Megakaryocyte- and Platelet-Derived Microparticles as Novel Diagnostic and Prognostic Biomarkers for Immune Thrombocytopenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Plasma Collection and Preparation
2.3. Quantification of MPs by Flow Cytometry
2.4. Statistical Analysis
3. Results
3.1. Study Population
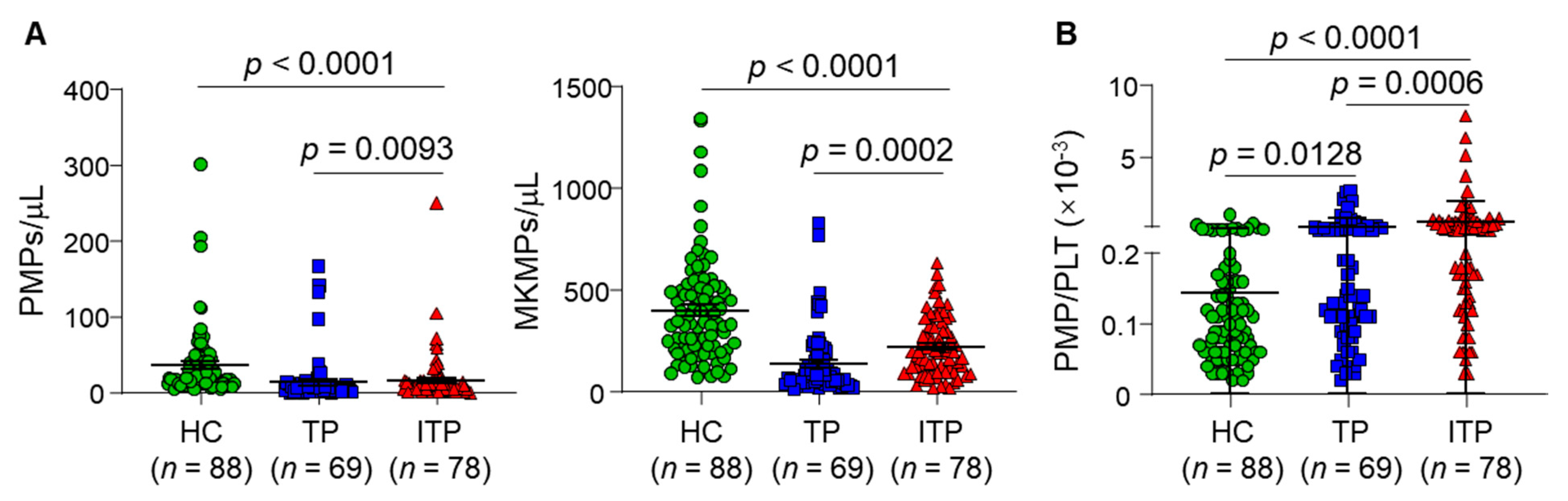
3.2. Plasma MP Levels in ITP, TP Patients and Healthy Controls
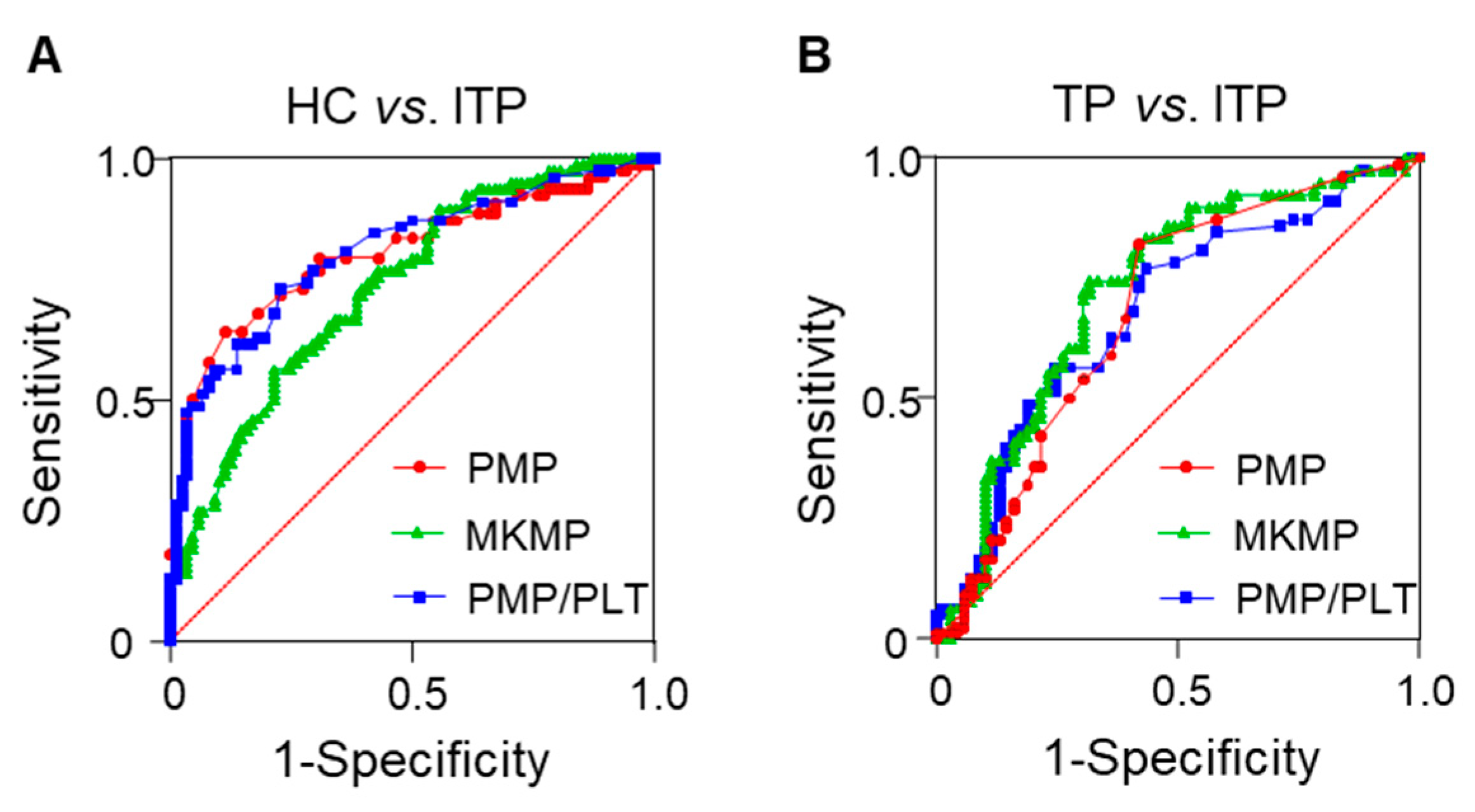
3.3. Diagnostic Values of Plasma MPs for ITP
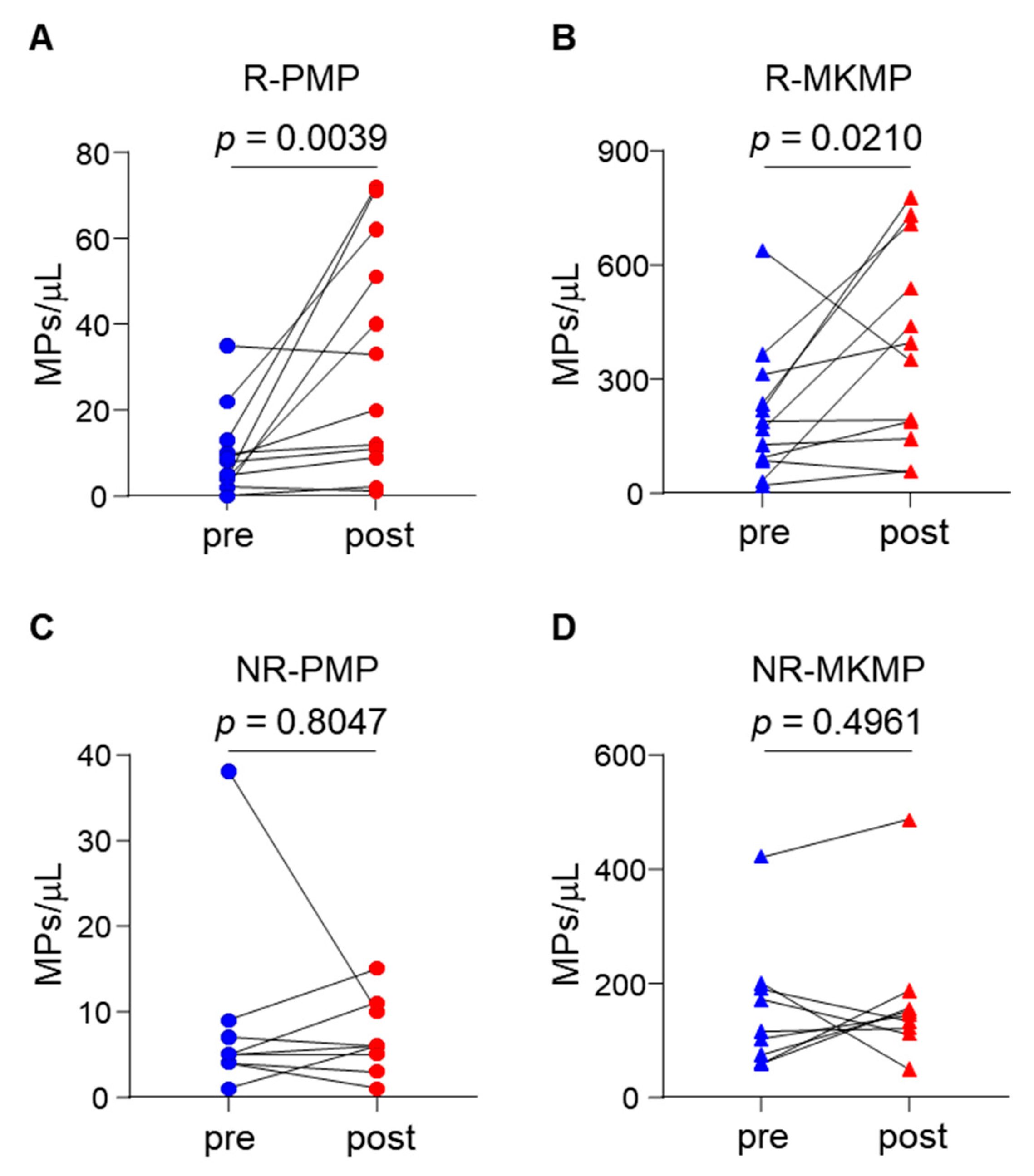
3.4. Elevated Plasma MKMP and PMP Levels in TPO-RA Responders
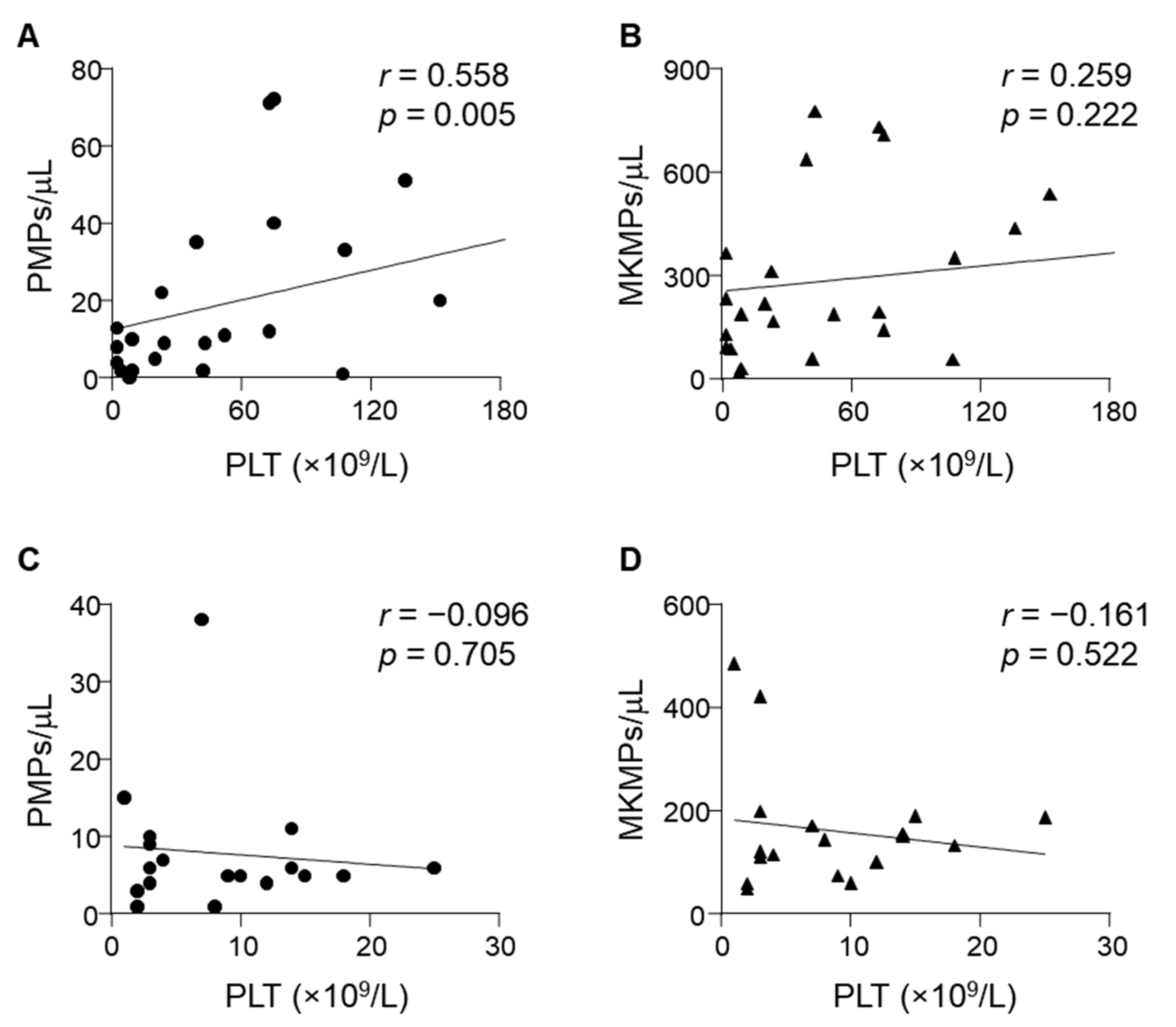
3.5. Correlation between Plasma MP Levels and ITP Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cines, D.B.; Bussel, J.B.; Liebman, H.A.; Prak, E.T.L. The ITP syndrome: Pathogenic and clinical diversity. Blood 2009, 113, 6511–6521. [Google Scholar] [CrossRef] [Green Version]
- Onisâi, M.; Vlădăreanu, A.M.; Spînu, A.; Găman, M.; Bumbea, H. Idiopathic thrombocytopenic purpura (ITP)—New era for an old disease. Rom. J. Intern. Med. 2019, 57, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Terrell, D.R.; Neunert, C.E.; Cooper, N.; Heitink-Pollé, K.M.; Kruse, C.; Imbach, P.; Kühne, T.; Ghanima, W. Immune Thrombo-cytopenia (ITP): Current Limitations in Patient Management. Medicina 2020, 56, 667. [Google Scholar] [CrossRef]
