Corneal Tonometric and Morphological Changes in Patients with Acromegaly
Abstract
:Highlights
- This study is the first prospective clinical trial to evaluate the anterior corneal parameters in patients with acromegaly before and after transsphenoidal pituitary adenoma resection on the basis of several months of postoperative follow-up.
- An increase in CD and a decrease in AVG and CCTNSM were observed after pituitary adenoma resection.
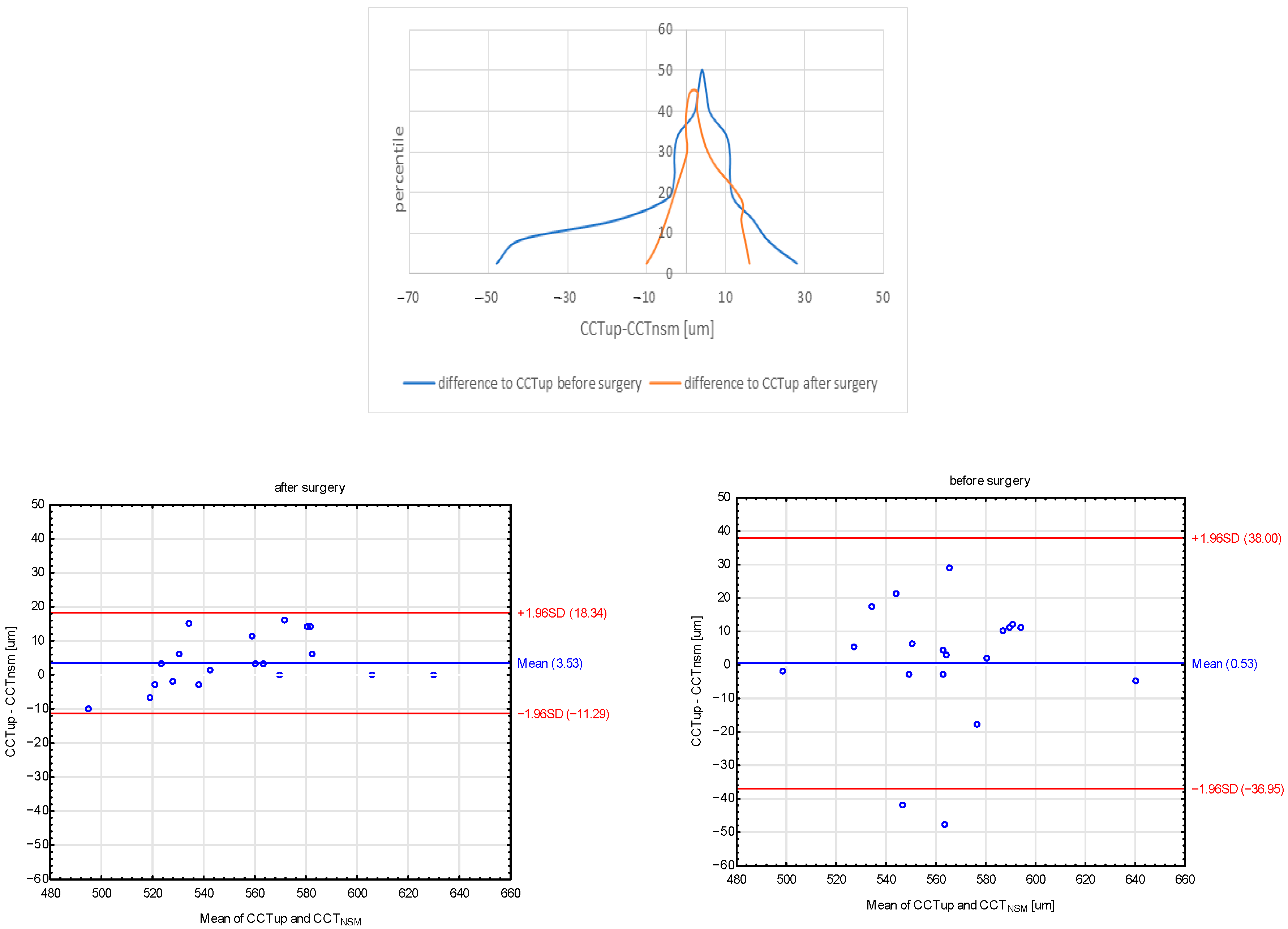
- The mean CCT measured both with an ultrasonic pachymeter and with non-contact specular microscopy was significantly lower as compared to the preoperative values.
