The Role of Novel Cardiac Imaging for Contemporary Management of Heart Failure
Abstract
1. Introduction
2. Is It Heart Failure?
3. Valvular Heart Disease
4. Left and Right Ventricular Function
5. Cardiac Imaging for Monitoring Heart Failure
6. Structure of the Myocardium
- Early and late tissue enhancement after contrast (gadolinium) application allows separation of intact myocardium from extracellular space, since intact myocardiocytes do not allow the intracellular accumulation of gadolinium. Thus, late gadolinium enhancement can detect myocardial scars as locally increased extracellular volume, such as infarcts, post-myocarditis damage, sarcoidosis, and other localized increases of extracellular volume (Figure 4). This technique relies on relative contrast intensity differences between myocardial regions, as in a myocardial infarction, but does not work in the presence of diffuse alterations of extracellular space. Though all of the cited etiologies result in the formation of localized increases of extracellular volume, often the “gestalt” of such a region of late contrast enhancement suggests a specific etiology. For example, subendocardial or transmural late enhancement following the “wavefront phenomenon” of myocardial ischemia in a coronary perfusion territory is strongly suggestive of post-infarct scar, and mid-wall late enhancement, especially in the lateral wall, suggests post-myocarditis damage. Note that similar principles enable contrast-enhanced CT to diagnose localized increases in extracellular space [36] in a similar manner to CMR, although the achieved contrast between normal and diseased regions is weaker than with CMR techniques.
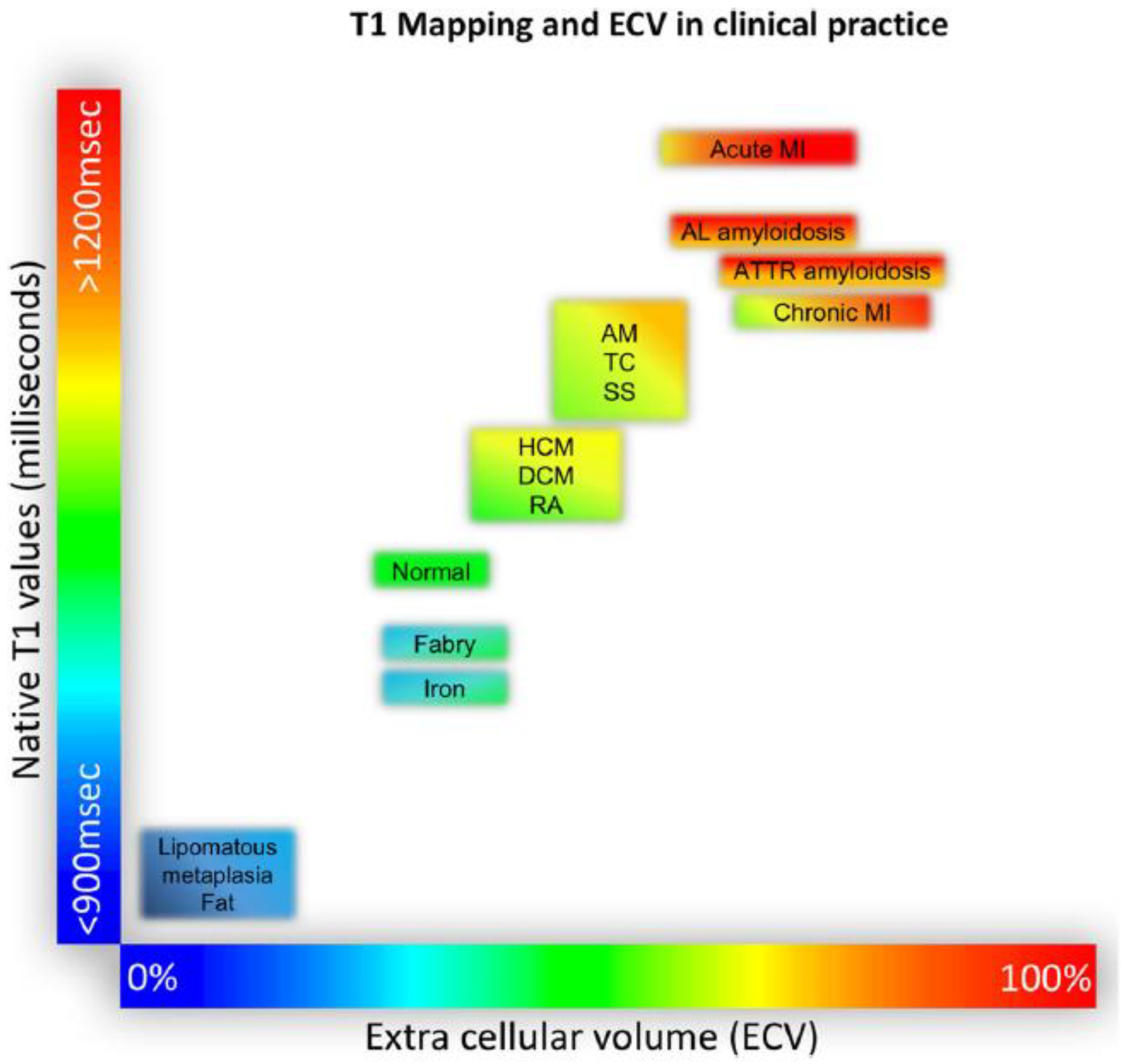
- Pixel-wise maps of the magnetic relaxation parameters T1, T2, and T2*, called “parametric imaging”, allow a limited characterization of underlying tissue ([37]; Figure 5). Additionally, by pre- and post-contrast registration of T1 in the myocardium and blood pool, a direct relative measure of extracellular volume (in percent) for each pixel can be calculated. For example, hemochromatosis leads to shortening and amyloidosis leads to lengthening of T1 relaxation times, which sets these pathologies apart from others. Myocardial edema leads to lengthening of T1 and T2 times and the expansion of extracellular volume, which in themselves are non-specific, but may aid in the diagnosis of, e.g., myocarditis, depending on clinical circumstances. Notably, increased extracellular space may be indicative of diffuse fibrosis but also, e.g., tissue edema. Hence, although, for example, an increased T1 value does correlate modestly with myocardial fibrosis and may, therefore, support the diagnosis of diastolic dysfunction and HFpEF, the relaxation parameters are multifactorial and should not be mistaken for true histology.