- Provan, D.; Stasi, R.; Newland, A.C.; Blanchette, V.S.; Bolton-Maggs, P.; Bussel, J.B.; Chong, B.H.; Cines, D.B.; Gernsheimer, T.B.; Godeau, B.; et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010, 115, 168–186. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Flaumenhaft, R.; Dilks, J.R.; Richardson, J.; Alden, E.; Patel-Hett, S.R.; Battinelli, E.; Klement, G.L.; Sola-Visner, M.; Italiano, J.E., Jr. Megakaryocyte-derived microparticles: Direct visualization and distinction from platelet-derived microparticles. Blood 2009, 113, 1112–1121. [Google Scholar] [CrossRef] [Green Version]
- Puhm, F.; Boilard, E.; Machlus, K.R. Platelet Extracellular Vesicles: Beyond the Blood. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 87–96. [Google Scholar] [CrossRef]
- Badimon, L.; Suades, R.; Fuentes, E.; Palomo, I.; Padró, T. Role of Platelet-Derived Microvesicles As Crosstalk Mediators in Ath-erothrombosis and Future Pharmacology Targets: A Link between Inflammation, Atherosclerosis, and Thrombosis. Front. Pharmacol. 2016, 7, 293. [Google Scholar] [CrossRef] [Green Version]
- Julich-Haertel, H.; Urban, S.K.; Krawczyk, M.; Willms, A.; Jankowski, K.; Patkowski, W.; Kruk, B.; Krasnodębski, M.; Ligocka, J.; Schwab, R.; et al. Cancer-associated circulating large extracellular vesicles in cholan-giocarcinoma and hepatocellular carcinoma. J. Hepatol. 2017, 67, 282–292. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Chen, Z.; Hu, Q.; Wang, C.; Yan, J.; Dotti, G.; Huang, P.; Gu, Z. Engineering PD-1-Presenting Platelets for Cancer Immunotherapy. Nano Lett. 2018, 18, 5716–5725. [Google Scholar] [CrossRef]
- Sewify, E.M.; Sayed, D.; Aal, R.F.A.; Ahmad, H.M.; Abdou, M.A. Increased circulating red cell microparticles (RMP) and platelet microparticles (PMP) in immune thrombocytopenic purpura. Thromb. Res. 2013, 131, e59–e63. [Google Scholar] [CrossRef]
