glagov, S. Juxtalumenal location of plaque necrosis and neoformation in symptomatic carotid stenosis. J. Vasc. Surg. 1997, 26, 585–594. [Google Scholar] [CrossRef]
- Trostdorf, F.; Buchkremer, M.; Harmjanz, A.; Kablau, M.; Jander, S.; Geiger, K.; Schmitz-Rixen, T.; Steinmetz, H.; Sitzer, M. Fibrous cap thickness and smooth muscle cell apoptosis in high-grade carotid artery stenosis. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2005, 29, 528–535. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Devuyst, G.; Ruchat, P.; Karapanayiotides, T.; Jonasson, L.; Cuisinaire, O.; Lobrinus, J.-A.; Pusztaszeri, M.; Kalangos, A.; Despland, P.-A.; Thiran, J.-P.; et al. Ultrasound Measurement of the Fibrous Cap in Symptomatic and Asymptomatic Atheromatous Carotid Plaques. Circulation 2005, 111, 2776–2782. [Google Scholar] [CrossRef] [PubMed]
- Fedak, A.; Ciuk, K.; Urbanik, A. Ultrasonography of vulnerable atherosclerotic plaque in the carotid arteries: B-mode imaging. J. Ultrason. 2020, 20, e135–e145. [Google Scholar] [CrossRef] [PubMed]
- Park, A.Y.; Seo, B.K.; Cho, K.R.; Woo, O.H. The Utility of MicroPure™ Ultrasound Technique in Assessing Grouped Microcalcifications without a Mass on Mammography. J. Breast Cancer 2016, 19, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cai, J.; He, Y.; Sun, C.; Lian, X. Usability of Ultrasonic MicroPure Imaging for Evaluating the Vulnerability of Carotid Atherosclerotic Plaques. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2021, 40, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimi, P.; Jashari, F.; Bajraktari, G.; Wester, P.; Henein, M.Y. Ultrasound assessment of carotid plaque echogenicity response to statin therapy: A systematic review and meta-analysis. Int. J. Mol. Sci. 2015, 16, 10734–10747. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, X.Q.; Liu, H.Y.; Bi, X.J.; Liu, Y.N.; Lv, W.Z.; Xiong, L.; Deng, Y.B. Relation of Carotid Plaque Features Detected with Ultrasonography-Based Radiomics to Clinical Symptoms. Transl. Stroke Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Jamthikar, A.D.; Gupta, D.; Saba, L.; Khanna, N.N.; Viskovic, K.; Mavrogeni, S.; Laird, J.R.; Sattar, N.; Johri, A.M.; Pareek, G.; et al. Artificial intelligence framework for predictive cardiovascular and stroke risk assessment models: A narrative review of integrated approaches using carotid ultrasound. Comput. Biol. Med. 2020, 126, 104043. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Caddeo, G.; Sanfilippo, R.; Montisci, R.; Mallarini, G. CT and ultrasound in the study of ulcerated carotid plaque compared with surgical results: Potentialities and advantages of multidetector row CT angiography. Am. J. Neuroradiol. 2007, 28, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Sitzer, M.; Müller, W.; Siebler, M.; Hort, W.; Kniemeyer, H.W.; Jäncke, L.; Steinmetz, H. Plaque ulceration and lumen thrombus are the main sources of cerebral microemboli in high-grade internal carotid artery stenosis. Stroke 1995, 26, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Muraki, M.; Mikami, T.; Yoshimoto, T.; Fujimoto, S.; Tokuda, K.; Kaneko, S.; Kashiwaba, T. New criteria for the sonographic diagnosis of a plaque ulcer in the extracranial carotid artery. Am. J. Roentgenol. 2012, 198, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- De Bray, J.M.; Baud, J.M.; Dauzat, M. Consensus Concerning the Morphology and the Risk of Carotid Plaques. Cerebrovasc. Dis. 1997, 7, 289–296. [Google Scholar] [CrossRef]