7. Myocardial Perfusion
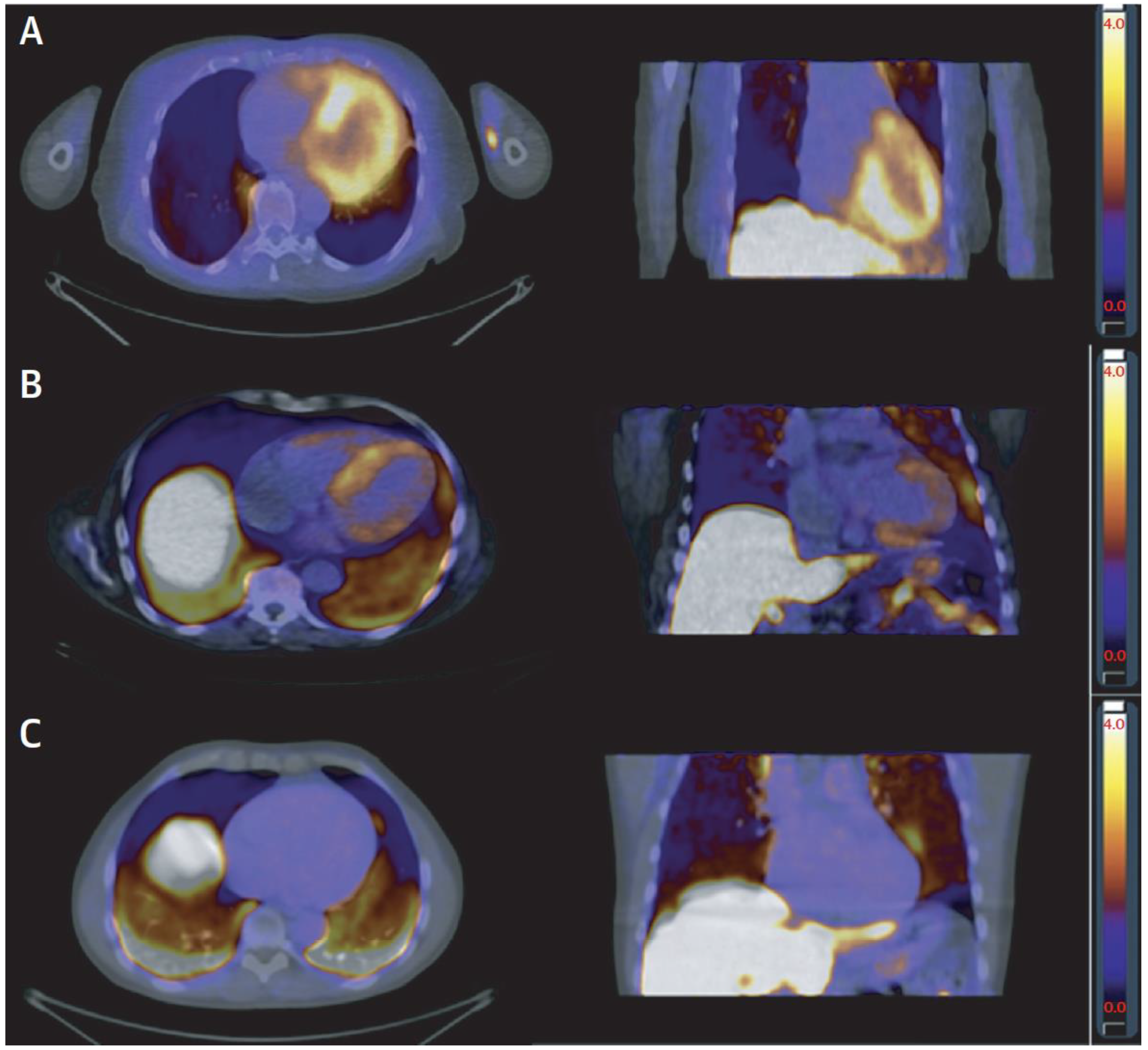
8. Other Nuclear Imaging
9. Cardiac Computed Tomography (CT)
10. Pulmonary Hypertension
11. How Should Cardiac Imaging Be Used “Wisely” in the Work-Up of Heart Failure?
12. Summary
Funding
Conflicts of Interest
References
- Scholten, M.; Midlöv, P.; Halling, A. Disparities in prevalence of heart failure according to age, multimorbidity level and socioeconomic status in southern Sweden: A cross-sectional study. BMJ Open 2022, 12, e051997. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
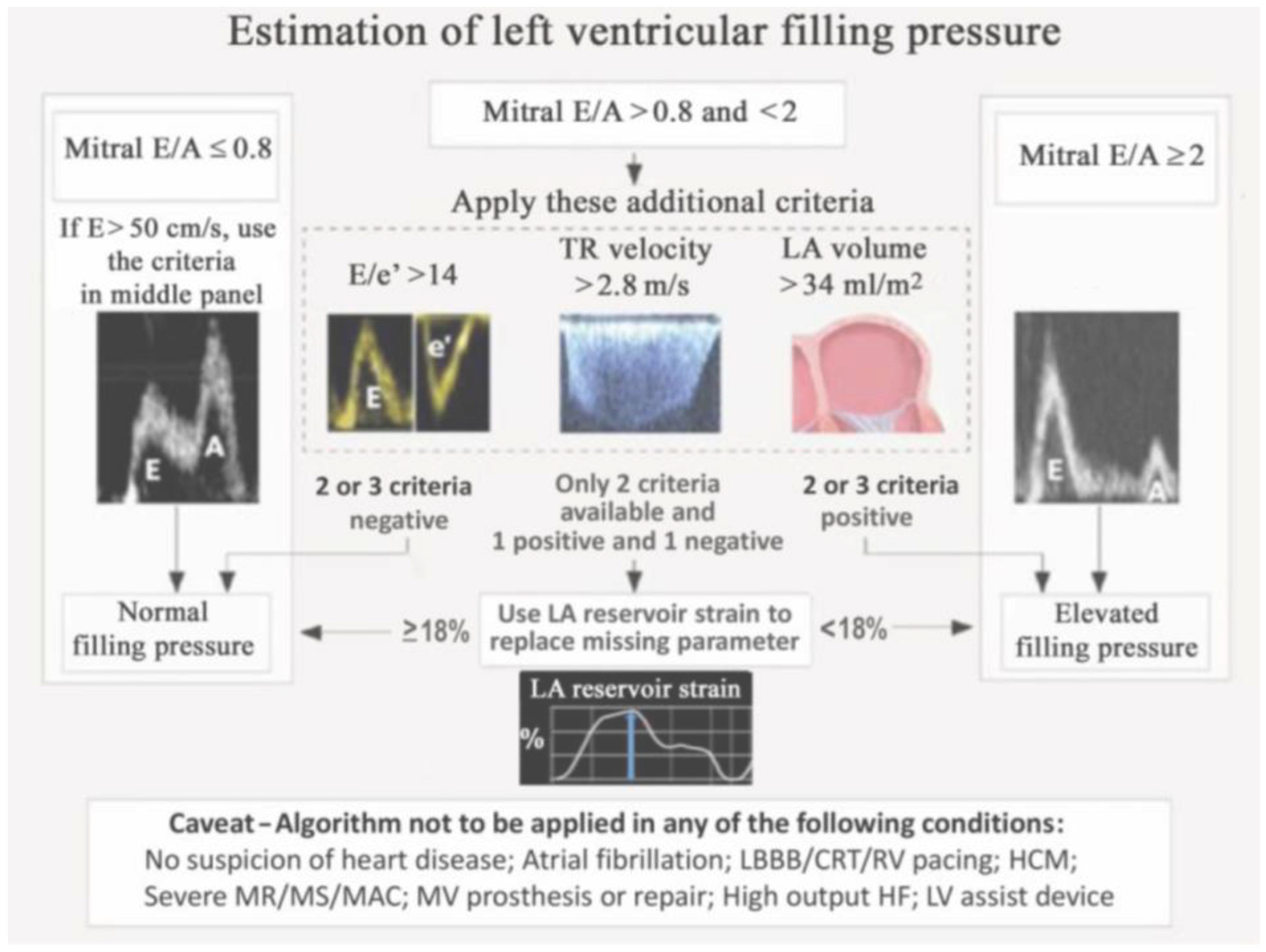
- Andersen, O.S.; Smiseth, O.A.; Dokainish, H.; Abudiab, M.M.; Schutt, R.C.; Kumar, A.; Sato, K.; Harb, S.; Gude, E.; Remme, E.W.; et al. Estimating left ventricular filling pressure by echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1937–1948. [Google Scholar] [CrossRef]
- Pak, M.; Kitai, T.; Kobori, A.; Sasaki, Y.; Okada, T.; Murai, R.; Toyota, T.; Kim, K.; Ehara, N.; Kinoshita, M.; et al. Diagnostic Accuracy of the 2016 Guideline-Based Echocardiographic Algorithm to Estimate Invasively-Measured Left Atrial Pressure by Direct Atrial Cannulation. J. Am. Coll. Cardiol. Img. 2022, 15, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Negishi, T.; Somerset, E.; Negishi, K.; Penicka, M.; Lemieux, J.; Aakhus, S.; Miyazaki, S.; Shirazi, M.; Galderisi, M.; et al. Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy. J. Am. Coll. Cardiol. 2021, 77, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Kalam, K.; Otahal, P.; Marwick, T.H. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014, 100, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
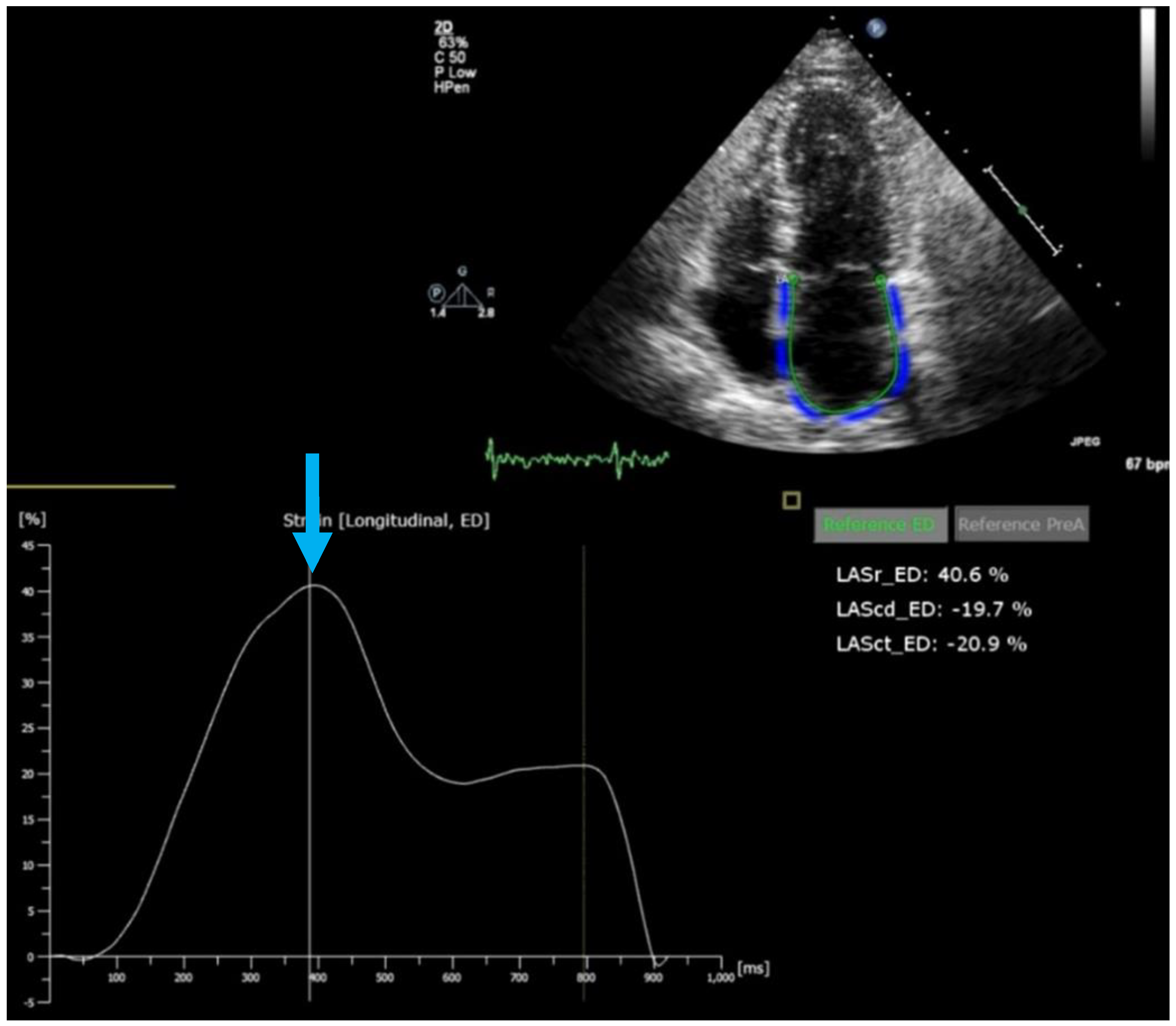
- Inoue, K.; Khan, F.H.; Remme, E.W.; Ohte, N.; Garcıa-Izquierdo, E.; Chetrit, M.; Moñivas-Palomero, V.; Mingo-Santos, S.; Andersen, Ø.S.; Gude, E.; et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, O.A.; Morris, D.A.; Cardim, N.; Cikes, M.; Delgado, V.; Donal, E.; Flachskampf, F.A.; Galderisi, M.; Gerber, B.L.; Gimelli, A.; et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e34–e61. [Google Scholar] [CrossRef]
- Obokata, M.; Negishi, K.; Kurosawa, K.; Arima, H.; Tateno, R.; Ui, G.; Tange, S.; Arai, M.; Kurabayashi, M. Incremental diagnostic value of la strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovasc. Imaging 2013, 6, 749–758. [Google Scholar] [CrossRef]
- Yamada, H.; Kusunose, K.; Nishio, S.; Bando, M.; Hotchi, J.; Hayashi, S.; Ise, T.; Yagi, S.; Yamaguchi, K.; Iwase, T.; et al. Preload Stress Echocardiography Predicts Outcomes in Patients with Preserved Ejection Fraction and Low-Gradient Aortic Stenosis. JACC Cardiovasc. Imaging 2014, 7, 641–649. [Google Scholar] [CrossRef]