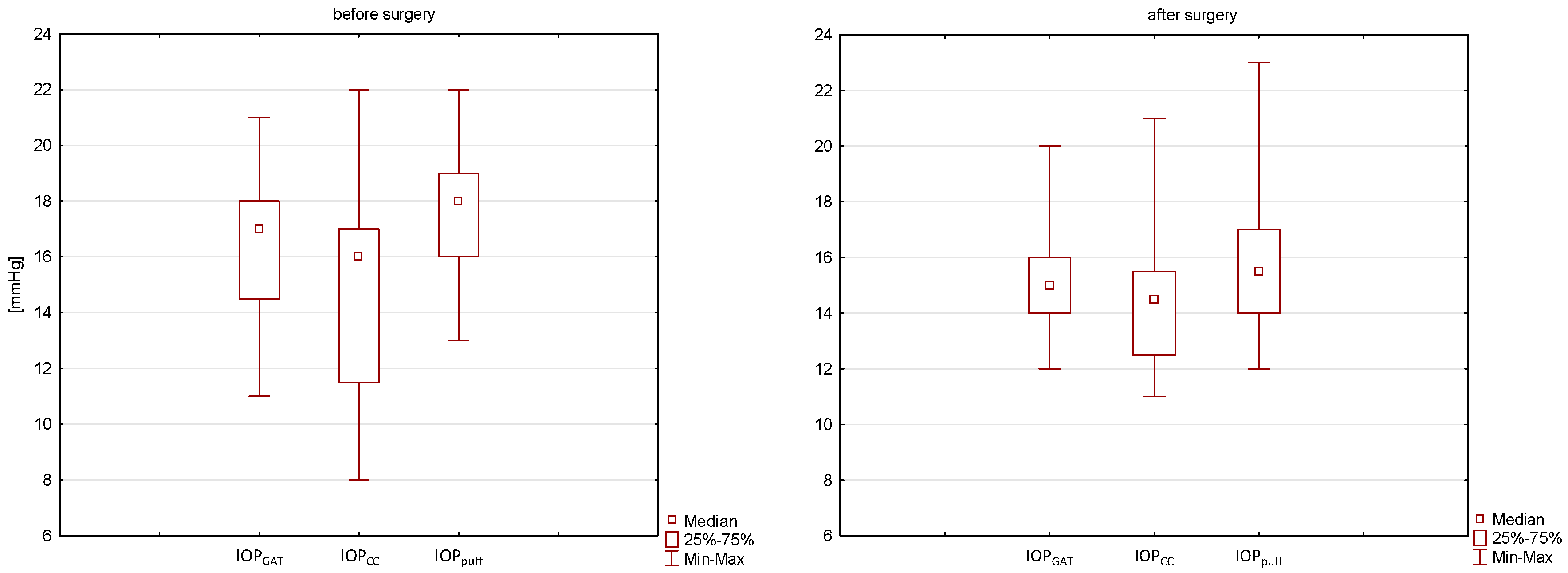
- IOP decreased statistically for the measured IOP air puff values. A downward trend was observed after treatment in the case of IOP GAT and IOPc, but statistical significance was not achieved.
- Statistically significant reduction in CH and CRF was found after the surgical treatment of acromegaly.
Abstract
1. Introduction
2. Materials and Methods
Examination Protocol and Measurements
3. Results
3.1. Demographic Characteristics
3.2. Specular Microscopy
3.3. ORA
3.4. Ultrasonic Pachymetry
4. Discussion
4.1. Corneal Endothelial Cell Parameters
4.2. Biomechanical Properties of the Cornea
4.3. CCT
4.4. IOP Measurements
5. Study Limitations
- The group after surgery could be further divided into two parts—complete biochemical remission (IGF-1/GH normal) and maintenance treatment with somatostatin analogs, but the study groups were too small to make such a comparative analysis. We will certainly conduct further observation on a larger group of patients to assess the long-term impact of acromegaly on the eye.
