Case Report with Systematic Literature Review on Vascular Complications of BCG Intravesical Therapy for Bladder Cancer
Abstract
:1. Introduction
2. Case
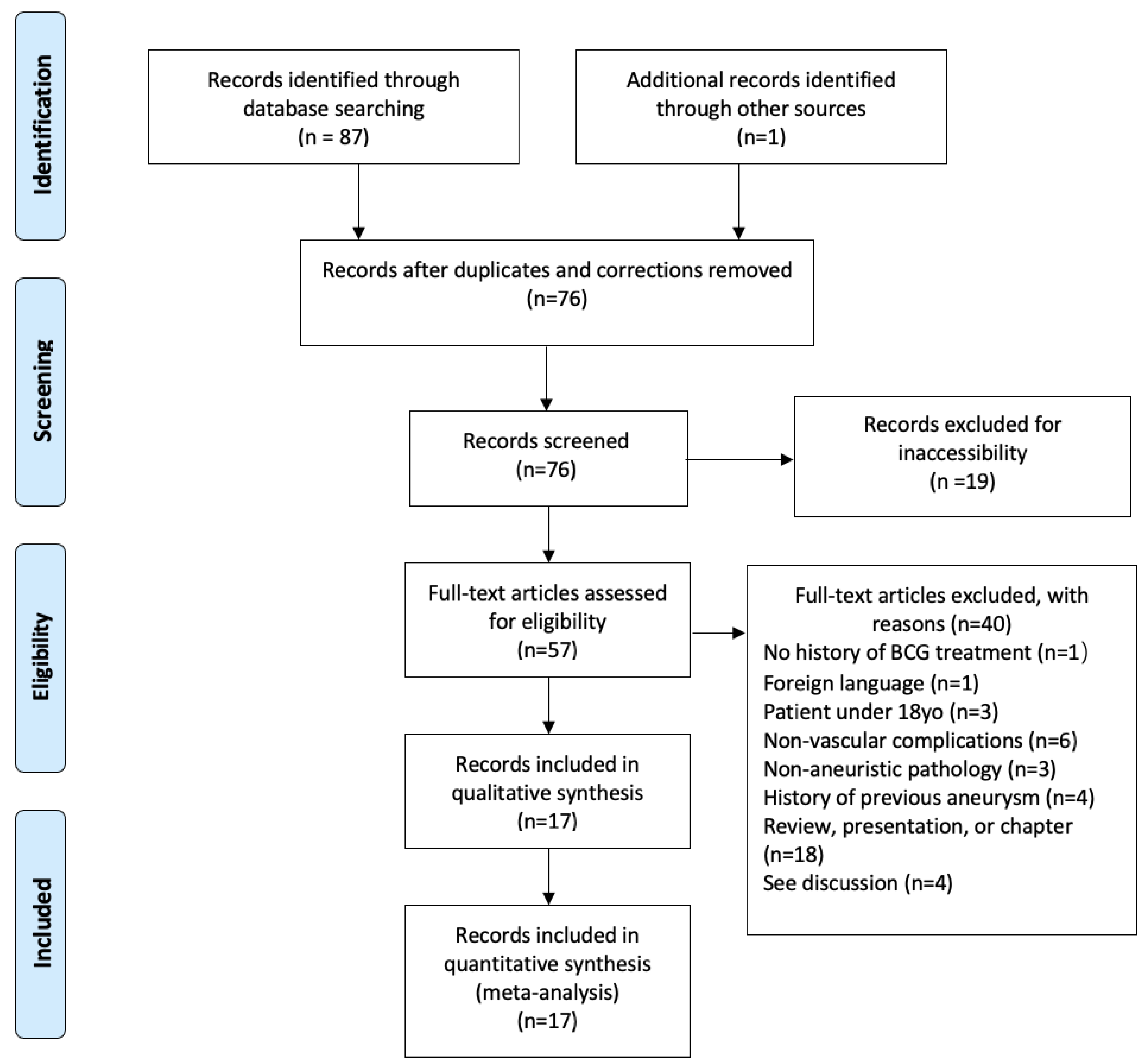
3. Materials and Methods
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rees, R.J. BCG vaccination in mycobacterial infections. Br. Med. Bull. 1969, 25, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef]
- Larsen, E.S.; Joensen, U.N.; Poulsen, A.M.; Goletti, D.; Johansen, I.S. Bacillus Calmette-Guérin immunotherapy for bladder cancer: A review of immunological aspects, clinical effects and BCG infections. Apmis 2020, 128, 92–103. (In Danish) [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamm, D.L.; Van Der Meijden, A.P.; Morales, A.; Brosman, S.A.; Catalona, W.J.; Herr, H.W.; Soloway, M.S.; Steg, A.; Debruyne, F.M. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J. Urol. 1992, 147, 596–600. [Google Scholar] [CrossRef]
- Cabas, P.; Rizzo, M.; Giuffrè, M.; Antonello, R.M.; Trombetta, C.; Luzzati, R.; Liguori, G.; Di Bella, S. BCG infection (BCGitis) following intravesical instillation for bladder cancer and time interval between treatment and presentation: A systematic review. Urol. Oncol. 2021, 39, 85–92. [Google Scholar] [CrossRef]
- Sörelius, K.; Budtz-Lilly, J.; Mani, K.; Wanhainen, A. Systematic Review of the Management of Mycotic Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 426–435. [Google Scholar] [CrossRef]
- Shirasu, T.; Kuno, T.; Yasuhara, J.; Yokoyama, Y.; Takagi, H.; Cullen, M.J.; CraigKent, K.; DarrinClouse, W. Meta-analysis finds recurrent infection is more common after endovascular than after open repair of infected abdominal aortic aneurysm. J. Vasc. Surg. 2022, 75, 348–355. [Google Scholar] [CrossRef]
- Wadhwani, A.; Moore, R.D.; Bakshi, D.; Mirakhur, A. Mycotic aortic aneurysms post-Intravesical BCG treatment for early-stage bladder carcinoma. CVIR Endovasc. 2018, 1, 28, Correction in CVIR Endovasc. 2019, 2, 43. [Google Scholar] [CrossRef]
- Akabane, K.; Uchida, T.; Matsuo, S.; Hirooka, S.; Kim, C.; Uchino, H.; Shimanuki, T. Hybrid operation for infectious thoracic and abdominal aortic aneurysms complicated with Bacillus Calmette-Guérin therapy for bladder cancer: A case report. Medicine 2021, 100, e24796. [Google Scholar] [CrossRef]
- Koterazawa, S.; Watanabe, J.; Uemura, Y.; Uegaki, M.; Shirahase, T.; Taki, Y. A case of infectious thoracic aortic aneurysm after intravesical Bacillus Calmette-Guérin instillation therapy for a superficial bladder cancer. Urol. Case Rep. 2021, 36, 101574. [Google Scholar] [CrossRef]