- Tossavainen, E.; Wikström, G.; Henein, M.Y.; Lundqvist, M.; Wiklund, U.; Lindqvist, P. Passive leg-lifting in heart failure patients predicts exercise-induced rise in left ventricular filling pressures. Clin. Res. Cardiol. 2020, 109, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Helle-Valle, T.; Remme, E.W.; Lyseggen, E.; Pettersen, E.; Vartdal, T.; Opdahl, A.; Smith, H.J.; Osman, N.F.; Ihlen, H.; Edvardsen, T.; et al. Clinical assessment of left ventricular rotation and strain: A novel approach for quantification of function in infarcted myocardium and its border zones. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H257–H267. [Google Scholar] [CrossRef] [PubMed]
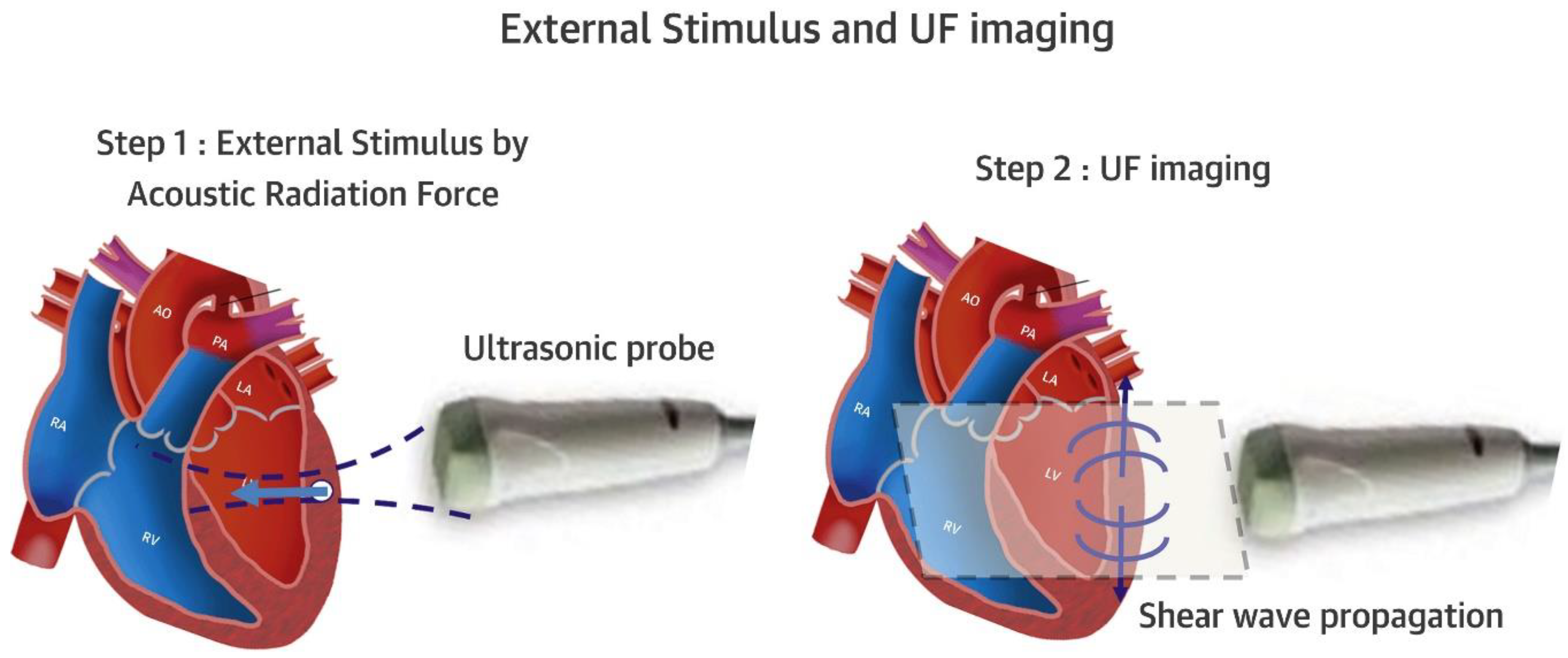
- Villemain, O.; Correia, M.; Mousseaux, E.; Baranger, J.; Zarka, S.; Podetti, I.; Soulat, G.; Damy, T.; Hagège, A.; Tanter, M.; et al. Myocardial stiffness evaluation using noninvasive shear wave imaging in healthy and hypertrophic cardiomyopathic adults. J. Am. Coll. Cardiovasc. Imaging 2019, 12, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Villemain, O.; Baranger, J.; Friedberg, M.K.; Papadacci, C.; Dizeux, A.; Messas, E.; Tanter, M.; Pernot, M.; Mertens, L. Ultrafast Ultrasound Imaging in Pediatric and Adult Cardiology. Techniques, Applications, and Perspectives. JACC Cardiovasc. Imaging 2020, 13, 1771–1791. [Google Scholar] [CrossRef] [PubMed]
- Doltra, A.; Bijnens, B.; Tolosana, J.M.; Borràs, R.; Khatib, M.; Penela, D.; De Caralt, T.M.; Castel, M.Á.; Berruezo, A.; Brugada, J.; et al. Mechanical abnormalities detected with conventional echocardiography are associated with response and midterm survival in CRT. JACC Cardiovasc. Imaging 2014, 7, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Baron, T.; Berglund, L.; Hedin, E.M.; Flachskampf, F.A. Test-retest reliability of new and conventional echocardiographic parameters of left ventricular systolic function. Clin. Res. Cardiol. 2019, 108, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Houard, L.; Militaru, S.; Tanaka, K.; Pasquet, A.; Vancraeynest, D.; Vanoverschelde, J.L.; Pouleur, A.C.; Gerber, B.L. Test-retest reliability of left and right ventricular systolic function by new and conventional echocardiographic and cardiac magnetic resonance parameters. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1157–1167. [Google Scholar] [CrossRef]
