Bone Loss in Patients with Pancreatic Neuroendocrine Tumors
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients
2.2. PNETs Grade
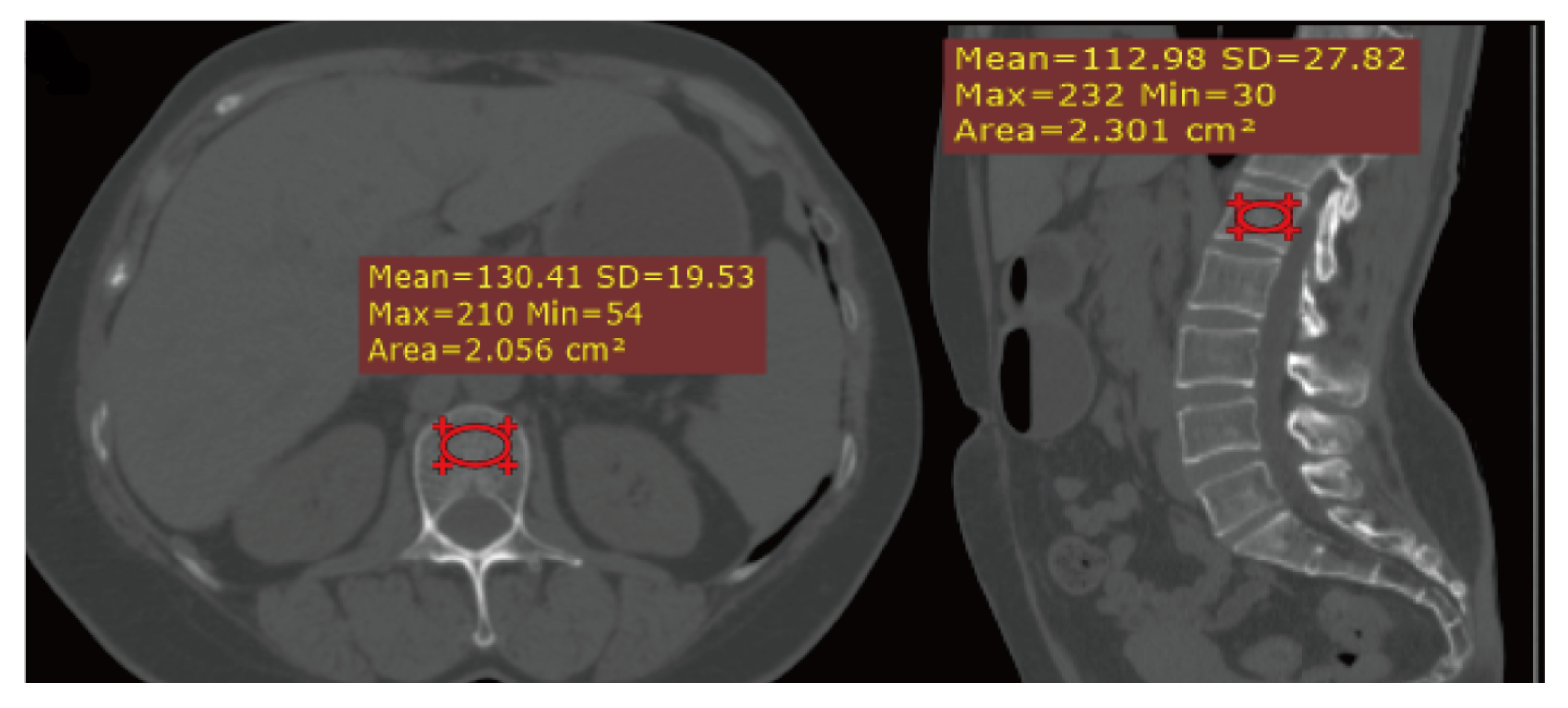
2.3. CT Scanning
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Patients
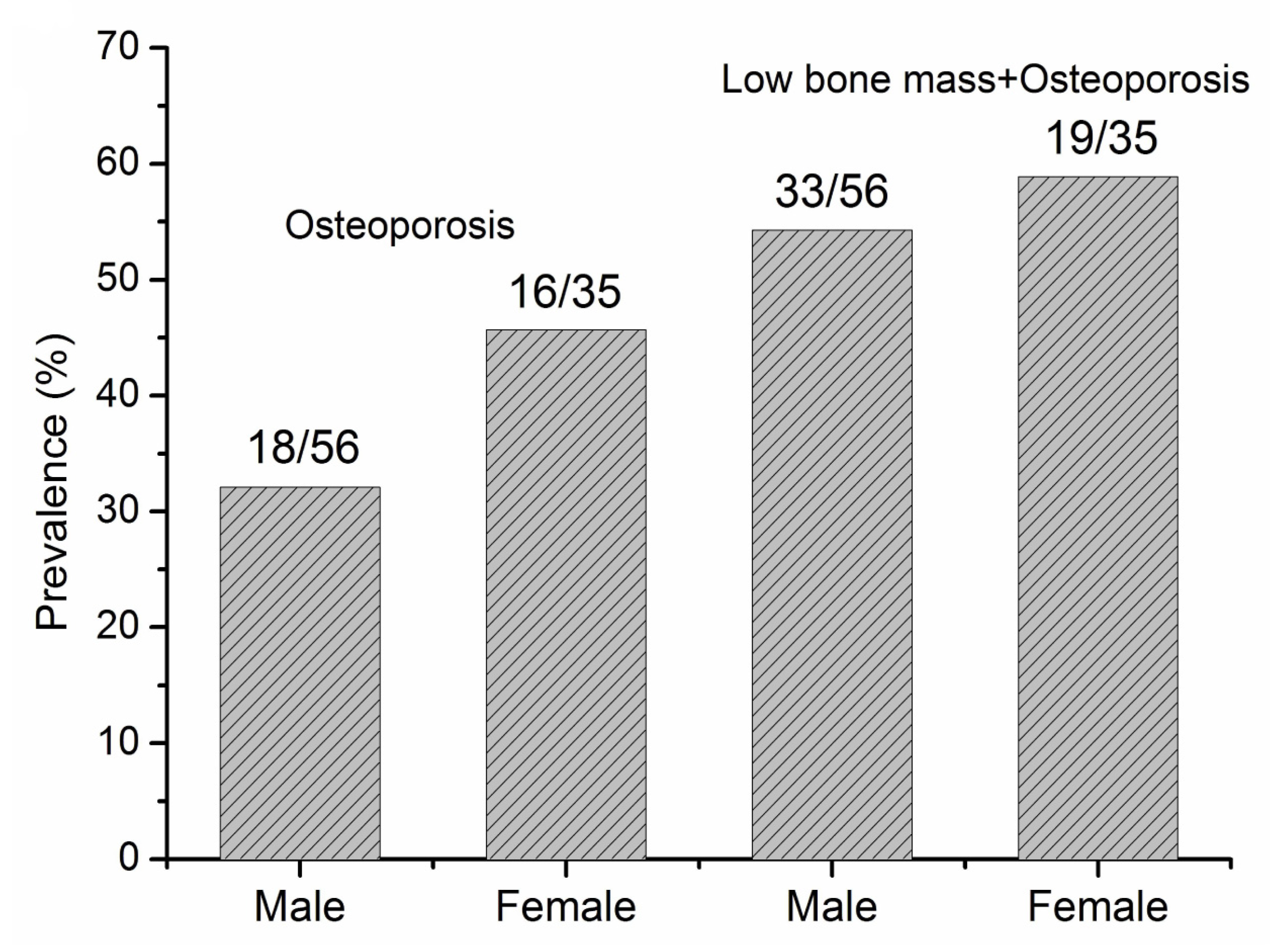
3.2. The Prevalence of Bone Loss
3.3. Risk Factors for Bone Loss
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ensrud, K.; Crandall, C. Osteoporosis. Ann. Intern. Med. 2017, 167, ITC17–ITC32. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Ryu, K.H.; Park, B.J.; Yoon, B.H. Osteoporosis and Osteoporotic Fractures in Gastrointestinal Disease. J. Bone Metab. 2018, 25, 213–217. [Google Scholar] [PubMed]
- Duggan, S.; Smyth, N.; Murphy, A.; Macnaughton, D.; O’Keefe, S.; Conlon, K.C. High prevalence of osteoporosis in patients with chronic pancreatitis: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 219–228. [Google Scholar] [CrossRef]
- Duggan, S.; Purcell, C.; Kilbane, M.; O’Keane, M.; McKenna, M.; Gaffney, P.; Ridgway, P.F.; Boran, G.; Conlon, K.C. An association between abnormal bone turnover, systemic inflammation, and osteoporosis in patients with chronic pancreatitis: A case-matched study. Am. J. Gastroenterol. 2015, 110, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.; Yadav, D.; Li, L.; Appana, S.; Fisher, W.; Fogel, E.; Forsmark, C.E.; Park, W.G.; Pandol, S.; Topazian, M.D.; et al. High Prevalence of Osteopathy in Chronic Pancreatitis: A Cross-sectional Analysis from the PROCEED Study. Clin. Gastroenterol. Hepatol. 2021, 20, 2005–2013. [Google Scholar] [CrossRef]
- Abou Saleh, M.; Alkhayyat, M.; Mansoor, E.; Khoudari, G.; Simons-Linares, C.R.; Vargo, J.; Chahal, P.; Stevens, T. The Risk of Vitamin D Deficiency, Osteoporosis, and Fractures in Acute Pancreatitis. Pancreas 2020, 49, 629–633. [Google Scholar] [CrossRef]