- The date of the follow-up examination was not the same for all patients. The patients came from all over Poland and the long distances made it difficult to come to the control visit at the appointed time. The COVID-19 pandemic, which began in the spring of 2020, has further aggravated the problem of timely visits.
- No studies were performed to assess the tear film, corneal topography and tomography, including the analysis of the corneal epithelium, which could provide more information about the abnormalities of the eye surface and changes in the corneal structure in patients with acromegaly undergoing surgical treatment.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akirov, A.; Asa, S.L.; Amer, L.; Shimon, I.; Ezzat, S. The Clinicopathological Spectrum of Acromegaly. J. Clin. Med. 2019, 8, 1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolla, M.; Jawiarczyk-Przybyłowska, A.; Halupczok-Żyła, J.; Kałużny, M.; Konopka, B.M.; Błoniecka, I.; Zieliński, G.; Bolanowski, M. Complications and Comorbidities of Acromegaly-Retrospective Study in Polish Center. Front. Endocrinol. 2021, 12, 642131. [Google Scholar] [CrossRef]
- Ahmed, S.; Elsheikh, M.; Stratton, I.M.; Page, R.C.; Adams, C.B.; Wass, J.A. Outcome of transphenoidal surgery for acromegaly and its relationship to surgical experience. Clin. Endocrinol. 1999, 50, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Rivoal, O.; Brézin, A.P.; Feldman-Billard, S.; Luton, J.P. Goldmann perimetry in acromegaly: A survey of 307 cases from 1951 through 1996. Ophthalmology 2000, 107, 991–997. [Google Scholar] [CrossRef]
- Kan, E.; Kan, E.K.; Atmaca, A.; Atmaca, H.; Colak, R. Visual field defects in 23 acromegalic patients. Int. Ophthalmol. 2013, 33, 521–5255. [Google Scholar] [CrossRef]
- Lee, A.G. Acromegaly and junctional visual field loss. Ophthalmology 2001, 108, 832–833. [Google Scholar] [CrossRef]
- Stuard, W.L.; Titone, R.; Robertson, D.M. The IGF/Insulin-IGFBP Axis in Corneal Development, Wound Healing, and Disease. Front. Endocrinol. 2020, 11, 24. [Google Scholar] [CrossRef] [Green Version]
- Rocha, E.M.; Cunha, D.A.; Carneiro, E.M.; Boschero, A.C.; Saad, M.J.; Velloso, L.A. Insulin, insulin receptor and insulin-like growth factor-I receptor on the human ocular surface. Adv. Exp. Med. Biol. 2002, 506 (Pt A), 607–610. [Google Scholar] [CrossRef]
- Quaranta, L.; Riva, I.; Mazziotti, G.; Porcelli, T.; Floriani, I.; Katsanos, A.; Giustina, A.; Konstas, A.G. Elevated intraocular pressure in patients with acromegaly. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1133–1139. [Google Scholar] [CrossRef] [Green Version]