- Kass, D.A.; Beyar, R. Evaluation of contractile state by maximal ventricular power divided by the square of end-diastolic volume. Circulation 1991, 84, 1698–1708. [Google Scholar] [CrossRef]
- Harada, T.; Yamaguchi, M.; Omote, K.; Iwano, H.; Mizuguchi, Y.; Amanai, S.; Yoshida, K.; Kato, T.; Kurosawa, K.; Nagai, T.; et al. Cardiac Power Output Is Independently and Incrementally Associated with Adverse Outcomes in Heart Failure with Preserved Ejection Fraction. Circ. Cardiovasc. Imaging 2022, 15, e013495. [Google Scholar] [CrossRef]
- Sörensen, J.; Harms, H.J.; Aalen, J.M.; Baron, T.; Smiseth, O.A.; Flachskampf, F.A. Myocardial Efficiency: A Fundamental Physiological Concept on the Verge of Clinical Impact. JACC Cardiovasc. Imaging 2020, 13, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Aalen, J.M.; Donal, E.; Larsen, C.K.; Duchenne, J.; Lederlin, M.; Cvijic, M.; Hubert, A.; Voros, G.; Leclercq, C.; Bogaert, J.; et al. Imaging predictors of response to cardiac resynchronization therapy: Left ventricular work asymmetry by echocardiography and septal viability by cardiac magnetic resonance. Eur. Heart J. 2020, 41, 3813–3823. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, O.A.; Donal, E.; Penicka, M.; Sletten, O.J. How to measure ventricular myocardial work by pressure-strain loops. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 259–261. [Google Scholar] [PubMed]
- Truong, V.T.; Vo, H.Q.; Ngo, T.N.M.; Mazur, J.; Nguyen, T.T.H.; Pham, T.T.M.; Le, T.K.; Phan, H.; Palmer, C.; Nagueh, S.F.; et al. Normal Ranges of Global Left Ventricular Myocardial Work Indices in Adults: A Meta-Analysis. J. Am. Soc. Echocardiogr. 2022, 35, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Flachskampf, F.A.; Chandrashekar, Y. Myocardial work and myocardial work index: Relatives, but different. JACC Cardiovascular. Imaging 2022, in press. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Temporelli, P.L.; Arena, R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1373–H1381. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Smith, J.; Grigoryan, K.; Lysne, V.; Rajani, R.; Chambers, J.B. The tricuspid annular plane systolic excursion to systolic pulmonary artery pressure index:Association with all-cause mortality in patients with moderate or severe tricuspid regurgitation. Int. J. Cardiol. 