Maxillomandibular Advancement and Upper Airway Stimulation for Treatment of Obstructive Sleep Apnea: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.2. Literature Search
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
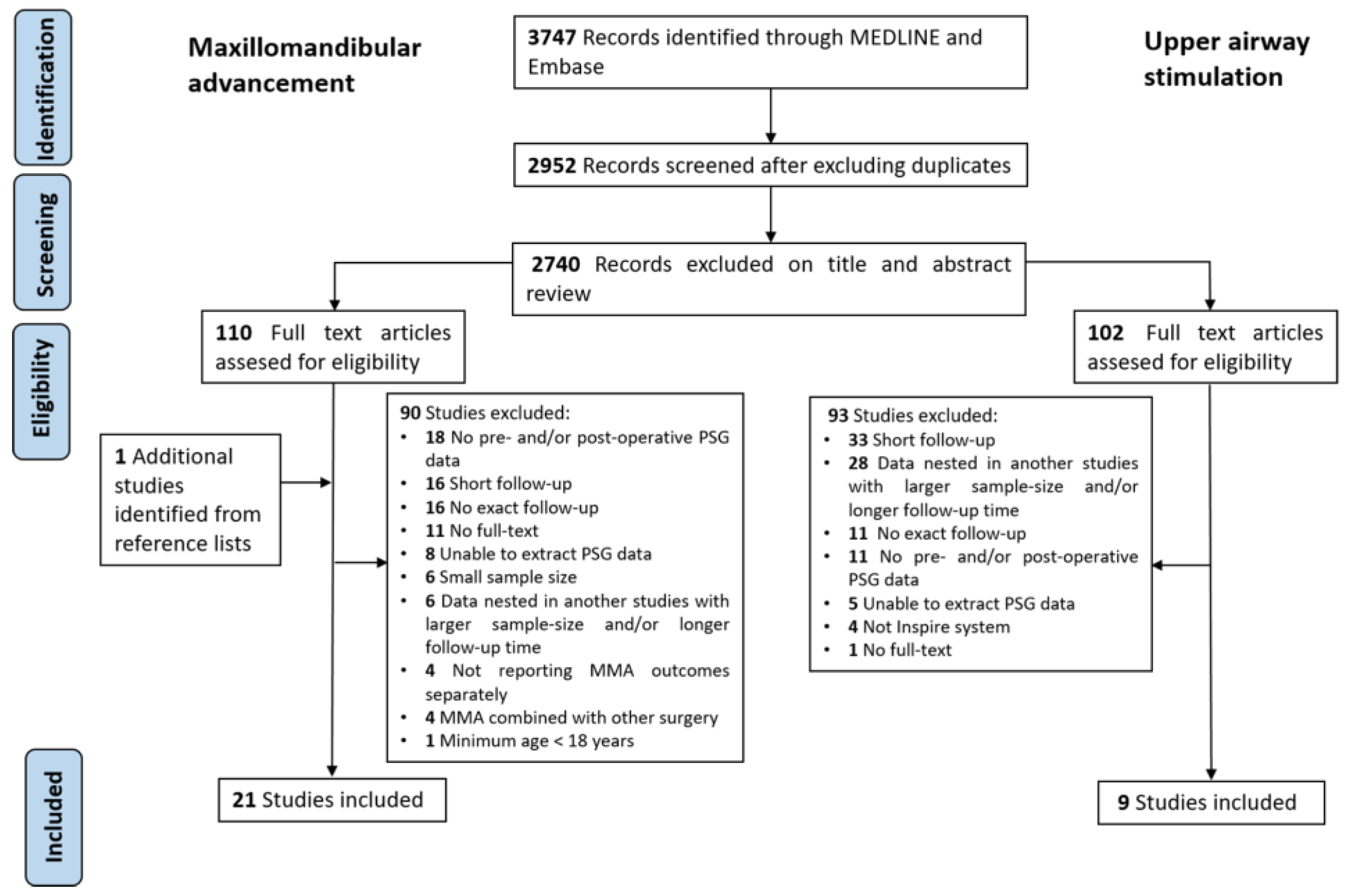
3.1. Search Results
| Study | Design | N | Age (Years) (Mean ± SD) | % Male | Degree of Advancement (mm) (Mean ± SD) | Follow-Up (Mean ± SD) | BMI (Mean ± SD) | AHI (Mean ± SD) | ODI (Mean ± SD) | ESS (Mean ± SD) | % Success | % Cure | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max | Mand | Pre-op | Post-op | Pre-op | Post-op | Pre-op | Post-op | Pre-op | Post-op | ||||||||
| Bettega et al., 2000 [28] | Retro | 20 | 44.4 ± 10.6 | 90 | 11.8 ± 0.5 | 11.8 ± 0.5 | 6 m | 26.9 ± 4.3 | 25.4 ± 3.3 | 59.3 ± 29.0 | 11.1 ± 8.9 | 75 c | |||||
| Bianchi et al., 2014 [29] | Retro | 10 | 45 ± 14 | 100 | 10 | 10 | 6 m | 56.8 ± 5.2 | 12.3 ± 5.5 | ||||||||
| Boyd et al., 2015 [30] | Pro | 14 | 7.0 ± 2.3 | 9.2 ± 3.3 | 6.6 ± 2.8 y | 50.0 ± 20.0 | 8.0 ± 10.7 | ||||||||||
| Conradt et al., 1997 [31] | Retro | 15 | 44 ± 12 | 93.3 | >2 y | 28.3 ± 3.4 | 51.4 ± 16.9 | 8.5 ± 9.4 | |||||||||
| Gerbino et al., 2014 [32] | Pro | 10 | 44.9 | 9.2 ± 1.2 | 10.4 ± 2.2 | 6 m | 31.6 ± 5.5 | 28 ± 1.4 | 69.8 ± 35.2 | 17.3 ± 16.7 | 59.5 ± 5.3 | 9.1 ± 8 | 80 d | ||||
| Goh et al., 2003 [33] | Pro | 11 | 42.8 ± 8.2 | 100 | 10 | 10 | 7.7 m | 29.4 ± 4.6 | 27.2 ± 3.3 | 70.7 ± 15.9 | 11.4 ± 7.4 | 81.8 | |||||
| Goodday et al., 2016 [34] | Retro | 13 | 37.8 ± 8.6 | 84.6 | 9.6 m | 38.8 ± 10.9 | 37.3 ± 8.0 | 117.9 ± 9.2 | 16.1 ± 26.2 | 12.9 ± 5.5 b | 5.0 ± 4.1 b | 76.9 | 46.2 | ||||
| Hsieh et al., 2014 [35] | Pro | 16 | 33 ± 7.9 | 75 | 12 ± 8 m | 22 ± 3.3 | 35.7 ± 18 | 4.8 ± 4.4 | 100 | ||||||||
| Kastoer et al., 2019 [36] | Pro | 14 | 51.1 ± 7.3 | 57.1 | 6 m | 25.7 ± 3.7 | 40.2 ± 25.6 | 9.9 ± 7.2 | 13.5 ± 18.6 | 4.0 ± 3.5 | 13 ± 6 | 9 ± 7 | |||||
| Li et al., 1999 [39] | Retro | 175 | 43.5 ± 11.5 | 83 | 6 m | 72.3 ± 26.7 a | 7.2 ± 7.5 a | 95 e | |||||||||
| Li et al., 2000 [38] | Retro | 40 | 45.6 ± 20.7 | 82.5 | 10.8 ± 2.7 | 10.8 ± 2.7 | 4.2 ± 2.7 y | 31.4 ± 6.7 | 32.2 ± 6.3 | 71.2 ± 27.0 a | 7.6 ± 5.1 a | 90 e | |||||
| Li et al., 2001 [40] | Retro | 52 | 46.6 ± 6.7 | 82.7 | 10.5 ± 1.5 | 6 m | 32.0 ± 6.0 | 61.6 ± 23.9 a | 9.2 ± 8 a | 90 f | |||||||