- Tantawy, A.A.G.; Matter, R.M.; Hamed, A.A.; Telbany, M.A.S.E.D.E. Platelet microparticles in immune thrombocytopenic purpura in pediatrics. Pediatr. Hematol. Oncol. 2010, 27, 283–296. [Google Scholar] [CrossRef]
- Álvarez-Román, M.T.; Fernández-Bello, I.; Jiménez-Yuste, V.; Martín-Salces, M.; Arias-Salgado, E.G.; Pollmar, M.I.R.; Sanz, R.J.; Butta, N.V. Procoagulant profile in patients with immune thrombocytopenia. Br. J. Haematol. 2016, 175, 925–934. [Google Scholar] [CrossRef]
- Kao, C.Y.; Papoutsakis, E.T. Engineering human megakaryocytic microparticles for targeted delivery of nucleic acids to hem-atopoietic stem and progenitor cells. Sci. Adv. 2018, 4, eaau6762. [Google Scholar] [CrossRef] [Green Version]
- Escobar, C.; Kao, C.-Y.; Das, S.; Papoutsakis, E.T. Human megakaryocytic microparticles induce de novo platelet biogenesis in a wild-type murine model. Blood Adv. 2020, 4, 804–814. [Google Scholar] [CrossRef] [Green Version]
- Houwerzijl, E.J.; Blom, N.R.; van der Want, J.J.; Esselink, M.T.; Koornstra, J.J.; Smit, J.W.; Louwes, H.; Vellenga, E.; de Wolf, J.T. Ultrastructural study shows morphologic features of apoptosis and para-apoptosis in megakaryocytes from patients with id-iopathic thrombocytopenic purpura. Blood 2004, 103, 500–506. [Google Scholar] [CrossRef]
- Neunert, C.; Lim, W.; Crowther, M.; Cohen, A.; Solberg, L.; Crowther, M.A., Jr. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011, 117, 4190–4207. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhao, Y.; Zhu, M.; Wu, Q.; Ruan, C. Detection of autoantibodies against platelet glycoproteins in patients with immune thrombocytopenic purpura by flow cytometric immunobead array. Clin. Chim. Acta 2013, 415, 176–180. [Google Scholar] [CrossRef]