- Saba, L.; Anzidei, M.; Marincola, B.C.; Piga, M.; Raz, E.; Bassareo, P.P.; Napoli, A.; Mannelli, L.; Catalano, C.; Wintermark, M. Imaging of the Carotid Artery Vulnerable Plaque. Cardiovasc. Interv. Radiol. 2014, 37, 572–585. [Google Scholar] [CrossRef]
- Lovett, J.K.; Gallagher, P.J.; Hands, L.J.; Walton, J.; Rothwell, P.M. Histological correlates of carotid plaque surface morphology on lumen contrast imaging. Circulation 2004, 110, 2190–2197. [Google Scholar] [CrossRef]
- Eliasziw, M.; Streifler, J.Y.; Fox, A.J.; Hachinski, V.C.; Ferguson, G.G.; Barnett, H.J. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Stroke 1994, 25, 304–308. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Gibson, R.; Warlow, C.P. Interrelation between plaque surface morphology and degree of stenosis on carotid angiograms and the risk of ischemic stroke in patients with symptomatic carotid stenosis. On behalf of the European Carotid Surgery Trialists’ Collaborative Group. Stroke 2000, 31, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Valton, L.; Larrue, V.; Arrué, P.; Géraud, G.; Bès, A. Asymptomatic cerebral embolic signals in patients with carotid stenosis. Correlation with appearance of plaque ulceration on angiography. Stroke 1995, 26, 813–815. [Google Scholar] [CrossRef]
- Orlandi, G.; Parenti, G.; Landucci Pellegrini, L.; Sartucci, F.; Paoli, C.; Puglioli, M.; Collavoli, P.; Murri, L. Plaque surface and microembolic signals in moderate carotid stenosis. Ital. J. Neurol. Sci. 1999, 20, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Ballotta, E.; Angelini, A.; Mazzalai, F.; Piatto, G.; Toniato, A.; Baracchini, C. Carotid endarterectomy for symptomatic low-grade carotid stenosis. J. Vasc. Surg. 2014, 59, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Barnett, H.J.M.; Taylor, D.W.; Haynes, R.B.; Sackett, D.L.; Peerless, S.J.; Ferguson, G.G.; Fox, A.J.; Rankin, R.N.; Hachinski, V.C.; Wiebers, D.O.; et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991, 325, 445–453. [Google Scholar] [CrossRef]
- Singh, T.D.; Kramer, C.L.; Mandrekar, J.; Lanzino, G.; Rabinstein, A.A. Asymptomatic Carotid Stenosis: Risk of Progression and Development of Symptoms. Cerebrovasc. Dis. 2015, 40, 236–243. [Google Scholar] [CrossRef]
- Tegos, T.J.; Kalodiki, E.; Daskalopoulou, S.S.; Nicolaides, A.N. Stroke: Epidemiology, clinical picture, and risk factors--Part I of III. Angiology 2000, 51, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Brajović, M.D.; Marković, N.; Loncar, G.; Sekularac, N.; Kordić, D.; Despotović, N.; Erceg, P.; Donfrid, B.; Stefanović, Z.; Bajcetić, M.; et al. The influence of various morphologic and hemodynamic carotid plaque characteristics on neurological events onset and deaths. Sci. World J. 2009, 9, 509–521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Handa, N.; Matsumoto, M.; Maeda, H.; Hougaku, H.; Kamada, T. Ischemic stroke events and carotid atherosclerosis. Results of the Osaka Follow-up Study for Ultrasonographic Assessment of Carotid Atherosclerosis (the OSACA Study). Stroke 1995, 26, 1781–1786. [Google Scholar] [CrossRef]
- Nakamura, T.; Tsutsumi, Y.; Shimizu, Y.; Uchiyama, S. Ulcerated carotid plaques with ultrasonic echolucency are causatively associated with thromboembolic cerebrovascular events. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2013, 22, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Warlow, C.P. Symptomatic patients: The European Carotid Surgery Trial (ECST). J. Des Mal. Vasc. 1993, 18, 198–201. [Google Scholar]