| Li et al., 2002 [37] | Pro | 12 | 47.3 ± 9.8 | 75 | 10.5 ± 1.2 | 10.5 ± 1.2 | 6 m | 33.5 ± 6.2 | 32.3 ± 4.1 | 75.3 ± 26.4 a | 10.4 ± 10.8 a | 83.3 f | |||||
| Liao et al., 2015 [41] | Pro | 20 | 33.4 ± 6.5 | 85 | 14 ± 9.3 m | 22.4 ± 3.4 | 41.6 ± 19.2 | 5.3 ± 4 | 11.9 ± 7.3 | 7 ± 3 | 100 c | ||||||
| Lin et al., 2020 [42] | Pro | 53 | 35.7 ± 11.7 | 75.7 | 4.3 ± 2.9 | 13.3 ± 3.8 | 24 m | 24.8 ± 3.3 | 23.9 ± 4.7 | 34.8 ± 26.0 | 7.4 ± 6.7 | 10.8 ± 5 | 10.2 ± 5.1 | 67.9 | |||
| Liu et al., 2016 [11] | Retro | 20 | 44 ± 12 | 85 | 7 ± 1.4 | 6 m | 27 ± 4.6 | 27.4 ± 4.6 | 53.6 ± 26.6 | 9.5 ± 7.4 | 38.7 ± 30.3 | 8.1 ± 9.2 | 17.0 ± 4.8 | 5.7 ± 2.7 | 90 | 50 | |
| Rubio-Bueno et al., 2017 [43] | Pro | 34 | 40.8 ± 13.9 | 41.2 | 4.9 ± 3.2 | 10.4 ± 3.9 | 6 m | 27.6 ± 4.5 | 25.5 ± 4.3 | 38.3 ± 10.7 | 6.5 ± 4.3 | 34.7 ± 12.5 | 5.4 ± 4.1 | 17.4 ± 5.4 | 0.8 ± 1.4 | 100 | 52.9 |
| Veys et al., 2017 [44] | Pro | 10 | 44.7 ± 9.5 | 80 | 4.8 ± 2.8 | 8.3 ± 2.3 | 6 m | 26.8 ± 12.7 | 12.3 ± 14.4 | 14.1 ± 5.9 | 5.7 ± 3.0 | 70 | 40 | ||||
| Vicini et al., 2010 [45] | RCT | 25 | 49.1 ± 9.1 | 92 | 11 | 13 ± 2.5 m | 32.7 ± 5.8 | 31.4 ± 6.5 | 56.8 ± 16.5 | 8.1 ± 7 | 11.6 ± 2.8 | 7.7 ± 1.3 | 88 | 36 | |||
| Vigneron et al., 2017 [46] | Retro | 29 | 40.7 ± 12.6 | 8.4 ± 4.1 | 11.7 ± 5.1 | 12.5 ± 3.5 y | 24.6 ± 4 | 56.6 ± 24 | 25.5 ± 20.6 | 7.5 ± 4.7 | 41.4 | ||||||
| Wu et al., 2019 [47] | Retro | 28 | 37.2 ± 11.8 | 53.6 | 2.0 ± 3.1 | 8.8 ± 3.7 | >1 y | 24.2 ± 5.1 | 59.3 ± 14.5 | 10.9 ± 3.3 | 12.8 ± 2.8 | 6.9 ± 2.5 | 85.7 | 46.4 | |||
| Study | Design | N | Age (Years) (Mean ± SD) | % Male | Follow-Up (Month) | BMI (Mean ± SD) | AHI (Mean ± SD) | ODI (Mean ± SD) | ESS (Mean ± SD) | % Success | % Cure | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-op | Post-op | Pre-op | Post-op | Pre-op | Post-op | Pre-op | Post-op | ||||||||
| Bachour et al., 2021 [55] | Retro | 15 | 52.9 ± 6.6 | 86.7 | 18 ± 9.6 | 29.1 ± 3.3 | 30.1 ± 4.5 | 33.0 ± 16.5 | 36.5 ± 23.8 | 25.3 ± 18.3 | 30.3 ± 21.1 | 11.5 ± 3.8 | 8.1 ± 4.5 | 26.7 | 6.7 |
| Heiser et al., 2017 [48] | Pro | 20 | 57 ± 12 | 100 | 12 | 28.1 ± 13.1 | 28.9 ± 7.6 | 6.6 ± 5.1 | |||||||
| Philip et al., 2018 [49] | Pro | 10 | 52.0 ± 9.4 | 100 | 6 | 28.8 ± 3.3 | 46.7 ± 12.2 | 14.5 ± 8.9 | 38.1 ± 21.1 | 10.5 ± 9.9 | 15.9 ± 3.5 | 10.0 ± 6.1 | |||
