Invasive Diagnostic and Therapeutic Management of Cerebral VasoSpasm after Aneurysmal Subarachnoid Hemorrhage (IMCVS)—A Phase 2 Randomized Controlled Trial
Abstract
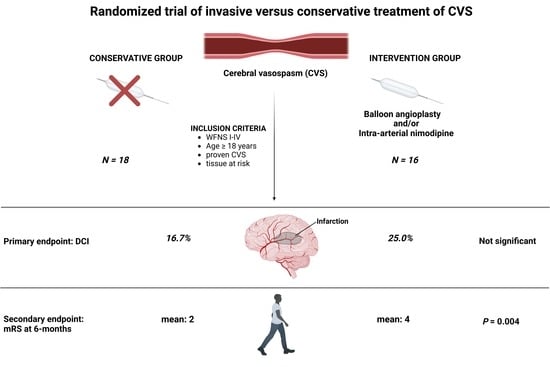
:1. Introduction
2. Materials and Methods
2.1. Definitions and Data Recording
2.2. Inclusion Criteria
2.3. Randomization
2.4. Conservative Treatment
2.5. Invasive Endovascular Treatment
2.6. Outcome Measurement and Endpoints
2.7. Sample Size Calculation
2.8. Statistics
3. Results
3.1. Patient Characteristics
3.2. Initial MRI
3.3. Follow-Up MRI
3.4. Frequency, Success, and Complications of Endovascular Treatment
3.5. Outcome
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, Y.B.; de Haan, R.J.; Beenen, L.F.; Groen, R.J.; Albrecht, K.W.; Vermeulen, M. Complications and outcome in patients with aneurysmal subarachnoid haemorrhage: A prospective hospital based cohort study in the Netherlands. J. Neurol. Neurosurg. Psychiatry 2000, 68, 337–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mees, S.D.; Rinkel, G.J.; Feigin, V.L.; Algra, A.; Bergh, W.M.V.D.; Vermeulen, M.; Van Gijn, J. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst. Rev. 2007, 2007, CD000277. [Google Scholar]
- Schmidt, J.M.; Wartenberg, K.E.; Fernandez, A.; Claassen, J.; Rincon, F.; Ostapkovich, N.D.; Badjatia, N.; Parra, A.; Connolly, E.S.; Mayer, S.A. Frequency and clinical impact of asymptomatic cerebral infarction due to vasospasm after subarachnoid hemorrhage. J. Neurosurg. 2008, 109, 1052–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muizelaar, J.P.; Becker, D.P. Induced hypertension for the treatment of cerebral ischemia after subarachnoid hemorrhage. Direct effect on cerebral blood flow. Surg. Neurol. 1986, 25, 317–325. [Google Scholar] [CrossRef]
- Bulsara, K.R.; Gunel, M.; Amin-Hanjani, S.; Chen, P.R.; Connolly, E.S.; Friedlander, R.M. Results of a national cerebrovascular neurosurgery survey on the management of cerebral vasospasm/delayed cerebral ischemia. J. Neurointerv. Surg. 2015, 7, 408–411. [Google Scholar] [CrossRef]
- Boulouis, G.; Labeyrie, M.A.; Raymond, J.; Rodriguez-Régent, C.; Lukaszewicz, A.C.; Bresson, D.; Ben Hassen, W.; Trystram, D.; Meder, J.F.; Oppenheim, C.; et al. Treatment of cerebral vasospasm following aneurysmal subarachnoid haemorrhage: A systematic review and meta-analysis. Eur. Radiol. 2017, 27, 3333–3342. [Google Scholar] [CrossRef]
- Vatter, H.; Güresir, E.; Berkefeld, J.; Beck, J.; Raabe, A.; Rochemont, R.D.M.D.; Seifert, V.; Weidauer, S. Perfusion-diffusion mismatch in MRI to indicate endovascular treatment of cerebral vasospasm after subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 2011, 82, 876–883. [Google Scholar] [CrossRef]
- Beck, J.; Raabe, A.; Lanfermann, H.; Berkefeld, J.; Rochemont, R.D.M.D.; Zanella, F.; Seifert, V.; Weidauer, S. Effects of balloon angioplasty on perfusion- and diffusion-weighted magnetic resonance imaging results and outcome in patients with cerebral vasospasm. J. Neurosurg. 2006, 105, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Turowski, B.; du Mesnil de Rochemont, R.; Beck, J.; Berkefeld, J.; Zanella, F.E. Assessment of changes in cerebral circulation time due to vasospasm in a specific arterial territory: Effect of angioplasty. Neuroradiology 2005, 47, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Raabe, A.; Lanfermann, H.; Seifert, V.; Weidauer, S. Tissue at risk concept for endovascular treatment of severe vasospasm after aneurysmal subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1779–1781. [Google Scholar] [CrossRef] [PubMed]