- Chang-Hong, H.; Xiao-Chen, X.; Cannata, J.M.; Yen, J.T.; Shung, K.K. Development of a real-time, high-frequency ultrasound digital beamformer for high-frequency linear array transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 317–323. [Google Scholar] [CrossRef]
- Comerota, A.J.; Katz, M.L.; White, J.V.; Grosh, J.D. The preoperative diagnosis of the ulcerated carotid atheroma. J. Vasc. Surg. 1990, 11, 505–510. [Google Scholar] [CrossRef]
- Snow, M.; Ben-Sassi, A.; Winter, R.K.; Verghese, A.; Hibberd, R.; Saad, R.A.; Morris Stiff, G.; Lewis, M.H. Can carotid ultrasound predict plaque histopathology? J. Cardiovasc. Surg. 2007, 48, 299–303. [Google Scholar]
- Sitzer, M.; Müller, W.; Rademacher, J.; Siebler, M.; Hort, W.; Kniemeyer, H.W.; Steinmetz, H. Color-flow doppler-assisted duplex imaging fails to detect ulceration in high-grade internal carotid artery stenosis. J. Vasc. Surg. 1996, 23, 461–465. [Google Scholar] [CrossRef][Green Version]
- Jung, E.M.; Kubale, R.; Ritter, G.; Gallegos, M.T.; Jungius, K.P.; Rupp, N.; Clevert, D.A. Diagnostics and characterisation of preocclusive stenoses and occlusions of the internal carotid artery with B-flow. Eur. Radiol. 2007, 17, 439–447. [Google Scholar] [CrossRef]
- Umemura, A.; Yamada, K. B-Mode Flow Imaging of the Carotid Artery. Stroke 2001, 32, 2055–2057. [Google Scholar] [CrossRef]
- Tegos, T.J.; Kalomiris, K.J.; Sabetai, M.M.; Kalodiki, E.; Nicolaides, A.N. Significance of Sonographic Tissue and Surface Characteristics of Carotid Plaques. Am. J. Neuroradiol. 2001, 22, 1605–1612. [Google Scholar] [PubMed]
- Kanber, B.; Hartshorne, T.C.; Horsfield, M.A.; Naylor, A.R.; Robinson, T.G.; Ramnarine, K.V. Quantitative assessment of carotid plaque surface irregularities and correlation to cerebrovascular symptoms. Cardiovasc. Ultrasound 2013, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, V.; Chryssogonidis, I.; Grisan, E.; Xerras, C.; Cheimariotis, G.A.; Tegos, T.; Rafailidis, D.; Sidhu, P.S.; Charitanti-Kouridou, A. Does Quantification of Carotid Plaque Surface Irregularities Better Detect Symptomatic Plaques Compared to the Subjective Classification? J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2019, 38, 3163–3171. [Google Scholar] [CrossRef]
- Kanber, B.; Hartshorne, T.C.; Horsfield, M.A.; Naylor, A.R.; Robinson, T.G.; Ramnarine, K.V. A Novel Ultrasound-Based Carotid Plaque Risk Index Associated with the Presence of Cerebrovascular Symptoms. Ultraschall Med. 2015, 36, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, V.; Chryssogonidis, I.; Xerras, C.; Grisan, E.; Cheimariotis, G.A.; Tegos, T.; Rafailidis, D.; Sidhu, P.S.; Charitanti-Kouridou, A. An Ultrasonographic Multiparametric Carotid Plaque Risk Index Associated with Cerebrovascular Symptomatology: A Study Comparing Color Doppler Imaging and Contrast-Enhanced Ultrasonography. Am. J. Neuroradiol. 2019, 40, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Heliopoulos, J.; Vadikolias, K.; Piperidou, C.; Mitsias, P. Detection of carotid artery plaque ulceration using 3-dimensional ultrasound. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2011, 21, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Madani, A.; Beletsky, V.; Tamayo, A.; Munoz, C.; Spence, J.D. High-risk asymptomatic carotid stenosis: Ulceration on 3D ultrasound vs. TCD microemboli. Neurology 2011, 77, 744–750. [Google Scholar] [CrossRef]