| Steffen et al., 2019 [50] | Retro | 18 | 51.5 | 24 | 27.9 ± 4.5 | 28.0 ± 4.7 | 26.3 ± 10.6 | 10.4 ± 10.1 | 12.8 ± 10.2 | 10.1 ± 12.0 | 12.7 ± 5.2 | 5.1 ± 3.8 | 77.8 | 33.3 | |
| Steffen et al., 2020 [51] | Pro | 38 | 58.0 ± 10.0 | 97.4 | 36 | 29.1 ± 3.9 | 28.6 ± 3.3 | 30.0 ± 13.7 | 13.1 ± 14.1 | 25.8 ± 16.7 | 11.6 ± 14.0 | 12.1 ± 5.8 | 6.0 ± 3.2 | 62 | 35 |
| Suurna et al., 2021 [54] | Pro | 782 | 14.3 ± 7.0 | 29.2 ± 4 | 35.8 ± 15.0 | 14.5 ± 14.9 | 11.4 ± 5.5 | 7.1 ± 4.6 | 69.7 | ||||||
| Van de Heyning et al., 2012 [52] | Pro | 28 | 55.1 ± 9.2 | 96.4 | 6 | 29.5 ± 2.5 | 42.3 ± 16.4 | 32.6 ± 29.1 | 30.7 ± 21.6 | 26.7 ± 27.0 | 11.0 ± 5.0 | 7.6 ± 4.3 | 50 | ||
| Vanderveken et al., 2013 [53] | Retro | 21 | 55 ± 11 | 95.2 | 6 | 28 ± 2 | 38.5 ± 11.8 | 20.3 ± 20.6 | 8.2 ± 5.0 a | 6.4 ± 4.3 a | 62 | ||||
| Woodson et al., 2018 [15] | Pro | 97 | 54.4 ± 10.3 | 60 | 28.6 ± 2.5 | 30.4 ± 9.4 b | 12.4 ± 16.3 | 27.2 ± 10.0 b | 9.9 ± 14.5 | 11.3 ± 5.2 | 6.9 ± 4.7 c | 74.6 b | 44 | ||
3.2. Quality Assessment
3.3. Respiratory Parameters
3.4. Subjective Parameters
3.5. Surgical Success and Cure
3.6. Long-Term Follow-Up Outcomes
3.7. Safety
4. Discussion
4.1. Objective Outcomes
4.2. Subjective Outcomes
4.3. Long-Term Outcomes
4.4. Safety
4.5. Clinical Relevance
4.6. Limitations
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef] [Green Version]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Bagai, K. Obstructive sleep apnea, stroke, and cardiovascular diseases. Neurologist 2010, 16, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Knauert, M.; Naik, S.; Gillespie, M.B.; Kryger, M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J. Otorhinolaryngol. Head Neck Surg. 2015, 1, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef]
- Rotenberg, B.W.; Vicini, C.; Pang, E.B.; Pang, K.P. Reconsidering first-line treatment for obstructive sleep apnea: A systematic review of the literature. J. Otolaryngol. Head Neck Surg. 2016, 45, 23. [Google Scholar] [CrossRef] [Green Version]
- Sharifian, M.R.; Zarrinkamar, M.; Alimardani, M.S.; Bakhshaee, M.; Asadpour, H.; Morovatdar, N.; Amini, M. Drug Induced Sleep Endoscopy in Obstructive Sleep Apnea. Tanaffos 2018, 17, 122–126. [Google Scholar]
- Li, K.K. Surgical management of obstructive sleep apnea. Clin. Chest Med. 2003, 24, 365–370. [Google Scholar] [CrossRef]