- Zhai, J.; Ding, M.; Yang, T.; Zuo, B.; Weng, Z.; Zhao, Y.; He, J.; Wu, Q.; Ruan, C.; He, Y. Flow cytometric immunobead assay for quantitative detection of platelet autoantibodies in immune thrombocytopenia patients. J. Transl. Med. 2017, 15, 214. [Google Scholar] [CrossRef] [Green Version]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Burnouf, T.; Chou, M.L.; Goubran, H.; Cognasse, F.; Garraud, O.; Seghatchian, J. An overview of the role of microparticles/microvesicles in blood components: Are they clinically beneficial or harmful? Transfus. Apher. Sci. 2015, 53, 137–145. [Google Scholar] [CrossRef]
- Montoro-García, S.; Shantsila, E.; Orenes-Piñero, E.; Lozano, M.L.; Lip, G.Y.H. An innovative flow cytometric approach for small-size platelet microparticles: Influence of calcium. Thromb. Haemost. 2012, 108, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Stoner, S.A.; Duggan, E.; Condello, D.; Guerrero, A.; Turk, J.R.; Narayanan, P.K.; Nolan, J.P. High sensitivity flow cytometry of membrane vesicles. Cytom. Part A J. Int. Soc. Anal. Cytol. 2016, 89, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Arraud, N.; Gounou, C.; Linares, R.; Brisson, A.R. A simple flow cytometry method improves the detection of phosphatidyl-serine-exposing extracellular vesicles. J. Thromb. Haemost. JTH 2015, 13, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Hicks, S.M.; Coupland, L.A.; Jahangiri, A.; Choi, P.Y.; Gardiner, E.E. Novel scientific approaches and future research directions in understanding ITP. Platelets 2020, 31, 315–321. [Google Scholar] [CrossRef]