- Berchiolli, R.; Mocellin, D.M.; Marconi, M.; Tomei, F.; Bargellini, I.; Zanca, R.; Erba, P.; Ferrari, M. Ruptured Mycotic Aneurysm After Intravesical Instillation for Bladder Tumor. Ann. Vasc. Surg. 2019, 59, 310.e7–310.e11. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.S.; Oldenburg, W.A.; Murray, P.M. Mycotic Aneurysm of the Ulnar Artery Secondary to Bacillus Calmette-Guérin Therapy for Bladder Cancer: A Rare Presentation of Hypothenar Hammer Syndrome. J. Hand. Surg. Am. 2019, 44, 905.e1–905.e4. [Google Scholar] [CrossRef]
- Darriet, F.; Bernioles, P.; Loukil, A.; Saidani, N.; Eldin, C.; Drancourt, M. Fluorescence in situ hybridization microscopic detection of Bacilli Calmette Guérin mycobacteria in aortic lesions: A case report. Medicine 2018, 97, e11321. [Google Scholar] [CrossRef] [PubMed]
- Roeke, T.; Hovsibian, S.; Schlejen, P.M.; Dinant, S.; Koster, T.; Waasdorp, E.J. A mycotic aneurysm of the abdominal aorta caused by Mycobacterium bovis after intravesical instillation with bacillus Calmette-Guérin. J. Vasc. Surg. Cases Innov. Tech. 2018, 4, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Kaur, G.; Poyck, P.; Vyas, V.; Redmond, A.; Quinn, S. Bacillus Calmette-Guérin immunotherapy: A rare cause for a ruptured common femoral artery. ANZ J. Surg. 2018, 88, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Pham, S.T.D.; Lee, A.; Struminger, J.S.; Belkoff, K.M.; Mendoza, B.; Berman, S.S. Mycotic infrarenal aortic aneurysm due to mycobacterium after intravesical treatment for bladder cancer. J. Vasc. Surg. Cases Innov. Tech. 2021, 7, 354–356. [Google Scholar] [CrossRef]
- Bilsen, M.P.; van Meijgaarden, K.E.; de Jong, H.K.; Joosten, S.A.; Prins, C.; Kroft, L.J.; Jonker, J.T.; Crobach, S.; Pelger, R.C.; Ottenhoff, T.H.; et al. A novel view on the pathogenesis of complications after intravesical BCG for bladder cancer. Int. J. Infect. Dis. 2018, 72, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Misch, E.A.; John-Paul, J.Y.; Brennan, M.B. Contiguous Mycotic Abdominal Aortic Aneurysm and Vertebral Osteomyelitis. Infect. Dis. Clin. Pract. 2021, 29, e254–e256. [Google Scholar] [CrossRef]
- Walkty, A.; Koulack, J.; Milligan, B.; Lu, M.; Mottola, J.; Embil, J. An 81-year-old Male with a Pulsatile Abdominal Mass. Clin. Infect. Dis. 2019, 69, 1456–1459. [Google Scholar] [CrossRef]
- Arif, S.; Jafri, F. A Case of Ruptured Mycotic Aneurysm after Disseminated Mycobacterium bovis Infection. Chest 2020, 158, A916. [Google Scholar] [CrossRef]
- Duvnjak, P.; Laguna, M. Left anterior descending coronary artery and multiple peripheral mycotic aneurysms due to Mycobacterium bovis following intravesical bacillus calmette-guerin therapy: A case report. J. Radiol. Case Rep. 2016, 10, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Costiniuk, C.T.; Sharapov, A.A.; Rose, G.W.; Veinot, J.P.; Desjardins, M.; Brandys, T.M.; Suh, K.N. Mycobacterium bovis abdominal aortic and femoral artery aneurysms following intravesical bacillus calmette-guerin therapy for bladder cancer. Cardiovasc. Pathol. 