- Weidauer, S.; Lanfermann, H.; Raabe, A.; Zanella, F.; Seifert, V.; Beck, J. Impairment of cerebral perfusion and infarct patterns attributable to vasospasm after aneurysmal subarachnoid hemorrhage: A prospective MRI and DSA study. Stroke 2007, 38, 1831–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidauer, S.; Vatter, H.; Beck, J.; Raabe, A.; Lanfermann, H.; Seifert, V.; Zanella, F. Focal laminar cortical infarcts following aneurysmal subarachnoid haemorrhage. Neuroradiology 2008, 50, 1–8. [Google Scholar] [CrossRef]
- Connolly, E.S., Jr.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muench, E.; Horn, P.; Bauhuf, C.; Roth, H.; Philipps, M.; Hermann, P.; Quintel, M.; Schmiedek, P.; Vajkoczy, P. Effects of hypervolemia and hypertension on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation after subarachnoid hemorrhage. Crit. Care Med. 2007, 35, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Suwatcharangkoon, S.; De Marchis, G.M.; Witsch, J.; Meyers, E.; Velazquez, A.; Falo, C.; Schmidt, J.M.; Agarwal, S.; Connolly, E.S.; Claassen, J.; et al. Medical Treatment Failure for Symptomatic Vasospasm After Subarachnoid Hemorrhage Threatens Long-Term Outcome. Stroke 2019, 50, 1696–1702. [Google Scholar] [CrossRef]
- Gathier, C.S.; van den Bergh, W.M.; van der Jagt, M.; Verweij, B.H.; Dankbaar, J.W.; Müller, M.C.; Oldenbeuving, A.W.; Rinkel, G.J.; Slooter, A.J. Induced Hypertension for Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage: A Randomized Clinical Trial. Stroke 2018, 49, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haegens, N.M.; Gathier, C.S.; Horn, J.; Coert, B.A.; Verbaan, D.; van den Bergh, W.M. Induced Hypertension in Preventing Cerebral Infarction in Delayed Cerebral Ischemia After Subarachnoid Hemorrhage. Stroke 2018, 49, 2630–2636. [Google Scholar] [CrossRef] [Green Version]
- Hosmann, A.; Angelmayr, C.; Hopf, A.; Rauscher, S.; Brugger, J.; Ritscher, L.; Bohl, I.; Schnackenburg, P.; Engel, A.; Plöchl, W.; et al. Detrimental effects of intrahospital transport on cerebral metabolism in patients suffering severe aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2021, 1–8. [Google Scholar] [CrossRef]
- Adami, D.; Berkefeld, J.; Platz, J.; Konczalla, J.; Pfeilschifter, W.; Weidauer, S.; Wagner, M. Complication rate of intraarterial treatment of severe cerebral vasospasm after subarachnoid hemorrhage with nimodipine and percutaneous transluminal balloon angioplasty: Worth the risk? J. Neuroradiol. 2019, 46, 15–24. [Google Scholar] [CrossRef]
- Güresir, E.; Welchowski, T.; Lampmann, T.; Brandecker, S.; Güresir, A.; Wach, J.; Lehmann, F.; Dorn, F.; Velten, M.; Vatter, H. Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage: The Results of Induced Hypertension Only after the IMCVS Trial—A Prospective Cohort Study. J. Clin. Med. 2022, 11, 5850. [Google Scholar] [CrossRef] [PubMed]


| Variable | Conservative (n = 18) | Invasive (n = 16) | p-Value |
|---|---|---|---|
| Age, Y ± SD, mean | 55 ± 10 | 56 ± 12 | |
| Smoker | 9/14 | 9/14 | 1.0 |
| Diabetes | 0/18 | 2/15 | 0.2 |
| Hypertension before SAH | 8/18 | 8/15 | 0.7 |
| Coronary heart disease | 2/18 | 0/16 | 0.5 |
| Adipositas | 1/12 | 1/15 | 1.0 |
| BMI | 27 ± 3 | 22 ± 0.4 | 0.4 |
| History of malignoma | 0 | 0 | |
| History of cerebral ischemia | 1/18 | 1/16 | |
