Management of Endometrial Cancer: French Society of Onco-Gynecology‘s Evaluation through a Delphi Survey
Abstract
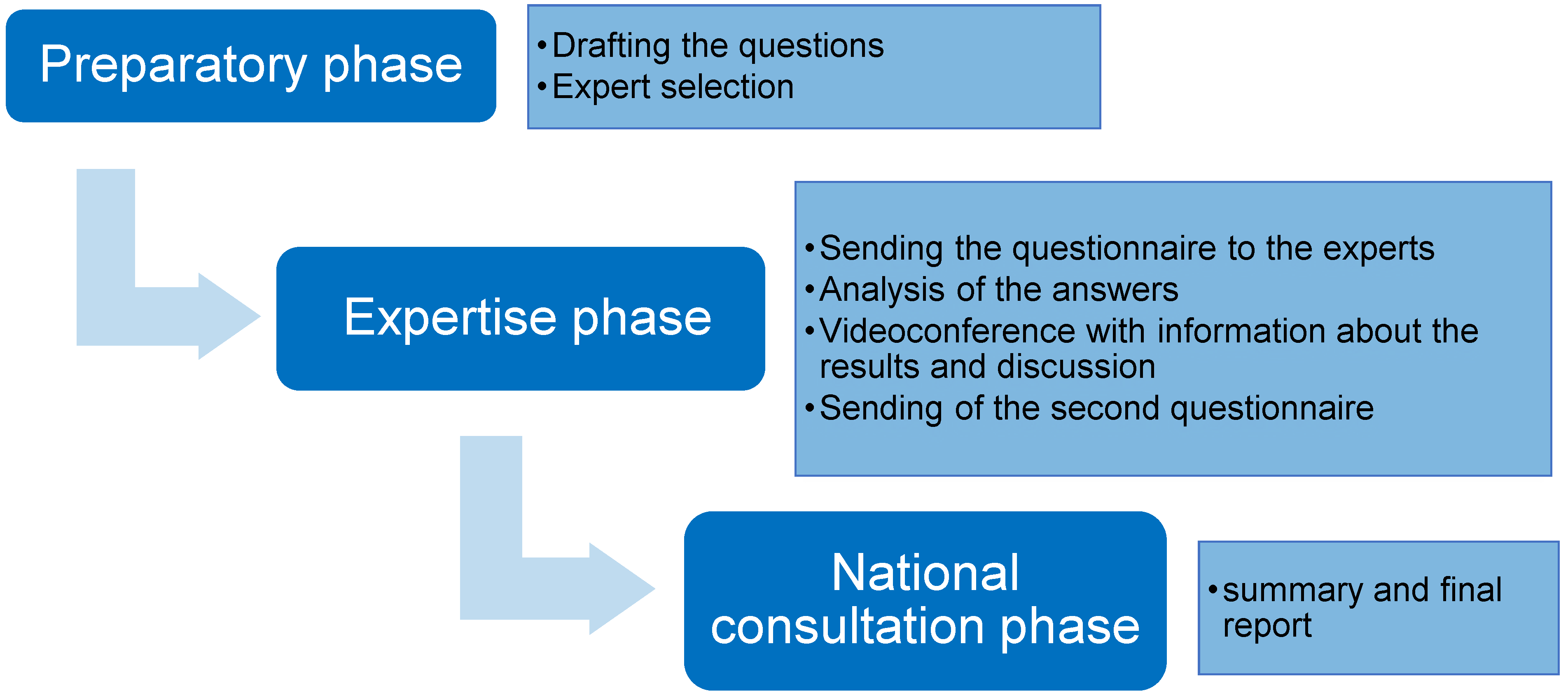
:1. Introduction
2. Materials and Methods
2.1. Preparatory Phase
2.2. Expertise Phase
- −
- panel median > 7 for validity
- −
- panel median > 6 for relevance
- −
- no disagreement within the panel
- −
- 1/3 or more of the ratings are between 1 and 3
- −
- 1/3 or more of the ratings are between 7 and 9.
3. Results
3.1. Fertility Preservation
3.2. Lymph Node Assessment
3.3. Adjuvant Treatment
3.3.1. Intermediate Risk
3.3.2. Intermediate–High Risk
3.3.3. High Risk
4. Discussion
4.1. Radiology
4.2. Pathology
- -
- Unnecessary: Low-grade tumors without embolus stage IA p53 normal and Stages III/IV
- -
- To be discussed on a case-by-case basis: In cases of non-endometrioid histology
- -
- Necessary: Stages I/II, especially high-grade endometrioid
4.3. Lymph Node Status
4.4. Adjuvant Treatment
4.5. Prospective
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Estimations Nationales de L’incidence et de la Mortalité par Cancer en France Métropolitaine Entre 1990 et 2018—Tumeurs Solides : Étude à Partir des Registres des Cancers du Réseau Francim. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/cancers/cancer-du-sein/documents/rapport-synthese/estimations-nationales-de-l-incidence-et-de-la-mortalite-par-cancer-en-france-metropolitaine-entre-1990-et-2018-volume-1-tumeurs-solides-etud (accessed on 29 June 2022).