2020, 317, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Karam, N.; Stolz, L.; Orban, M.; Deseive, S.; Praz, F.; Kalbacher, D.; Westermann, D.; Braun, D.; Näbauer, M.; Neuss, M.; et al. Impact of Right Ventricular Dysfunction on Outcomes After Transcatheter Edge-to-Edge Repair for Secondary Mitral Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Karam, N.; Mehr, M.; Taramasso, M.; Besler, C.; Ruf, T.; Connelly, K.A.; Weber, M.; Yzeiraj, E.; Schiavi, D.; Mangieri, A.; et al. Value of Echocardiographic Right Ventricular and Pulmonary Pressure Assessment in Predicting Transcatheter Tricuspid Repair Outcome. JACC Cardiovasc. Interv. 2020, 13, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.Y.; Brooksbank, K.J.M.; Wetherall, K.; Mangion, K.; Roditi, G.; Campbell, R.T.; Berry, C.; Chong, V.; Coyle, L.; Docherty, K.F.; et al. Effect of Empagliflozin on Left Ventricular Volumes in Patients with Type 2 Diabetes, or Prediabetes, and Heart Failure With Reduced Ejection Fraction (SUGAR-DM-HF). Circulation 2021, 143, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, N.K.; Mistry, N.; Puar, P.; Verma, R.; Anker, S.; Mazer, C.D.; Verma, S. SGLT2 inhibitors and cardiac remodelling: A systematic review and meta-analysis of randomized cardiac magnetic resonance imaging trials. ESC Heart Fail. 2021, 8, 4693–4700. [Google Scholar] [CrossRef] [PubMed]
- Sado, D.M.; White, S.K.; Piechnik, S.K.; Banypersad, S.M.; Treibel, T.; Captur, G.; Fontana, M.; Maestrini, V.; Flett, A.S.; Robson, M.D.; et al. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ. Cardiovasc. Imaging 2013, 6, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Phelan, D.; Collier, P.; Thavendiranathan, P.; Popović, Z.B.; Hanna, M.; Plana, J.C.; Marwick, T.H.; Thomas, J.D. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012, 98, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Scully, P.R.; Patel, K.P.; Kammerlander, A.A.; Koschutnik, M.; Dona, C.; Wollenweber, T.; Ahmed, N.; Thornton, G.D.; Kelion, A.D.; et al. Prevalence and Outcomes of Concomitant Aortic Stenosis and Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2021, 77, 128–139. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Peker, E.; Chandrashekhar, Y.; Nagel, E. T1 Mapping in Characterizing Myocardial Disease: A Comprehensive Review. Circ. Res. 2016, 119, 277–299. [Google Scholar] [CrossRef]
- Rodriguez-Granillo, G.A. Delayed enhancement cardiac computed tomography for the assessment of myocardial infarction: From bench to bedside. Cardiovasc. Diagn. Ther. 2017, 7, 159–170. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar]
- Patel, A.R.; Kramer, C.M. Role of Cardiac Magnetic Resonance in the Diagnosis and Prognosis of Nonischemic Cardiomyopathy. JACC Cardiovasc. Imaging 2017, 10 Pt A, 1180–1193. [Google Scholar]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef]