- Altinkaynak, H.; Duru, N.; Ersoy, R.; Akcay, E.K.; Ugurlu, N.; Cagil, N.; Cakir, B. Topographic and biomechanical evaluation of cornea in patients with acromegaly. Cornea 2015, 34, 65–70. [Google Scholar] [CrossRef]
- Kilic, D.; Akmaz, B.; Akay, F.; Guven, Y.Z.; Oruk, G.G. Changes in anterior segment parameters and presence of dry eye disease in patients with acromegaly: A Sirius topography study combined with meibography. Growth Horm. IGF Res. 2021, 60, 101424. [Google Scholar] [CrossRef]
- Ciresi, A.; Amato, M.C.; Morreale, D.; Lodato, G.; Galluzzo, A.; Giordano, C. Cornea in acromegalic patients as a possible target of growth hormone action. J. Endocrinol. Investig. 2011, 34, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Sen, E.; Tutuncu, Y.; Elgin, U.; Balikoglu-Yilmaz, M.; Berker, D.; Aksakal, F.N.; Ozturk, F.; Guler, S. Comparing acromegalic patients to healthy controls with respect to intraocular pressure, central corneal thickness, and optic disc topography findings. Indian J. Ophthalmol. 2014, 62, 841–845. [Google Scholar] [CrossRef]
- Erol, R.S.; Tiryaki, S.; Şen, E.Ç.; Öztürk, F.Y.; Canat, M.M.; Yıldız, D.; Batman, A.; Güven, D.; Altuntas, Y.; Süren, E.; et al. Alteration in choroidal microvasculature determined by optical coherence tomography angiography in patients with acromegaly. Photodiagnosis Photodyn. 2021, 36, 102590. [Google Scholar] [CrossRef] [PubMed]
- Polat, S.B.; Ugurlu, N.; Ersoy, R.; Oguz, O.; Duru, N.; Cakir, B. Evaluation of central corneal and central retinal thicknesses and intraocular pressure in acromegaly patients. Pituitary 2014, 17, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Ozkok, A.; Hatipoglu, E.; Tamcelik, N.; Balta, B.; Gundogdu, A.S.; Ozdamar, M.A.; Kadioglu, P. Corneal biomechanical properties of patients with acromegaly. Br. J. Ophthalmol. 2014, 98, 651–657. [Google Scholar] [CrossRef]
- Hatipoglu, E.; Arici, C.; Arslan, O.S.; Dikkaya, F.; Sultan, P.; Kadioglu, P.; Gundogdu, S. Corneal endothelial cell density and morphology in patients with acromegaly. Growth Horm. IGF Res. 2014, 24, 260–263. [Google Scholar] [CrossRef]
- Arici, C.; Hatipoglu, E.; Iskeleli, G.; Sultan, P.; Yuksel, C.; Gundogdu, S.; Kadioglu, P. Tear Osmolarity and Tear Function Changes in Patients with Acromegaly. Curr. Eye Res. 2015, 40, 863–869. [Google Scholar] [CrossRef]
- Kan, E.; Kan, E.K.; Okuyucu, A. The evaluation of central corneal thickness and intraocular pressure in conjunction with tear IGF-1 levels in patients with acromegaly. Eur. J. Ophthalmol. 2017, 27, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Sen, E.; Tutuncu, Y.; Balikoglu-Yilmaz, M.; Elgin, U.; Berker, D.; Ozturk, F.; Guler, S. Corneal biomechanical properties measured by the ocular response analyzer in acromegalic patients. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1283–1288. [Google Scholar] [CrossRef]