- Dunmore, B.J.; McCarthy, M.J.; Naylor, A.R.; Brindle, N.P. Carotid plaque instability and ischemic symptoms are linked to immaturity of microvessels within plaques. J. Vasc. Surg. 2007, 45, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Ladich, E.R.; Burke, A.P.; Kolodgie, F.D. Histopathology of carotid atherosclerotic disease. Neurosurgery 2006, 59, S219–S227; discussion S213. [Google Scholar] [CrossRef]
- Huang, R.; Abdelmoneim, S.S.; Ball, C.A.; Nhola, L.F.; Farrell, A.M.; Feinstein, S.; Mulvagh, S.L. Detection of Carotid Atherosclerotic Plaque Neovascularization Using Contrast Enhanced Ultrasound: A Systematic Review and Meta-Analysis of Diagnostic Accuracy Studies. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2016, 29, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Balan, P.; Weinberg, M.; Reddy, V.; Neems, R.; Feinstein, M.; Dainauskas, J.; Meyer, P.; Goldin, M.; Feinstein, S.B. Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: A new surrogate marker of atherosclerosis? Vasc. Med. 2007, 12, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, W.; Guo, D.; Chen, L.; Jin, X.; Wang, W.; Huang, B.; Wang, W. Quantification of carotid plaque neovascularization using contrast-enhanced ultrasound with histopathologic validation. Ultrasound Med. Biol. 2014, 40, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Van den Oord, S.C.; Akkus, Z.; Bosch, J.G.; Hoogi, A.; ten Kate, G.L.; Renaud, G.; Sijbrands, E.J.; Verhagen, H.J.; van der Lugt, A.; Adam, D.; et al. Quantitative contrast-enhanced ultrasound of intraplaque neovascularization in patients with carotid atherosclerosis. Ultraschall Med. 2015, 36, 154–161. [Google Scholar] [CrossRef]
- Hoogi, A.; Adam, D.; Hoffman, A.; Kerner, H.; Reisner, S.; Gaitini, D. Carotid plaque vulnerability: Quantification of neovascularization on contrast-enhanced ultrasound with histopathologic correlation. Am. J. Roentgenol. 2011, 196, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Faggioli, G.L.; Pini, R.; Mauro, R.; Pasquinelli, G.; Fittipaldi, S.; Freyrie, A.; Serra, C.; Stella, A. Identification of carotid ‘vulnerable plaque’ by contrast-enhanced ultrasonography: Correlation with plaque histology, symptoms and cerebral computed tomography. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2011, 41, 238–248. [Google Scholar] [CrossRef]
- McCabe, J.J.; Camps-Renom, P.; Giannotti, N.; McNulty, J.P.; Coveney, S.; Murphy, S.; Barry, M.; Harbison, J.; Cronin, S.; Williams, D.; et al. Carotid Plaque Inflammation Imaged by PET and Prediction of Recurrent Stroke at 5 Years. Neurology 2021, 97, e2282–e2291. [Google Scholar] [CrossRef]
- Demeure, F.; Bouzin, C.; Roelants, V.; Bol, A.; Verhelst, R.; Astarci, P.; Gerber, B.L.; Pouleur, A.C.; Pasquet, A.; de Meester, C.; et al. Head-to-Head Comparison of Inflammation and Neovascularization in Human Carotid Plaques: Implications for the Imaging of Vulnerable Plaques. Circ. Cardiovasc. Imaging 2017, 10, e005846. [Google Scholar] [CrossRef] [PubMed]
- Shalhoub, J.; Monaco, C.; Owen, D.R.J.; Gauthier, T.; Thapar, A.; Leen, E.L.S.; Davies, A.H. Late-Phase Contrast-Enhanced Ultrasound Reflects Biological Features of Instability in Human Carotid Atherosclerosis. Stroke 2011, 42, 3634–3636. [Google Scholar] [CrossRef][Green Version]
- Baragetti, A.; Mattavelli, E.; Grigore, L.; Pellegatta, F.; Magni, P.; Catapano, A.L. Targeted Plasma Proteomics to Predict the Development of Carotid Plaques. Stroke 2022, 53, e411–e414. [Google Scholar] [CrossRef]