- Jeong, S.M.; Shin, D.W.; Lee, J.E.; Jin, S.M.; Kim, S. Increased Risk of Osteoporosis in Gastric Cancer Survivors Compared to General Population Control: A Study with Representative Korean Population. Cancer Res. Treat. 2019, 51, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Atsumi, Y.; Rino, Y.; Wada, H.; Kitani, Y.; Ozawa, Y.; Aoyama, T.; Oshima, T.; Yukawa, N.; Yoshikawa, T.; Masuda, M. Changes in bone metabolism after gastric cancer surgery in male patients: A prospective observational study. Gastric Cancer 2019, 22, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Hess, G.; Chen, C.; Liu, Z.; Yao, J.; Phan, A.; Hill, J. Clinical burden of illness in patients with neuroendocrine tumors. Pancreas 2012, 41, 1058–1062. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar]
- Megdanova-Chipeva, V.G.; Lamarca, A.; Backen, A.; McNamara, M.G.; Barriuso, J.; Sergieva, S.; Gocheva, L.; Mansoor, W.; Manoharan, P.; Valle, J.W. Systemic Treatment Selection for Patients with Advanced Pancreatic Neuroendocrine Tumours (PanNETs). Cancers 2020, 12, 1988. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Kim, S.; Jeong, H.; Rhee, Y.; Lee, W.; Kang, C. ACTH-producing neuroendocrine tumor of the pancreas: A case report and literature review. Ann. Hepatobiliary. Pancreat. Surg. 2017, 21, 61–65. [Google Scholar]
- Altieri, B.; Di Dato, C.; Martini, C.; Sciammarella, C.; Di Sarno, A.; Colao, A.; Faggiano, A. Bone Metastases in Neuroendocrine Neoplasms: From Pathogenesis to Clinical Management. Cancers 2019, 11, 1332. [Google Scholar] [CrossRef] [Green Version]
- Feola, T.; Puliani, G.; Sesti, F.; Modica, R.; Centello, R.; Minotta, R.; Cannavale, G.; Di Meglio, S.; Di Vito, V.; Lauretta, R.; et al. Risk factors for gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): A three-centric case-control study. J. Endocrinol. Investig. 