- Akay, F.; Akmaz, B.; Işik, M.U.; Güven, Y.Z.; Örük, G.G. Evaluation of the retinal layers and microvasculature in patients with acromegaly: A case-control OCT angiography study. Eye 2021, 35, 523–527. [Google Scholar] [CrossRef]
- Katznelson, L.; Laws, E.R., Jr.; Melmed, S.; Molitch, M.E.; Murad, M.H.; Utz, A.; Wass, J.A. Endocrine Society. Acromegaly: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 3933–3951. [Google Scholar] [CrossRef] [PubMed]
- Katznelson, L. Alterations in body composition in acromegaly. Pituitary 2009, 12, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Titone, R.; Zhu, M.; Robertson, D.M. Insulin mediates de novo nuclear accumulation of the IGF-1/insulin Hybrid Receptor in corneal epithelial cells. Sci. Rep. 2018, 8, 4378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smedowski, A.; Weglarz, B.; Tarnawska, D.; Kaarniranta, K.; Wylegala, E. Comparison of three intraocular pressure measurement methods including biomechanical properties of the cornea. Investig. Ophthalmol. Vis. Sci. 2014, 55, 666–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramm, L.; Herber, R.; Spoerl, E.; Pillunat, L.E.; Terai, N. Measurement of Corneal Biomechanical Properties in Diabetes Mellitus Using the Ocular Response Analyzer and the Corvis ST. Cornea 2019, 38, 595–599. [Google Scholar] [CrossRef]
- Moghimi, S.; Safizadeh, M.; Mazloumi, M.; Hosseini, H.; Vahedian, Z.; Rajabi, M.T. Evaluation of Corneal Biomechanical Properties in Patients With Thyroid Eye Disease Using Ocular Response Analyzer. J. Glaucoma 2016, 25, 269–273. [Google Scholar] [CrossRef]
- Berthaut, A.; Mirshahi, P.; Benabbou, N.; Ducros, E.; Agra, A.; Therwath, A.; Legeais, J.M.; Mirshahi, M. Insulin growth factor promotes human corneal fibroblast network formation in vitro. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7647–7653. [Google Scholar] [CrossRef] [Green Version]
- Kawana, K.; Tokunaga, T.; Miyata, K.; Okamoto, F.; Kiuchi, T.; Oshika, T. Comparison of corneal thickness measurements using Orbscan II, non-contact specular microscopy, and ultrasonic pachymetry in eyes after laser in situ keratomileusis. Br. J. Ophthalmol. 2004, 88, 466–468. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Rho, C.R.; Cho, Y.W.; Shin, J. Comparison of corneal thickness measurements using ultrasound pachymetry, noncontact tonopachy, Pentacam HR, and Fourier-domain OCT. Medicine 2021, 100, 25638. [Google Scholar] [CrossRef]