- World Health Organization. GLOBOCAN 2018: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/24-Corpus-uteri-fact-sheet.pdf (accessed on 29 June 2021).
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO–ESGO–ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Dalkey, N.; Helmer, O. An experimental application of the Delphi method to the use of experts. Manag. Sci. 1959, 6, 458–467. [Google Scholar] [CrossRef]
- Nasa, P.; Jain, R.; Juneja, D. Delphi methodology in healthcare research: How to decide its appropriateness. World J. Methodol. 2021, 11, 116–129. [Google Scholar] [CrossRef]
- Jones, J.; Hunter, D. Consensus methods for medical and health services research. BMJ 1995, 311, 376. [Google Scholar] [CrossRef]
- Frei, K.A.; Kinkel, K. Staging endometrial cancer: Role of magnetic resonance imaging. J. Magn. Reason. Imaging 2001, 13, 850–855. [Google Scholar] [CrossRef]
- Legros, M.; Margueritte, F.; Tardieu, A.; Deluche, E.; Mbou, V.B.; Lacorre, A.; Ceuca, A.; Aubard, Y.; Moneteil, J.; Sallee, C.; et al. Para-aortic Lymph Node Invasion in High-risk Endometrial Cancer: Performance of 18FDG PET-CT. Anticancer Res. 2019, 39, 619–625. [Google Scholar] [CrossRef]
- Tanaka, T.; Terai, Y.; Yamamoto, K.; Yamada, T.; Ohmichi, M. The diagnostic accuracy of fluorodeoxyglucose-positron emission tomography/computed tomography and sentinel node biopsy in the prediction of pelvic lymph node metastasis in patients with endometrial cancer: A retrospective observational study. Medicine 2018, 97, e12522. [Google Scholar] [CrossRef]
- Oaknin, A.; Bosse, T.J.; Creutzberg, C.L.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.R.; et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 860–877. [Google Scholar] [CrossRef]
- Cho, K.R.; Cooper, K.; Croce, S.; Djordevic, B.; Herrington, S.; Howitt, B.; Hui, P.; Ip, P.; Koebel, M.; Lax, S.; et al. International Society of Gynecological Pathologists (ISGyP) Endometrial Cancer Project: Guidelines from the Special Techniques and Ancillary Studies Group. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. S1), S114–S122. [Google Scholar] [CrossRef]
- Panici, P.B.; Basile, S.; Maneschi, F.; Lissoni, A.A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Magili, G.; Katsaros, D.; et al. Systematic Pelvic Lymphadenectomy vs No Lymphadenectomy in Early-Stage Endometrial Carcinoma: Randomized Clinical Trial. JNCI J. Natl. Cancer Inst. 2008, 100, 1707–17016. [Google Scholar] [CrossRef] [Green Version]
- Kitchener, H.; Swart, A.M.; Qian, Q.; Amos, C.; Parmar, M.K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2008, 373, 125–136. [Google Scholar]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef]
- Soliman, P.T.; Westin, S.N.; Dioun, S.; Sun, C.C.; Euscher, E.; Munsell, M.F.; Fleming, N.D.; Levenback, C.; Frumovitz, M.; Ramirez, P.T.; et al. A prospective validation study of sentinel lymph node mapping for high-risk endometrial cancer. Gynecol. Oncol. 2017, 146, 234–239. [Google Scholar] [CrossRef]
- Cusimano, M.C.; Vicus, D.; Pulman, K.; Maganti, M.; Bernardini, M.Q.; Bouchard-Fortier, G.; Laframboise, S.; May, T.; Hogen, L.F.; Covens, A.L.; et al. Assessment of Sentinel Lymph Node Biopsy vs Lymphadenectomy for Intermediate- and High-Grade Endometrial Cancer Staging. JAMA Surg. 2021, 156, 157–164. [Google Scholar] [CrossRef]
- Persson, J.; Salehi, S.; Bollino, M.; Lönnerfors, C.; Falconer, H.; Geppert, B. Pelvic Sentinel lymph node detection in High-Risk Endometrial Cancer (SHREC-trial)—The final step towards a paradigm shift in surgical staging. Eur. J. Cancer 2019, 116, 77–85. [Google Scholar] [CrossRef]
- Bogani, G.; Murgia, F.; Ditto, A.; Raspagliesi, F. Sentinel node mapping vs. lymphadenectomy in endometrial cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2019, 153, 676–683. [Google Scholar] [CrossRef]
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef] [Green Version]
- León-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef]