- Weil, D.; Di Martino, V.; Mourey, G.; Biichle, S.; Renaudin, A.; Laheurte, C.; Cypriani, B.; Delabrousse, E.; Grandclément, E.; Thévenot, T.; et al. Small Annexin V–Positive Platelet-Derived Microvesicles Affect Prognosis in Cirrhosis: A Longitudinal Study. Clin. Transl. Gastroenterol. 2021, 12, e00333. [Google Scholar] [CrossRef]
- Van Der Zee, P.M.; Biró, É; Ko, Y.; De Winter, R.J.; Hack, C.E.; Sturk, A.; Nieuwland, R. P-Selectin- and CD63-Exposing Platelet Microparticles Reflect Platelet Activation in Peripheral Arterial Disease and Myocardial Infarction. Clin. Chem. 2006, 52, 657–664. [Google Scholar] [CrossRef]
- Michelsen, A.E.; Brodin, E.; Brosstad, F.; Hansen, J.B. Increased level of platelet microparticles in survivors of myocardial in-farction. Scand. J. Clin. Lab. Investig. 2008, 68, 386–392. [Google Scholar] [CrossRef]
- Mooberry, M.J.; Key, N.S. Microparticle analysis in disorders of hemostasis and thrombosis. Cytom. Part A J. Int. Soc. Anal. Cytol. 2016, 89, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Jy, W.; Horstman, L.L.; Arce, M.; Ahn, Y.S. Clinical significance of platelet microparticles in autoimmune thrombocytopenias. J. Lab. Clin. Med. 1992, 119, 334–335. [Google Scholar]