- Iezzi, R.; Petrone, G.; Ferrante, A.; Lauriola, L.; Vincenzoni, C.; la Torre, M.F.; Snider, F.; Rindi, G.; Bonomo, L. The role of contrast-enhanced ultrasound (CEUS) in visualizing atherosclerotic carotid plaque vulnerability: Which injection protocol? Which scanning technique? Eur. J. Radiol. 2015, 84, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Amamoto, T.; Sakata, N.; Ogata, T.; Shimada, H.; Inoue, T. Intra-Plaque Vessels on Contrast-Enhanced Ultrasound Sonography Predict Carotid Plaque Histology. Cerebrovasc. Dis. 2018, 46, 265–269. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, M.; Chiarandini, S.; Pipitone, M.D.; Fisicaro, M.; Calvagna, C.; Bussani, R.; Rotelli, A.; Ziani, B. Contrast Enhanced Ultrasound (CEUS) Is Not Able to Identify Vulnerable Plaques in Asymptomatic Carotid Atherosclerotic Disease. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Vavuranakis, M.; Sigala, F.; Vrachatis, D.A.; Papaioannou, T.G.; Filis, K.; Kavantzas, N.; Kalogeras, K.I.; Massoura, C.; Toufektzian, L.; Kariori, M.G.; et al. Quantitative analysis of carotid plaque vasa vasorum by CEUS and correlation with histology after endarterectomy. VASA Z. Gefasskrankh. 2013, 42, 184–195. [Google Scholar] [CrossRef]
- Rafailidis, V.; Chryssogonidis, I.; Tegos, T.; Kouskouras, K.; Charitanti-Kouridou, A. Imaging of the ulcerated carotid atherosclerotic plaque: A review of the literature. Insights Imaging 2017, 8, 213–225. [Google Scholar] [CrossRef]
- Hamada, O.; Sakata, N.; Ogata, T.; Shimada, H.; Inoue, T. Contrast-enhanced ultrasonography for detecting histological carotid plaque rupture: Quantitative analysis of ulcer. Int. J. Stroke 2016, 11, 791–798. [Google Scholar] [CrossRef] [PubMed]
- ten Kate, G.L.; van Dijk, A.C.; van den Oord, S.C.; Hussain, B.; Verhagen, H.J.; Sijbrands, E.J.; van der Steen, A.F.; van der Lugt, A.; Schinkel, A.F. Usefulness of contrast-enhanced ultrasound for detection of carotid plaque ulceration in patients with symptomatic carotid atherosclerosis. Am. J. Cardiol. 2013, 112, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.A.; Gourineni, V.; Feinstein, S.B. The Use of Contrast-enhanced Ultrasonography for Imaging of Carotid Atherosclerotic Plaques: Current Evidence, Future Directions. Neuroimaging Clin. N. Am. 2016, 26, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, V.; Charitanti, A.; Tegos, T.; Rafailidis, D.; Chryssogonidis, I. Swirling of microbubbles: Demonstration of a new finding of carotid plaque ulceration on contrast-enhanced ultrasound explaining the arterio-arterial embolism mechanism. Clin. Hemorheol. Microcirc. 2016, 64, 245–250. [Google Scholar] [CrossRef]
- Rafailidis, V.; Chryssogonidis, I.; Xerras, C.; Nikolaou, I.; Tegos, T.; Kouskouras, K.; Rafailidis, D.; Charitanti-Kouridou, A. A comparative study of color Doppler imaging and contrast-enhanced ultrasound for the detection of ulceration in patients with carotid atherosclerotic disease. Eur. Radiol. 2019, 29, 2137–2145. [Google Scholar] [CrossRef]
- Lyu, Q.; Tian, X.; Ding, Y.; Yan, Y.; Huang, Y.; Zhou, P.; Hui, P. Evaluation of Carotid Plaque Rupture and Neovascularization by Contrast-Enhanced Ultrasound Imaging: An Exploratory Study Based on Histopathology. Transl. Stroke Res. 2021, 12, 49–56. [Google Scholar] [CrossRef]