2022, 45, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.D.; Morin, S.N.; Majumdar, S.R.; Lix, L.M. Effects of obesity and diabetes on rate of bone density loss. Osteoporos. Int. 2018, 29, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Gao, Q.; Wang, C.; Dai, J. Diabetes mellitus and risk of low-energy fracture: A meta-analysis. Aging Clin. Exp. Res. 2020, 32, 2173–2186. [Google Scholar]
- Koromani, F.; Oei, L.; Shevroja, E.; Trajanoska, K.; Schoufour, J.; Muka, T.; Franco, O.H.; Ikram, M.A.; Zillikens, M.C.; Uitterlinden, A.G.; et al. Vertebral Fractures in Individuals With Type 2 Diabetes: More Than Skeletal Complications Alone. Diabetes Care 2020, 43, 137–144. [Google Scholar]
- Muscogiuri, G.; Altieri, B.; Albertelli, M.; Dotto, A.; Modica, R.; Barrea, L.; Fanciulli, G.; Feola, T.; Baldelli, R.; Ruggeri, R.M.; et al. Epidemiology of pancreatic neuroendocrine neoplasms: A gender perspective. Endocrine 2020, 69, 441–450. [Google Scholar] [CrossRef]
- Guilmette, J.M.; Nosé, V. Neoplasms of the Neuroendocrine Pancreas: An Update in the Classification, Definition, and Molecular Genetic Advances. Adv. Anat. Pathol. 2019, 26, 13–30. [Google Scholar]
- Rebello, D.; Anjelly, D.; Grand, D.J.; Machan, J.T.; Beland, M.D.; Furman, M.S.; Shapiro, J.; LeLeiko, N.; Sands, B.E.; Mallette, M.; et al. Opportunistic screening for bone disease using abdominal CT scans obtained for other reasons in newly diagnosed IBD patients. Osteoporos. Int. 2018, 29, 1359–1366. [Google Scholar] [CrossRef]
- Altieri, B.; Di Dato, C.; Modica, R.; Bottiglieri, F.; Di Sarno, A.; Pittaway, J.; Martini, C.; Faggiano, A.; Colao, A. Bone Metabolism and Vitamin D Implication in Gastroenteropancreatic Neuroendocrine Tumors. Nutrients 2020, 12, 1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Zhao, K.; Zha, X.; Du, X.; Li, Y.; Chen, S.; Wu, Y.; Li, S.; Lu, Y.; Zhang, Y.; et al. Opportunistic Screening Using Low-Dose CT and the Prevalence of Osteoporosis in China: A Nationwide, Multicenter Study. J. Bone Miner. Res. 2021, 36, 427–435. [Google Scholar]