- Psaila, B.; Bussel, J.B.; Frelinger, A.L.; Babula, B.; Linden, M.D.; Li, Y.; Barnard, M.R.; Tate, C.; Feldman, E.J.; Michelson, A.D. Differences in platelet function in patients with acute myeloid leukemia and myelodysplasia compared to equally thrombocyto-penic patients with immune thrombocytopenia. J. Thromb. Haemost. JTH 2011, 9, 2302–2310. [Google Scholar] [CrossRef]
- Boulware, R.; Refaai, M.A. Why do patients with immune thrombocytopenia (ITP) experience lower bleeding events despite thrombocytopenia? Thromb. Res. 2020, 187, 154–158. [Google Scholar] [CrossRef]
- Barsam, S.J.; Psaila, B.; Forestier, M.; Page, L.K.; Sloane, P.A.; Geyer, J.T.; Villarica, G.O.; Ruisi, M.M.; Gernsheimer, T.B.; Beer, J.H.; et al. Platelet production and platelet destruction: Assessing mechanisms of treatment effect in immune thrombocytopenia. Blood 2011, 117, 5723–5732. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, E.E.; Thom, J.Y.; Al-Tamimi, M.; Hughes, A.; Berndt, M.C.; Andrews, R.K.; Baker, R. Restored platelet function after romiplostim treatment in a patient with immune thrombocytopenic purpura. Br. J. Haematol. 2010, 149, 625–628. [Google Scholar] [CrossRef]
- Brisson, A.R.; Tan, S.; Linares, R.; Gounou, C.; Arraud, N. Extracellular vesicles from activated platelets: A semiquantitative cryo-electron microscopy and immuno-gold labeling study. Platelets 2017, 28, 263–271. [Google Scholar] [CrossRef]

| Baseline Characteristics | Discovery Set | Therapy Set | |||
|---|---|---|---|---|---|
| HC (n = 88) | TP (n = 69) | ITP (n = 78) | Non-Responder (n = 9) | Responder (n = 12) | |
| Age, yrs. | 43 [37–50] | 44 [33–58] | 49 [34–58] | 61 [31–68] | 49 [27–59] |
| Male, n (%) | 52 (59.1) | 35 (50.7) | 38 (48.7) | 5 (55.6) | 5 (41.7) |