- Kaspar, M.; Partovi, S.; Aschwanden, M.; Imfeld, S.; Baldi, T.; Uthoff, H.; Staub, D. Assessment of microcirculation by contrast-enhanced ultrasound: A new approach in vascular medicine. Swiss Med. Wkly. 2015, 145, w14047. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.F.L.; Bosch, J.G.; Staub, D.; Adam, D.; Feinstein, S.B. Contrast-Enhanced Ultrasound to Assess Carotid Intraplaque Neovascularization. Ultrasound Med. Biol. 2020, 46, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, S.; Malato, A.; Saccullo, G.; Iorio, A.; Di Ianni, M.; Caracciolo, C.; Coco, L.L.; Raso, S.; Santoro, M.; Guarneri, F.P.; et al. Residual vein thrombosis for assessing duration of anticoagulation after unprovoked deep vein thrombosis of the lower limbs: The extended DACUS study. Am. J. Hematol. 2011, 86, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Garcia, B.; Ruiz, C.; Chacon, P.; Sabin, J.A.; Matas, M. High-sensitivity C-reactive protein in high-grade carotid stenosis: Risk marker for unstable carotid plaque. J. Vasc. Surg. 2003, 38, 1018–1024. [Google Scholar] [CrossRef]
- Chang, X.; Feng, J.; Ruan, L.; Shang, J.; Yang, Y.; Sun, J.; Dang, Y.; Song, Y. Positive correlation between neovascularization degree of carotid atherosclerosis determined by contrast-enhanced ultrasound and level of serum C-reactive protein. VASA Z. Gefasskrankh. 2015, 44, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yin, X.; Xu, W.; Jin, L.; Lu, M.; Wang, Y. Assessment of carotid plaque neovascularization by contrast-enhanced ultrasound and high sensitivity C-reactive protein test in patients with acute cerebral infarction: A comparative study. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2016, 37, 1107–1112. [Google Scholar] [CrossRef]
- Li, Z.; Bai, Y.; Li, W.; Gao, F.; Kuang, Y.; Du, L.; Luo, X. Carotid vulnerable plaques are associated with circulating leukocytes in acute ischemic stroke patients: An clinical study based on contrast-enhanced ultrasound. Sci. Rep. 2018, 8, 8849. [Google Scholar] [CrossRef]
- Piri, R.; Gerke, O.; Høilund-Carlsen, P.F. Molecular imaging of carotid artery atherosclerosis with PET: A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2016–2025. [Google Scholar] [CrossRef]
- Hjelmgren, O.; Johansson, L.; Prahl, U.; Schmidt, C.; Fredén-Lindqvist, J.; Bergström, G.M.L. A study of plaque vascularization and inflammation using quantitative contrast-enhanced US and PET/CT. Eur. J. Radiol. 2014, 83, 1184–1189. [Google Scholar] [CrossRef]
- Staub, D.; Patel, M.B.; Tibrewala, A.; Ludden, D.; Johnson, M.; Espinosa, P.; Coll, B.; Jaeger, K.A.; Feinstein, S.B. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke 2010, 41, 41–47. [Google Scholar] [CrossRef]
- Yan, H.; Wu, X.; He, Y.; Staub, D.; Wen, X.; Luo, Y. Carotid Intraplaque Neovascularization on Contrast-Enhanced Ultrasound Correlates with Cardiovascular Events and Poor Prognosis: A Systematic Review and Meta-analysis. Ultrasound Med. Biol. 2021, 47, 167–176. [Google Scholar] [CrossRef]
- Deyama, J.; Nakamura, T.; Takishima, I.; Fujioka, D.; Kawabata, K.; Obata, J.E.; Watanabe, K.; Watanabe, Y.; Saito, Y.; Mishina, H.; et al. Contrast-enhanced ultrasound imaging of carotid plaque neovascularization is useful for identifying high-risk patients with coronary artery disease. Circ. J. Off. J. Jpn. Circ. Soc. 2013, 77, 1499–1507. [Google Scholar] [CrossRef]
- Mantella, L.E.; Colledanchise, K.N.; Hétu, M.F.; Feinstein, S.B.; Abunassar, J.; Johri, A.M. Carotid intraplaque neovascularization predicts coronary artery disease and cardiovascular events. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1239–1247. [Google Scholar] [CrossRef]