- Bramsen, T.; Klauber, A.; Bjerre, P. Central corneal thickness and intraocular tension in patients with acromegaly. Acta Ophthalmol. 1980, 58, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Van Setten, G.; Brismar, K.; Algvere, P. Elevated intraocular levels of insulin-like growth factor I in a diabetic patient with acromegaly. Orbit 2002, 21, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Kan, E.; Elif, K.K.; Ali, O. Comparing two acromegalic patients with respect to central corneal thickness, intraocular pressure, and tear insulin-like growth factor levels before and after treatment. Indian J. Ophthalmol. 2015, 63, 731–732. [Google Scholar] [CrossRef]
- Atmaca, M.; Kızıldağ, E.; Candan, Z.; Özbay, M.F.; Seven, İ. Ocular findings in Sheehan’s syndrome. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Ciresi, A.; Morreale, R.; Radellini, S.; Cillino, S.; Giordano, C. Corneal thickness in children with growth hormone deficiency: The effect of GH treatment. Growth Horm. IGF Res. 2014, 24, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Lomoriello, S.D.; Lombardo, M.; Tranchina, L.; Oddone, F.; Serrao, S.; Ducoli, P. Repeatability of intra-ocular pressure and central corneal thickness measurements provided by a non-contact method of tonometry and pachymetry. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 429–434. [Google Scholar] [CrossRef]
- Tranchina, L.; Lombardo, M.; Oddone, F.; Serrao, S.; Schiano Lomoriello, S.D.; Ducoli, P. Influence of corneal biomechanical properties on intraocular pressure differences between an air-puff tonometer and the Goldmann applanation tonometer. J. Glaucoma 2013, 22, 416–421. [Google Scholar] [CrossRef]
- Gordon, M.O.; Beiser, J.A.; Brandt, J.D.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., 2nd; Wilson, M.R.; et al. The Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 714–720; discussion 829–830. [Google Scholar] [CrossRef]
| Before Surgery | After Surgery | ||||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Range | Mean ± SD | Median | Range | p Value | |
| Age [years] | 49.7 ± 13.4 | 49 | 29–75 | ||||
| Gender—male [%] | 35% | ||||||
| Remission [%] | 0% | 75% | |||||
| Disease duration [years] | 8.3 ± 5.2 | 7 | 2–20 | ||||
| Tumor height [mm] | 14.0 ± 7.4 | 12.5 | 2–33 | 2.8 ± 5.1 | 0 | 0–16 | 0.001 * |
| BMI | 28.0 ± 4.1 | 27.3 | 21.4–38.5 | 27.2 ± 4.7 | 26.6 | 19.1–36.1 | 0.112 |
| IGF-1 [ng/mL] | 474 ± 299 | 359.5 | 102–1268 | 267 ± 265 | 173 | 88–1219 | 0.001 * |
| GH [µg/l] | 18.2 ± 23.3 | 6.1 | 0.2–74.0 | 3.6 ± 6.7 | 1.4 | 0.1–30 | 0.001 * |
| Glucose [mmol/mL] | 6.0 ± 0.9 | 5.8 | 5.1–9.4 | 5.1 ± 0.6 | 5 | 4.3–6.1 | <0.001 * |
| BCVA | 1.0 ± 0.1 | 1 | 0.8–1.0 | 1.0 ± 0.1 | 1 | 0.8–1.0 | 0.789 |
| SE [D] | −0.46 ± 1.33 | −0.38 | −3.50–2.00 | −0.45 ± 1.85 | −0.63 | −3.63–2.75 | 0.802 |