| Disease course, n (%) | |||||
| Newly diagnosed | NA | NA | 36 (46.2) | 1 (11.1) | 6 (50) |
| Persistent | NA | NA | 17 (21.8) | 5 (55.6) | 1 (8.3) |
| Chronic | NA | NA | 25 (32.1) | 3 (33.3) | 5 (41.7) |
| FCIA, n (%) | |||||
| Negative | NA | NA | 53 (67.9) | 5 (55.6) | 10 (83.3) |
| GPIb | NA | NA | 4 (5.1) | 1 (11.1) | 0 (0) |
| GPIIb | NA | NA | 3 (3.8) | 0 (0) | 1 (8.3) |
| GPIIIa | NA | NA | 7 (9.0) | 1 (11.1) | 0 (0) |
| Multiple Abs | NA | NA | 11 (14.1) | 2 (22.2) | 1 (8.3) |
| Platelet count (×109/L) | |||||
| Pre-treatment | 238 [204–288] | 24 [17–37] * | 28 [10–56] * | 7 [3–11] | 9 [2–22] |
| Post-treatment | NA | NA | NA | 8 [3–16] | 75 [57–129] § |
| RP % | NA | NA | 19 [9.4–28.7] | 11 [7.9–37.6] | 23 [13.3–31.6] |
| MPV (fL) | 10.5 [9.9–11.1] | 12 [11.1–12.7] * | 12.5 [11.6–13.9] * | 11.2 [10.4–11.7] | 11.4 [11.1–13.5] |
| TPO (pg/mL) | NA | NA | 110.2 [52.2–172.8] | 92.8 [41.4–147.8] | 187 [169.4–279.7] |
| WBC (×109/L) | 6.3 [5.1–7.0] | 1.5 [0.5–3.1] * | 8.6 [6.3–11.1] # | 8.0 [5.0–10.9] | 9.1 [6.8–10.5] |
| Hemoglobin (g/L) | 145 [134–154] | 75 [59–93] * | 125 [110–140] * | 125 [113–138] | 131 [84–139] |
| Treatment, n (%) | |||||
| Steroids | NA | NA | 77 (98.7) | 9 (100) | 12 (100) |
| Immune suppressant | NA | NA | 11 (14.1) | 4 (44.4) | 2 (16.7) |
| Splenectomy | NA | NA | 1 (1.3) | 0 (0) | 1 (8.3) |
| Others | NA | NA | 70 (89.7) | 9 (100) | 12 (100) |
| Parameters | HC vs. ITP | TP vs. ITP | ||||
|---|---|---|---|---|---|---|
| PMP | MKMP | PMP/PLT | PMP | MKMP | PMP/PLT | |
| AUC | 0.805 | 0.735 | 0.808 | 0.682 | 0.723 | 0.681 |