- Halfdanarson, T.R.; Bamlet, W.R.; McWilliams, R.R.; Hobday, T.J.; Burch, P.A.; Rabe, K.G.; Petersen, G.M. Risk factors for pancreatic neuroendocrine tumors (PNETs): A clinic-based case-control study. Pancreas 2014, 43, 1219–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Gong, Y.; Huang, Q.; Yang, C.; Cheng, H.; Jin, K.; Fan, K.; Ni, Q.; Yu, X.; Luo, G.; et al. Diabetes Is Associated With the Metastasis of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Dammann, H.; Kruse, H.; Kuhlencordt, F.; Montz, R.; Schreiber, H. Resections of the pancreas: Their effects on bone and calcium metabolism. Z. Gastroenterol. 1977, 15, 577–585. [Google Scholar]
- Shapiro, C.; Van Poznak, C.; Lacchetti, C.; Kirshner, J.; Eastell, R.; Gagel, R.; Smith, S.; Edwards, B.J.; Frank, E.; Lyman, G.H.; et al. Management of Osteoporosis in Survivors of Adult Cancers With Nonmetastatic Disease: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 2916–2946. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ran, L.; Zha, X.; Zhao, K.; Yang, Y.; Shuang, Q.; Liu, Y.; Hind, K.; Cheng, X.; Blake, G.M. Adjustment of DXA BMD measurements for anthropometric factors and its impact on the diagnosis of osteoporosis. Arch. Osteoporos. 2020, 15, 155. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.; Pooler, B.; Lauder, T.; del Rio, A.; Bruce, R.; Binkley, N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann. Intern. Med. 2013, 158, 588–595. [Google Scholar] [PubMed] [Green Version]

| PNET (n = 91) | |
|---|---|
| Age | 55.76 ± 12.97 |
| Gender (women/men) | 35/56 |
| Functional tumor | 8 |
| Clinical symptoms | |
| Abdominal pain | 29 |
| Weight loss | 4 |
| Jaundice | 3 |
| Back pain | 4 |
| Abdominal mass | 0 |
| Glucopenia | 6 |
| Asymptomatic | 48 |