- Huang, R.; DeMarco, J.K.; Ota, H.; Macedo, T.A.; Abdelmoneim, S.S.; Huston, J., 3rd; Pellikka, P.A.; Mulvagh, S.L. Prognostic Value of Intraplaque Neovascularization Detected by Carotid Contrast-Enhanced Ultrasound in Patients Undergoing Stress Echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2021, 34, 614–624. [Google Scholar] [CrossRef]
- Camps-Renom, P.; Prats-Sánchez, L.; Casoni, F.; González-de-Echávarri, J.M.; Marrero-González, P.; Castrillón, I.; Marín, R.; Jiménez-Xarrié, E.; Delgado-Mederos, R.; Martínez-Domeño, A.; et al. Plaque neovascularization detected with contrast-enhanced ultrasound predicts ischaemic stroke recurrence in patients with carotid atherosclerosis. Eur. J. Neurol. 2020, 27, 809–816. [Google Scholar] [CrossRef]
- Song, Y.; Dang, Y.; Wang, J.; Cai, H.; Feng, J.; Zhang, H.; Ruan, L. Carotid Intraplaque Neovascularization Predicts Ischemic Stroke Recurrence in Patients with Carotid Atherosclerosis. Gerontology 2021, 67, 144–151. [Google Scholar] [CrossRef]
- Bamber, J.; Cosgrove, D.; Dietrich, C.F.; Fromageau, J.; Bojunga, J.; Calliada, F.; Cantisani, V.; Correas, J.M.; D’Onofrio, M.; Drakonaki, E.E.; et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013, 34, 169–184. [Google Scholar] [CrossRef]
- Hansen, H.H.G.; de Borst, G.J.; Bots, M.L.; Moll, F.L.; Pasterkamp, G.; de Korte, C.L. Validation of Noninvasive In Vivo Compound Ultrasound Strain Imaging Using Histologic Plaque Vulnerability Features. Stroke 2016, 47, 2770–2775. [Google Scholar] [CrossRef]
- Sarvazyan, A.P.; Rudenko, O.V.; Swanson, S.D.; Fowlkes, J.B.; Emelianov, S.Y. Shear wave elasticity imaging: A new ultrasonic technology of medical diagnostics. Ultrasound Med. Biol. 1998, 24, 1419–1435. [Google Scholar] [CrossRef]
- Chai, C.-K.; Akyildiz, A.C.; Speelman, L.; Gijsen, F.J.H.; Oomens, C.W.J.; van Sambeek, M.R.H.M.; van der Lugt, A.; Baaijens, F.P.T. Local axial compressive mechanical properties of human carotid atherosclerotic plaques—Characterisation by indentation test and inverse finite element analysis. J. Biomech. 2013, 46, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Golemati, S.; Cokkinos, D.D. Recent advances in vascular ultrasound imaging technology and their clinical implications. Ultrasonics 2022, 119, 106599. [Google Scholar] [CrossRef]
- Di Leo, N.; Venturini, L.; de Soccio, V.; Forte, V.; Lucchetti, P.; Cerone, G.; Alagna, G.; Caratozzolo, M.; Messineo, D.; Di Gioia, C.; et al. Multiparametric ultrasound evaluation with CEUS and shear wave elastography for carotid plaque risk stratification. J. Ultrasound 2018, 21, 293–300. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, Z.; Huang, C.; Huang, M.; Huang, L.; Xu, D.; Zhang, H.; Yuan, C.; Luo, J. Interoperator Reproducibility of Carotid Elastography for Identification of Vulnerable Atherosclerotic Plaques. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 505–516. [Google Scholar] [CrossRef]
- Liu, F.; Yong, Q.; Zhang, Q.; Liu, P.; Yang, Y. Real-Time Tissue Elastography for the Detection of Vulnerable Carotid Plaques in Patients Undergoing Endarterectomy: A Pilot Study. Ultrasound Med. Biol. 2015, 41, 705–712. [Google Scholar] [CrossRef]
- Lou, Z.; Yang, J.; Tang, L.; Jin, Y.; Zhang, J.; Liu, C.; Li, Q. Shear Wave Elastography Imaging for the Features of Symptomatic Carotid Plaques: A Feasibility Study. J. Ultrasound Med. 2017, 36, 1213–1223. [Google Scholar] [CrossRef]
- Shang, J.; Wang, W.; Feng, J.; Luo, G.G.; Dang, Y.; Sun, J.; Yang, Y.Q.; Ruan, L.T. Carotid Plaque Stiffness Measured with Supersonic Shear Imaging and Its Correlation with Serum Homocysteine Level in Ischemic Stroke Patients. Korean J. Radiol. 2018, 19, 15–22. [Google Scholar] [CrossRef]