| 95% CI | 0.736–0.874 | 0.661–0.810 | 0.741–0.875 | 0.593–0.770 | 0.639–0.808 | 0.593–0.768 |
| Cut-off values | <9.500 (MPs/μL) | <209.0 (MPs/μL) | >0.165 (×10−3) | >3.500 (MPs/μL) | >112.5 (MPs/μL) | >0.145 (×10−3) |
| Sensitivity (%) | 64.1 | 56.4 | 73.1 | 82.1 | 74.4 | 76.9 |
| Specificity (%) | 88.6 | 78.4 | 77.3 | 58.0 | 68.1 | 56.5 |
| p-value | <0.0001 | <0.0001 | <0.0001 | 0.0001 | <0.0001 | 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zuo, B.; Wang, Y.; Li, X.; Weng, Z.; Zhai, J.; Wu, Q.; He, Y. Megakaryocyte- and Platelet-Derived Microparticles as Novel Diagnostic and Prognostic Biomarkers for Immune Thrombocytopenia. J. Clin. Med. 2022, 11, 6776. https://doi.org/10.3390/jcm11226776
Wang W, Zuo B, Wang Y, Li X, Weng Z, Zhai J, Wu Q, He Y. Megakaryocyte- and Platelet-Derived Microparticles as Novel Diagnostic and Prognostic Biomarkers for Immune Thrombocytopenia. Journal of Clinical Medicine. 2022; 11(22):6776. https://doi.org/10.3390/jcm11226776
Chicago/Turabian StyleWang, Wen, Bin Zuo, Yao Wang, Xinyu Li, Zhen Weng, Juping Zhai, Qingyu Wu, and Yang He. 2022. "Megakaryocyte- and Platelet-Derived Microparticles as Novel Diagnostic and Prognostic Biomarkers for Immune Thrombocytopenia" Journal of Clinical Medicine 11, no. 22: 6776. https://doi.org/10.3390/jcm11226776
APA StyleWang, W., Zuo, B., Wang, Y., Li, X., Weng, Z., Zhai, J., Wu, Q., & He, Y. (2022). Megakaryocyte- and Platelet-Derived Microparticles as Novel Diagnostic and Prognostic Biomarkers for Immune Thrombocytopenia. Journal of Clinical Medicine, 11(22), 6776. https://doi.org/10.3390/jcm11226776