2010, 19, e29–e32. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.G.; Wolf, D.G.; Higginbottom, P.A.; Dilley, R.B. Infection of a ruptured aortic aneurysm and an aortic graft with bacille calmette-guerin after intravesical administration for bladder cancer. J. Vasc. Surg. 1995, 22, 80–84. [Google Scholar] [CrossRef]

| Case | Age/Sex | Aneurysm Location | Extravascular Spread | Months from Last Tx to Admit | # of Instillations | Cx Site | Cx Results | Surgical Treatment | Discharge Therapy | Reported Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 73M | Thoracic AAA | Retrocrural/ posterior mediastinum collection | 6 | 5 | Retrocrural abscess | M. bovis | TEVAR and percutaneous drainage | RIPE +B6 | Asymptomatic at 6 mo |
| 2 | 67M | AAA | Infrarenal periaortic collection | 4 | 15 | AAA | M. bovis | Open repair with autologous graft | RIPE +B6 | Asymptomatic at 6 mo |
| 3 | 76M | Thoracic AA and AAA | 12 | NR | AAA AFB stain, T-RT, and DnaSP | BCG strain M. bovis | TEVAR and open repair with prosthetic graft | RIE | Asymptomatic at 6 mo | |
| 4 | 80M | Thoracic AA | Infiltrative lung consolidation | 10 | 8 at 80 mg | Sputum PCR | M. bovis | TEVAR | RIE | No recurrence at 24 mo |
| 5 | 70M | AAA | 3 | NR | NA | NA | EVAR | NR | Graft-site aortoenteric fistula at 10 d postop, critical colic ischemia with total colectomy, and unspecified death at 25 d | |
| 6 | 72M | R UAA | NR | 9 | R UAA | M. tb | Open repair with autologous graft | Ethambutol 9 mo | NR | |
| 7 | 66M | AAA | PE and R psoas muscle hematoma | NR | 6 | AAA PCR & FISH + ZN | BCG strain M. bovis, M. tb | “Surgical flattening of the aneurysm” | RIE 2 mo then RI 8mo | Aneurysm size reduction at 6 mo |
| 8 | 73M | AApA | L iliopsoas | 14 | 6 | AApA | BCG strain M. bovis | Open repair with bovine graft | RIE 3 mo then RE + moxifloxacin | Postop severe inflammatory response syndrome, renal insufficiency, and chylous ascites treated w/HD, TPN, paracentesis, and octreotide; mycobacterium fluid collection at graft and left psoas muscle at 3 mo |
| 9 | 80M | L CFApA | 8 | NR | L CFApA PCR | BCG strain M. bovis | Open repair with autologous graft | RIE 9 mo | NR | |
| 10 | 76M | AAA | NR | “biannual infusions for the prior 2 years” | AAA DNA probe | M. tb | Open repair with bypass | RIE (rifampin switched to rifabutin at 6 wks) | Pneumonia, sepsis, death at 4 mo | |
| 11 | 70M | AAA | 2 | 18 | AAA PCR | M. bovis | EVAR | NA | Aortic leak and splenomegaly treated with aortic repair, resection of para-aortic lymph nodes, and splenectomy at 10 mo, aortic rupture to death 1w postop | |