- Ramnarine, K.V.; Garrard, J.W.; Kanber, B.; Nduwayo, S.; Hartshorne, T.C.; Robinson, T.G. Shear wave elastography imaging of carotid plaques: Feasible, reproducible and of clinical potential. Cardiovasc. Ultrasound 2014, 12, 49. [Google Scholar] [CrossRef]
- Garrard, J.W.; Ummur, P.; Nduwayo, S.; Kanber, B.; Hartshorne, T.C.; West, K.P.; Moore, D.; Robinson, T.G.; Ramnarine, K.V. Shear Wave Elastography May Be Superior to Greyscale Median for the Identification of Carotid Plaque Vulnerability: A Comparison with Histology. Ultraschall Med. 2015, 36, 386–390. [Google Scholar] [CrossRef]
- Doherty, J.R.; Dahl, J.J.; Kranz, P.G.; Husseini, N.E.; Chang, H.C.; Chen, N.k.; Allen, J.D.; Ham, K.L.; Trahey, G.E. Comparison of Acoustic Radiation Force Impulse Imaging Derived Carotid Plaque Stiffness With Spatially Registered MRI Determined Composition. IEEE Trans. Med. Imaging 2015, 34, 2354–2365. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; He, Q.; Huang, M.; Huang, L.; Zhao, X.; Yuan, C.; Luo, J. Non-Invasive Identification of Vulnerable Atherosclerotic Plaques Using Texture Analysis in Ultrasound Carotid Elastography: An in vivo Feasibility Study Validated by Magnetic Resonance Imaging. Ultrasound Med. Biol. 2017, 43, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Czernuszewicz, T.J.; Homeister, J.W.; Caughey, M.C.; Wang, Y.; Zhu, H.; Huang, B.Y.; Lee, E.R.; Zamora, C.A.; Farber, M.A.; Fulton, J.J.; et al. Performance of acoustic radiation force impulse ultrasound imaging for carotid plaque characterization with histologic validation. J. Vasc. Surg. 2017, 66, 1749–1757.e1743. [Google Scholar] [CrossRef] [PubMed]
- Nordenfur, T.; Caidahl, K.; Grishenkov, D.; Maksuti, E.; Marlevi, D.; Urban, M.W.; Larsson, M. Safety of arterial shear wave elastography-ex-vivo assessment of induced strain and strain rates. Biomed. Phys. Eng. Express 2022, 8, 055012. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandratou, M.; Papachristodoulou, A.; Li, X.; Partovi, S.; Davidhi, A.; Rafailidis, V.; Prassopoulos, P.; Kamperidis, V.; Koutroulou, I.; Tsivgoulis, G.; et al. Advances in Noninvasive Carotid Wall Imaging with Ultrasound: A Narrative Review. J. Clin. Med. 2022, 11, 6196. https://doi.org/10.3390/jcm11206196
Alexandratou M, Papachristodoulou A, Li X, Partovi S, Davidhi A, Rafailidis V, Prassopoulos P, Kamperidis V, Koutroulou I, Tsivgoulis G, et al. Advances in Noninvasive Carotid Wall Imaging with Ultrasound: A Narrative Review. Journal of Clinical Medicine. 2022; 11(20):6196. https://doi.org/10.3390/jcm11206196
Chicago/Turabian StyleAlexandratou, Maria, Angeliki Papachristodoulou, Xin Li, Sasan Partovi, Andjoli Davidhi, Vasileios Rafailidis, Panos Prassopoulos, Vasileios Kamperidis, Ioanna Koutroulou, Georgios Tsivgoulis, and et al. 2022. "Advances in Noninvasive Carotid Wall Imaging with Ultrasound: A Narrative Review" Journal of Clinical Medicine 11, no. 20: 6196. https://doi.org/10.3390/jcm11206196
APA StyleAlexandratou, M., Papachristodoulou, A., Li, X., Partovi, S., Davidhi, A., Rafailidis, V., Prassopoulos, P., Kamperidis, V., Koutroulou, I., Tsivgoulis, G., Grigoriadis, N., Krogias, C., & Karapanayiotides, T. (2022). Advances in Noninvasive Carotid Wall Imaging with Ultrasound: A Narrative Review. Journal of Clinical Medicine, 11(20), 6196. https://doi.org/10.3390/jcm11206196