| IOP air puff [mmHg] | 17.7 ± 2.3 | 18 | 13–22 | 16.2 ± 2.9 | 16 | 12–23 | 0.012 * |
| IOP GAT [mmHg] | 16.2 ± 2.4 | 17 | 11–21 | 15.3 ± 2.3 | 15 | 12–20 | 0.083 |
| IOPc [mmHg] | 14.9 ± 3.9 | 16 | 8–22 | 14.5 ± 2.6 | 14.5 | 11–21 | 0.356 |
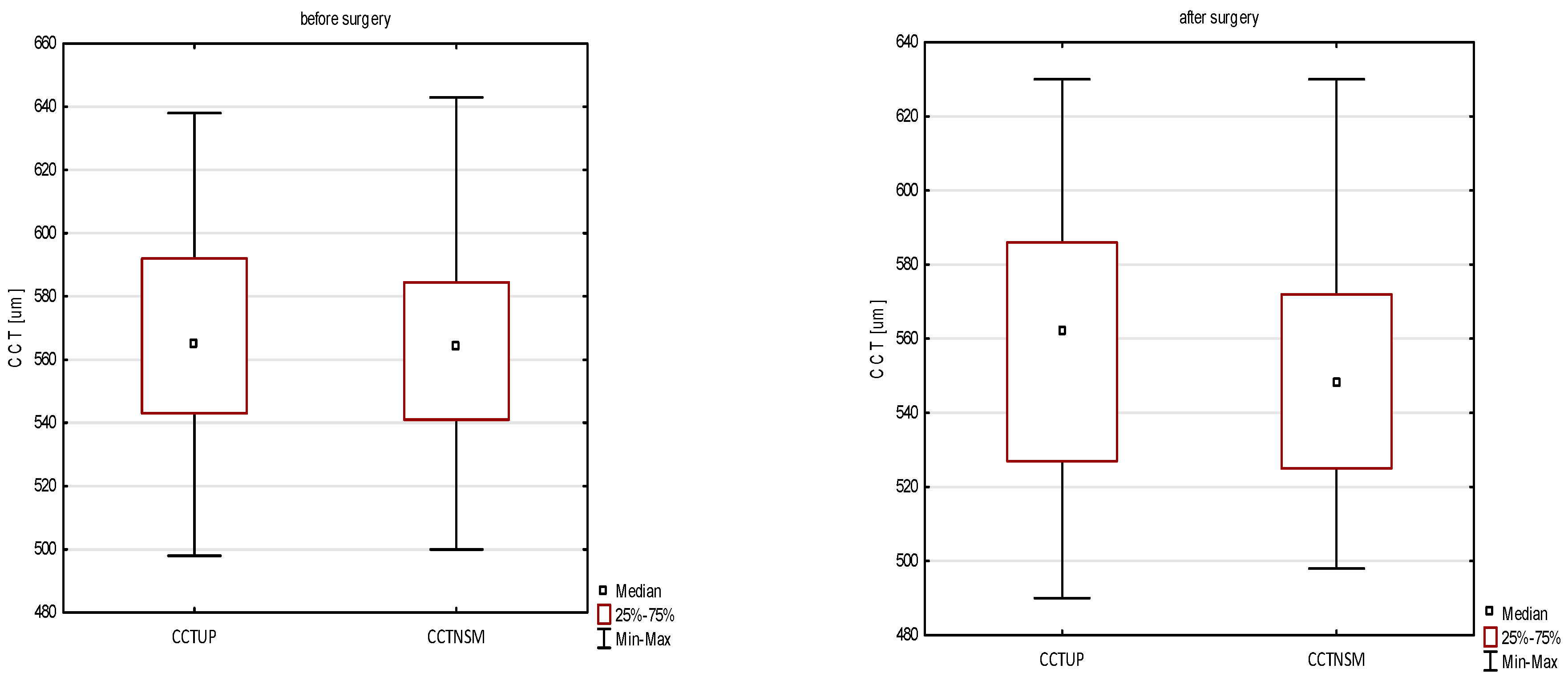
| CCTUP [μm] | 565 ± 32 | 565 | 498–638 | 557 ± 35 | 562 | 490–630 | 0.007 * |
| Before Surgery | After Surgery | ||||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Range | Mean ± SD | Median | Range | p Value | |
| CD [cell/mm2] | 2404 ± 214 | 2346 | 2110–2883 | 2525 ± 218 | 2532 | 2123–2914 | 0.001 * |
| AVG [μm2] | 415 ± 34 | 416 | 347–473 | 399 ± 34 | 395 | 343–470 | 0.009 * |
| CCTNSM [μm] | 559 ± 34 | 563 | 500–643 | 547 ± 35 | 542 | 490–630 | 0.004 * |
| Before Surgery | After Surgery | ||||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Range | Mean ± SD | Median | Range | p Value | |
| IOPcc [mmHg] | 15.7 ± 2.7 | 14.9 | 11.4–22.8 | 15.1 ± 2.6 | 15.4 | 10.9–19.3 | 0.314 |
| IOPg [mmHg] | 15.8 ± 3.4 | 14.9 | 11.9–25.8 | 14.4 ± 2.8 | 14.2 | 8.8–20.2 | 0.011 * |
| CH [mmHg] | 10.9 ± 1.4 | 10.8 | 8.4–14.3 | 10.3 ± 1.4 | 10.0 | 8.8–13.6 | 0.016 * |
| CRF [mmHg] | 11.0 ± 1.8 | 10.3 | 8.9–15.3 | 10.0 ± 1.6 | 9.7 | 7.9–14.4 | 0.001 * |
| GH | IGF-1 | Glucose | Tumor Height | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORA | Before Surgery | After Surgery | Before Surgery | After Surgery | Before Surgery | After Surgery | Before Surgery | |||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| CH | 0.373 | 0.116 | 0.038 | 0.878 | 0.508 | 0.026 | −0.020 | 0.936 | 0.634 | 0.004 | 0.153 | 0.532 | 0.624 | 0.004 |
| CRF | 0.394 | 0.095 | 0.279 | 0.248 | 0.548 | 0.015 | −0.023 | 0.926 | 0.548 | 0.015 | 0.346 | 0.146 | 0.573 | 0.010 |
| IOPg | 0.317 | 0.186 | 0.399 | 0.091 | 0.365 | 0.124 | 0.080 | 0.745 | 0.282 | 0.242 | 0.515 | 0.024 | 0.218 | 0.371 |
| IOPcc | 0.239 | 0.325 | 0.239 | 0.325 | 0.208 | 0.394 | −0.018 | 0.943 | −0.078 | 0.752 | 0.355 | 0.136 | 0.225 | 0.355 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrzypiec, I.; Wierzbowska, J.; Sobol, M.; Zieliński, G. Corneal Tonometric and Morphological Changes in Patients with Acromegaly. J. Clin. Med. 2022, 11, 6750. https://doi.org/10.3390/jcm11226750
Skrzypiec I, Wierzbowska J, Sobol M, Zieliński G. Corneal Tonometric and Morphological Changes in Patients with Acromegaly. Journal of Clinical Medicine. 2022; 11(22):6750. https://doi.org/10.3390/jcm11226750
Chicago/Turabian StyleSkrzypiec, Izabela, Joanna Wierzbowska, Maria Sobol, and Grzegorz Zieliński. 2022. "Corneal Tonometric and Morphological Changes in Patients with Acromegaly" Journal of Clinical Medicine 11, no. 22: 6750. https://doi.org/10.3390/jcm11226750