- Harries, I.; Berlot, B.; Ffrench-Constant, N.; Williams, M.; Liang, K.; De Garate, E.; Baritussio, A.; Biglino, G.; Plana, J.C.; Bucciarelli-Ducci, C. Cardiovascular magnetic resonance characterisation of anthracycline cardiotoxicity in adults with normal left ventricular ejection fraction. Int. J. Cardiol. 2021, 343, 180–186. [Google Scholar] [CrossRef]
- Lisi, M.; Cameli, M.; Mandoli, G.E.; Pastore, M.C.; Righini, F.M.; D’Ascenzi, F.; Focardi, M.; Rubboli, A.; Mondillo, S.; Henein, M.Y. Detection of myocardial fibrosis by speckle-tracking echocardiography: From prediction to clinical applications. Heart Fail. Rev. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Steeds, R.P.; Wheeler, R.; Bhattacharyya, S.; Reiken, J.; Nihoyannopoulos, P.; Senior, R.; Monaghan, M.J.; Sharma, V. Stress echocardiography in coronary artery disease: A practical guideline from the British Society of Echocardiography. Echo Res. Pract. 2019, 6, G17–G33. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.N.; Chahal, N.S.; Bhattacharyya, S.; Li, W.; Roussin, I.; Khattar, R.S.; Senior, R. The feasibility and clinical utility of myocardial contrast echocardiography in clinical practice: Results from the incorporation of myocardial perfusion assessment into clinical testing with stress echocardiography study. J. Am. Soc. Echocardiogr. 2014, 27, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.Y.; Groves, D.W.; Aletras, A.H.; Kellman, P.; Arai, A.E. A quantitative pixel-wise measurement of myocardial blood flow by contrast-enhanced first-pass CMR perfusion imaging: Microsphere validation in dogs and feasibility study in humans. JACC Cardiovasc. Imaging 2012, 5, 154–166. [Google Scholar] [CrossRef]
- Arai, A.E.; Schulz-Menger, J.; Berman, D.; Mahrholdt, H.; Han, Y.; Bandettini, W.P.; Gutberlet, M.; Abraham, A.; Woodard, P.K.; Selvanayagam, J.B.; et al. Gadobutrol-enhanced magnetic resonance imaging for detection of coronary artery disease. J. Am. Coll. Cardiol. 2020, 76, 1536–1547. [Google Scholar] [CrossRef]
- Kotecha, T.; Martinez-Naharro, A.; Boldrini, M. Automated pixel-wise quantitative myocardial perfusion mapping by CMR to detect obstructive coronary artery disease and coronary microvascular dysfunction. J. Am. Coll. Cardiovasc. Imaging 2019, 12, 1958–1969. [Google Scholar] [CrossRef]
- Kotecha, T.; Chacko, L.; Chehab, O.; O’Reilly, N.; Martinez-Naharro, A.; Lazari, J.; Knott, K.D.; Brown, J.; Knight, D.; Muthurangu, V.; et al. Assessment of Multivessel Coronary Artery Disease Using Cardiovascular Magnetic Resonance Pixelwise Quantitative Perfusion Mapping. JACC Cardiovasc. Imaging 2020, 13, 2546–2557. [Google Scholar] [CrossRef]