- Vermij, L.; Léon-Castillo, A.; Singh, N.; Powell, M.E.; Edmondson, R.J.; Genestie, C.; Khaw, P.; Pyman, J.; McLachlin, C.M.; Ghatage, P.; et al. p53 immunohistochemistry in endometrial cancer: Clinical and molecular correlates in the PORTEC-3 trial. Mod. Pathol. 2022, 35, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Piskorz, A.M.; Bosse, T.; Jimenez-Linan, M.; Rous, B.; Brenton, J.D.; Gilks, C.B.; Köbel, M. p53 immunohistochemistry is an accurate surrogate for TP53 mutational analysis in endometrial carcinoma biopsies. J. Pathol. 2020, 250, 336–345. [Google Scholar] [CrossRef] [PubMed]
| Degree of Agreement of Experts (Positive Answers) | Validation in Round 1 or 2 | |
|---|---|---|
| Radiology | ||
| 100.0% | Round 1 |
| 100.0% | Round 1 |
| 75.0% | Round 1 |
| 100.0% | Round 1 |
| 44.4% | Round 2 |
| 75.0% in favour of PET | Round 1 |
| -clinical follow-up: 52.6% -no follow-up: 26.3% -MRI: 10.3% | Round 2 |
| -PET scan: 31.6% -Clinical follow-up: 21.1% | Round 2 |
| Pathology | ||
| 83.3% | Round 1 |
| Performance of a complete MLH1/PMS2/MSH2/MSH6 panel in IHC and confirmation in molecular biology if loss of expression: 78.9% | Round 2 |
| ultrastadification HES + IHC: 66.7% | Round 1 |
| 16.7% | Round 1 |
| Fertility preservation | ||
| Oral progestin: 63.6% | Round 1 |
| 79.0% | Round 2 |
| 87.2% | Round 1 |
| Surgery of early stages | ||
| Curietherapy and external radiotherapy: 61.1% | Round 2 |
| Assessment of lymph node status | ||
| 20:84.6% | Round 1 |
| 84.6% | Round 1 |
| 34.5% | Round 1 |
| 80.7% | Round 1 |
| 80.8% | Round 1 |
| 60.0% | Round 2 |
| 89.4% | Round 2 |
| 69.2% | Round 1 |
| Intermediate risk | ||
| 66.6% | Round 1 |
| 33.3% | Round 1 |
| 33.3% | Round 1 |
| 33.3% | Round 1 |
| Intermediate–high risk | ||
| 45 Gy: 100.0% | Round 1 |
| Intermediate–high risk (pN0 after lymph node staging) | ||
| 88.9% | Round 1 |
| 84.6% | Round 1 |
| 22.5% | Round 1 |
| Intermediate–high risk (cN0/Nx) | ||
| 94.8% | Round 1 |
| 92.4% | Round 1 |
| 55.5% | Round 1 |
| High risk | ||
| PORTEC: 66.7% | Round 2 |
| 86.8% | Round 1 |
| 85.8% | Round 1 |
| 39.0% | Round 2 |
| 39.0% | Round 1 |
| 95.3% | Round 1 |
| 90.2% | Round 1 |
| Most pejorative: 93.6% | Round 1 |
| 66.8% | Round 2 |
| 88.9% | Round 2 |
| Locally advanced/metastatic tumours | ||
| 80.8% | Round 1 |
| 100.0% | Round 1 |
| 72.3% | Round 2 |
| 35.0% | Round 1 |
| 54.5% | Round 1 |
| 90.9% | Round 1 |
| 92.3% | Round 1 |
| 4 courses with carboplatin-taxol: 44.4% | Round 2 |
| 4 courses with carboplatine-taxol: 50.0% | Round 2 |
| 6 courses with carboplatin-taxol: 72.2% | Round 2 |
| 6 courses with carboplatine-taxol: 66.7% | Round 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marti, C.; Deluche, E.; Jochum, F.; Bendifallah, S.; Azais, H.; Deidier, J.; Cockenpot, V.; Menoux, I.; Balaya, V.; Betrian, S.; et al. Management of Endometrial Cancer: French Society of Onco-Gynecology‘s Evaluation through a Delphi Survey. J. Clin. Med. 2022, 11, 6765. https://doi.org/10.3390/jcm11226765
Marti C, Deluche E, Jochum F, Bendifallah S, Azais H, Deidier J, Cockenpot V, Menoux I, Balaya V, Betrian S, et al. Management of Endometrial Cancer: French Society of Onco-Gynecology‘s Evaluation through a Delphi Survey. Journal of Clinical Medicine. 2022; 11(22):6765. https://doi.org/10.3390/jcm11226765
Chicago/Turabian StyleMarti, Carolin, Elise Deluche, Floriane Jochum, Sofiane Bendifallah, Henri Azais, Jonas Deidier, Vincent Cockenpot, Inès Menoux, Vincent Balaya, Sarah Betrian, and et al. 2022. "Management of Endometrial Cancer: French Society of Onco-Gynecology‘s Evaluation through a Delphi Survey" Journal of Clinical Medicine 11, no. 22: 6765. https://doi.org/10.3390/jcm11226765
APA StyleMarti, C., Deluche, E., Jochum, F., Bendifallah, S., Azais, H., Deidier, J., Cockenpot, V., Menoux, I., Balaya, V., Betrian, S., Chargari, C., Gouy, S., Genestie, C., Feki, A., Uzan, C., Guyon, F., Devouassoux-Shisheboran, M., Body, N., Akladios, C., ... on behalf of the SFOG and the SFOG Campus. (2022). Management of Endometrial Cancer: French Society of Onco-Gynecology‘s Evaluation through a Delphi Survey. Journal of Clinical Medicine, 11(22), 6765. https://doi.org/10.3390/jcm11226765