| 12 | 71M | AApA | Paraspinal muscles, retroperitoneum, and L4 & 5 ventral bodies | NR | NR | AApA PCR | M. tb RNA, BCG strain M. bovis | Open repair with nonautologous biologic graft | RIE 12 mo (ethambutol held for 1 mo) | Chills, night sweats, back pain and weight loss resolving 3 mo |
| 13 | 81M | AAA | 12 | NR | AAA | BCG strain M. bovis | Open repair with bypass | NA | Aspiration-induced respiratory failure, death | |
| 14 | 86M | AAA | L psoas & retroperitoneum | NR | NR | L Psoas abscess | M. bovis | EVAR | NR | Renal failure to hospice |
| 15 | 63M | L popliteal, L & R CFAA, and R CIAA | 7 | 16 | CFAA AFB | M. bovis | L pop stent, R CIAA EVAR with stent graft and coil embolization, and open CFA repair with graft | RIE 9 mo | Bilateral groin wound washout and VAC at 2 mo, LAD aneurysm with DNR at 4 mo | |
| 16 | 75M | AAA and L SFAA | 14 | 5 | AAA ZN and L SFAA | AFB, BCG strain M. bovis | Open repair with dacron graft bypass | RIE 12 mo | SFA hematoma and para-grafts granulation tissue tx: percutaneous SFA drainage at 1 mo, PAs at both grafts and R CIApA tx: SFA stent, AApA bypass and graft removal, and oversewing RCIA at 4 mo | |
| 17 | 80M | AAA | R psoas muscle | NR | 7 | AAA DNA probe (12 mo) | BCG strain M. bovis | Open repair with dacron bifurcation graft | RIE at 12 mo for 20 mo | Acute MI tx: PTCA, stent, and CABG at 5 mo; Para-graft R psoas fluid collection at 12 mo; graft-enteric fistula tx: graft removal and at 20 mo |
| Our Case | 78M | AAA | L psoas abscess | 30 | 9 | AAA (25 d) | M. tb with pyrazinamide resistance | Open repair with allograft | NA | Cardiac arrest leading to death intraoperatively |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, B.; Singh, D.; Rathore, A.; Flenner, R.; Flemmer, M. Case Report with Systematic Literature Review on Vascular Complications of BCG Intravesical Therapy for Bladder Cancer. J. Clin. Med. 2022, 11, 6226. https://doi.org/10.3390/jcm11206226
King B, Singh D, Rathore A, Flenner R, Flemmer M. Case Report with Systematic Literature Review on Vascular Complications of BCG Intravesical Therapy for Bladder Cancer. Journal of Clinical Medicine. 2022; 11(20):6226. https://doi.org/10.3390/jcm11206226
Chicago/Turabian StyleKing, Brianna, Dhanveer Singh, Animesh Rathore, Ronald Flenner, and Mark Flemmer. 2022. "Case Report with Systematic Literature Review on Vascular Complications of BCG Intravesical Therapy for Bladder Cancer" Journal of Clinical Medicine 11, no. 20: 6226. https://doi.org/10.3390/jcm11206226
APA StyleKing, B., Singh, D., Rathore, A., Flenner, R., & Flemmer, M. (2022). Case Report with Systematic Literature Review on Vascular Complications of BCG Intravesical Therapy for Bladder Cancer. Journal of Clinical Medicine, 11(20), 6226. https://doi.org/10.3390/jcm11206226