- Bonow, R.O.; Maurer, G.; Lee, K.L.; Holly, T.A.; Binkley, P.F.; Desvigne-Nickens, P.; Drozdz, J.; Farsky, P.S.; Feldman, A.M.; Doenst, T.; et al. Myocardial viability and survival in ischemic left ventricular dysfunction. N. Engl. J. Med. 2011, 364, 1617–1625. [Google Scholar] [CrossRef]
- Almeida, A.G.; Carpenter, J.P.; Cameli, M.; Donal, E.; Dweck, M.R.; Flachskampf, F.A.; Maceira, A.M.; Muraru, D.; Neglia, D.; Pasquet, A.; et al. Multimodality imaging of myocardial viability: An expert consensus document from the European Association of Cardiovascular Imaging (EACVI). Eur. Heart J. Cardiovasc. Imaging 2021, 22, e97–e125. [Google Scholar] [CrossRef]
- Rosengren, S.; Skibsted Clemmensen, T.; Tolbod, L.; Granstam, S.O.; Eiskjær, H.; Wikström, G.; Vedin, O.; Kero, T.; Lubberink, M.; Harms, H.J.; et al. Diagnostic Accuracy of [11C]PIB Positron Emission Tomography for Detection of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2020, 13, 1337–1347. [Google Scholar] [CrossRef]
- Mathew, R.C.; Gottbrecht, M.; Salerno, M. Computed Tomography Fractional Flow Reserve to Guide Coronary Angiography and Intervention. Interv. Cardiol. Clin. 2018, 7, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Aziz, W.; Claridge, S.; Ntalas, I.; Gould, J.; de Vecchi, A.; Razeghi, O.; Toth, D.; Mountney, P.; Preston, R.; Rinaldi, C.A.; et al. Emerging role of cardiac computed tomography in heart failure. ESC Heart Fail. 2019, 6, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Kovacs, G.; Vaidya, A.; Bhatt, D.L.; Nishimura, R.A.; Mak, S.; Guazzi, M.; Tedford, R.J. Cardiopulmonary Hemodynamics in Pulmonary Hypertension and Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 2671–2681. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Martinez-Naharro, A.; Chacko, L.; Rowczenio, D.; Gilbertson, J.A.; Whelan, C.J.; Strehina, S.; Lane, T.; Moon, J.; Hutt, D.F.; et al. Reduction in CMR Derived Extracellular Volume with Patisiran Indicates Cardiac Amyloid Regression. JACC Cardiovasc. Imaging 2021, 14, 189–199. [Google Scholar] [CrossRef]
- Available online: https://www.choosingwisely.org/societies/american-college-of-cardiology/ (accessed on 22 July 2022).

| Modality | Goals | Strengths | Weaknesses/Limitations |
|---|---|---|---|