| Extensive alcohol consumption | 2/18 | 1/16 | |
| History of myocardial infarction | 2/18 | 0 | |
| History of SAH (from the same aneurysm) | 1/18 | 0 | |
| mRS before SAH | 0 | 0 | |
| Karnofsky before SAH | 100 ± 0 | 99 ± 3 | 0.1 |
| Hydrocephalus at admission | 13/16 | 12/16 | 1.0 |
| GCS | 9 ± 5 | 8 ± 4 | 0.6 |
| WFNS grade | 4 ± 2 | 4 ± 1 | 0.6 |
| Fisher score | 3 ± 0.5 | 3 ± 0.4 | 0.2 |
| IVH | 5/16 | 4/14 | 1.0 |
| ICH < 3 cm | 2/15 | 5/15 | 0.1 |
| ICH > 3 cm | 2/15 | 5/15 | 0.1 |
| Clipping | 10/18 | 8/18 * | |
| Coiling | 8/18 | 10/18 * |
| Frequency of DSA | No. of pts. | Endovascular Treatment (i.a. Nimodipine/PTA) | No. of pts. | Treatment Success According to Interventionalist |
|---|---|---|---|---|
| 1 | 16 | i.a. nimodipine | 16 | Good (16/16) |
| 2 | 11 | i.a. nimodipine | 7/11 | Good (4/10) |
| Fair (3/10) | ||||
| PTA | 3/11 | Good (3/10) | ||
| none | 1/11 | Massive thromboembolic event | ||
| 3 | 4 | i.a. nimodipine | 3/4 | Good (1/4) |
| Fair (1/4) | ||||
| No success (1/4) | ||||
| PTA | 1/4 | Good (1/4) | ||
| 4 | 2 | i.a. nimodipine | 2 | Good (0/2) |
| Fair (1/2) | ||||
| No success (1/2) | ||||
| 5 | 1 | i.a. nimodipine | 1 | Fair (1/1) |
| Frequency of DSA | No. of pts. | Complications | No. of pts. | Severity of Complication/Necessity of Treatment |
|---|---|---|---|---|
| 1 | 16 | None | ||
| 2 | 11 | Dissection | 1 | Stent implantation |
| Bleeding | 0 | |||
| Thromboembolic event | 1 | Minor supratentorial infarction | ||
| Embolic infarction | 1 | Massive cerebellar and brainstem infarction | ||
| 3 | 4 | Dissection | 1 | Stent implantation |
| Bleeding | 0 | |||
| Thromboembolic event | 1 | Major supratentorial infarction | ||
| Embolic infarction | 0 | |||
| 4 | 2 | Dissection | 0 | |
| Bleeding | 0 | |||
| Thromboembolic event | 0 | |||
| Embolic infarction | 0 | |||
| 5 | 1 | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vatter, H.; Güresir, E.; König, R.; Durner, G.; Kalff, R.; Schuss, P.; Mayer, T.E.; Konczalla, J.; Hattingen, E.; Seifert, V.; et al. Invasive Diagnostic and Therapeutic Management of Cerebral VasoSpasm after Aneurysmal Subarachnoid Hemorrhage (IMCVS)—A Phase 2 Randomized Controlled Trial. J. Clin. Med. 2022, 11, 6197. https://doi.org/10.3390/jcm11206197
Vatter H, Güresir E, König R, Durner G, Kalff R, Schuss P, Mayer TE, Konczalla J, Hattingen E, Seifert V, et al. Invasive Diagnostic and Therapeutic Management of Cerebral VasoSpasm after Aneurysmal Subarachnoid Hemorrhage (IMCVS)—A Phase 2 Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(20):6197. https://doi.org/10.3390/jcm11206197
Chicago/Turabian StyleVatter, Hartmut, Erdem Güresir, Ralph König, Gregor Durner, Rolf Kalff, Patrick Schuss, Thomas E. Mayer, Jürgen Konczalla, Elke Hattingen, Volker Seifert, and et al. 2022. "Invasive Diagnostic and Therapeutic Management of Cerebral VasoSpasm after Aneurysmal Subarachnoid Hemorrhage (IMCVS)—A Phase 2 Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 20: 6197. https://doi.org/10.3390/jcm11206197
APA StyleVatter, H., Güresir, E., König, R., Durner, G., Kalff, R., Schuss, P., Mayer, T. E., Konczalla, J., Hattingen, E., Seifert, V., & Berkefeld, J. (2022). Invasive Diagnostic and Therapeutic Management of Cerebral VasoSpasm after Aneurysmal Subarachnoid Hemorrhage (IMCVS)—A Phase 2 Randomized Controlled Trial. Journal of Clinical Medicine, 11(20), 6197. https://doi.org/10.3390/jcm11206197