| Others | 8 |
| Post-menopausal status * | 27 |
| TNM stage | |
| T1 | 27 |
| T2 | 52 |
| T3 | 12 |
| N0 | 76 |
| N1 | 15 |
| CT value (HU) | 158.0 ± 50.11 |
| Location (head-neck/body-tail) | 49/42 |
| Size | 3.14 ± 1.67 |
| Grade (G1/G2/G3) | 20/24/14 |
| CT values (HU) | |
| >160 | 36 |
| 135–160 | 21 |
| <135 | 34 |
| Osteoporosis | Low Bone Mass | |||
|---|---|---|---|---|
| Univariate (OR, 95% CI) | Multivariate (OR, 95% CI) | Univariate (OR, 95% CI) | Multivariate (OR, 95% CI) | |
| Age | 1.11 (1.05–1.17) | 1.14 (1.03–1.20) | 1.12 (1.06–1.18) | 1.13 (1.04–1.22) |
| Gender (women vs. men) | 1.78 (0.75–4.24) | 2.36 (0.68–9.78) | 0.83 (0.35–1.94) | 0.81 (0.32–2.75) |
| Location | 1.60 (0.53–4.85) | 1.74 (0.49–7.69) | 1.0 (0.43–2.00) | 1.24 (0.43–4.36) |
| Diabetes mellitus | 10.64 (1.32–118.7) | 13.56 (1.02–132.4) | / | / |
| Grade (G3 vs. G1/G2) | 0.45 (0.12–1.84) | 0.36 (0.04–1.13) | 1.28 (0.42–4.54) | 0.54 (0.13–4.56) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, H.; Wang, M.; Liu, J.; Guo, C.; Wang, Z.; Wang, J.; Chen, X. Bone Loss in Patients with Pancreatic Neuroendocrine Tumors. J. Clin. Med. 2022, 11, 6701. https://doi.org/10.3390/jcm11226701
Tong H, Wang M, Liu J, Guo C, Wang Z, Wang J, Chen X. Bone Loss in Patients with Pancreatic Neuroendocrine Tumors. Journal of Clinical Medicine. 2022; 11(22):6701. https://doi.org/10.3390/jcm11226701
Chicago/Turabian StyleTong, He, Miaomiao Wang, Jingjing Liu, Chuangen Guo, Zhongqiu Wang, Jianhua Wang, and Xiao Chen. 2022. "Bone Loss in Patients with Pancreatic Neuroendocrine Tumors" Journal of Clinical Medicine 11, no. 22: 6701. https://doi.org/10.3390/jcm11226701
APA StyleTong, H., Wang, M., Liu, J., Guo, C., Wang, Z., Wang, J., & Chen, X. (2022). Bone Loss in Patients with Pancreatic Neuroendocrine Tumors. Journal of Clinical Medicine, 11(22), 6701. https://doi.org/10.3390/jcm11226701