| Echocardiography | Measurement of systolic and diastolic function parameters (EF, GLS, etc.), identification of etiology (e.g., cardiomyopathies, coronary artery disease, hypertrophy, valvular heart disease) | Ubiquitous and prompt availability (first-line imaging modality for heart failure), good assessment of valvular heart disease, cost-effective, no side effects | High observer and test–retest variability, no tissue characterization, only indirect assessment of coronary artery disease by stress test, often limited by image quality |
| Cardiovascular Magnetic Resonance | Measurement of systolic and diastolic function parameters (LV and RV volumes, EF, GLS, etc.), identification of etiology of heart failure (ischemic scar, cardiomyopathy, myocarditis, storage disease, sarcoidosis, and others) | Tissue characterization by late enhancement and tissue relaxation parameters (T1, T2, T*); high volumetric accuracy (gold standard); ability to reliably quantify valvular regurgitation | Not everywhere available, expensive, limited in atrial fibrillation, some contraindications (renal failure for contrast application, some implanted devices, claustrophobia) |
| Cardiac Computed Tomography | Assessment of coronary artery disease; second-line imaging of structural disease (cardiomyopathies, valve disease) | Very high sensitivity for coronary artery disease; very high morphologic resolution | Radiation exposure, renal failure contraindication for contrast application |
| Nuclear Perfusion Imaging | Assessment of myocardial perfusion (coronary or microvascular disease); assessment of amyloidosis with specific tracers; sarcoidosis | PET is gold standard for myocardial perfusion; identification of perfusion/metabolism mismatch, as in hibernating myocardium; high accuracy for amyloidosis and sarcoidosis; DPD imaging (planar/SPECT) is sensitive for cardiac ATTR amyloidosis | PET limited to centers with cyclotron; radiation exposure |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flachskampf, F.A.; Baron, T. The Role of Novel Cardiac Imaging for Contemporary Management of Heart Failure. J. Clin. Med. 2022, 11, 6201. https://doi.org/10.3390/jcm11206201
Flachskampf FA, Baron T. The Role of Novel Cardiac Imaging for Contemporary Management of Heart Failure. Journal of Clinical Medicine. 2022; 11(20):6201. https://doi.org/10.3390/jcm11206201
Chicago/Turabian StyleFlachskampf, Frank A., and Tomasz Baron. 2022. "The Role of Novel Cardiac Imaging for Contemporary Management of Heart Failure" Journal of Clinical Medicine 11, no. 20: 6201. https://doi.org/10.3390/jcm11206201
APA StyleFlachskampf, F. A., & Baron, T. (2022). The Role of Novel Cardiac Imaging for Contemporary Management of Heart Failure. Journal of Clinical Medicine, 11(20), 6201. https://doi.org/10.3390/jcm11206201