- Riley, R.W.; Powell, N.B.; Guilleminault, C.; Nino-Murcia, G. Maxillary, mandibular, and hyoid advancement: An alternative to tracheostomy in obstructive sleep apnea syndrome. Otolaryngol. Head Neck Surg. 1986, 94, 584–588. [Google Scholar] [CrossRef]
- Gokce, S.M.; Gorgulu, S.; Gokce, H.S.; Bengi, A.O.; Karacayli, U.; Ors, F. Evaluation of pharyngeal airway space changes after bimaxillary orthognathic surgery with a 3-dimensional simulation and modeling program. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 477–492. [Google Scholar] [CrossRef]
- Liu, S.Y.; Huon, L.K.; Iwasaki, T.; Yoon, A.; Riley, R.; Powell, N.; Torre, C.; Capasso, R. Efficacy of Maxillomandibular Advancement Examined with Drug-Induced Sleep Endoscopy and Computational Fluid Dynamics Airflow Modeling. Otolaryngol. Head Neck Surg. 2016, 154, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Strollo, P.J.; Soose, R.J.; Maurer, J.T.; de Vries, N.; Cornelius, J.; Froymovich, O.; Hanson, R.D.; Padhya, T.A.; Steward, D.L.; Gillespie, M.B.; et al. Upper-Airway Stimulation for Obstructive Sleep Apnea. N. Engl. J. Med. 2014, 370, 139–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffen, A.; Heiser, C.; Galetke, W.; Herkenrath, S.D.; Maurer, J.T.; Günther, E.; Stuck, B.A.; Woehrle, H.; Löhler, J.; Randerath, W. Hypoglossal nerve stimulation for obstructive sleep apnea: Updated position paper of the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 61–66. [Google Scholar] [CrossRef]
- Vanderveken, O.M.; Beyers, J.; Op de Beeck, S.; Dieltjens, M.; Willemen, M.; Verbraecken, J.A.; De Backer, W.A.; Van de Heyning, P.H. Development of a Clinical Pathway and Technical Aspects of Upper Airway Stimulation Therapy for Obstructive Sleep Apnea. Front. Neurosci. 2017, 11, 523. [Google Scholar] [CrossRef] [Green Version]
- Woodson, B.T.; Strohl, K.P.; Soose, R.J.; Gillespie, M.B.; Maurer, J.T.; de Vries, N.; Padhya, T.A.; Badr, M.S.; Lin, H.S.; Vanderveken, O.M.; et al. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngol. Head Neck Surg. 2018, 159, 194–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dedhia, R.C.; Strollo, P.J.; Soose, R.J. Upper Airway Stimulation for Obstructive Sleep Apnea: Past, Present, and Future. Sleep 2015, 38, 899–906. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.S.; Ibrahim, B.; Riley, R.W.; Liu, S.Y. Maxillomandibular Advancement and Upper Airway Stimulation: Extrapharyngeal Surgery for Obstructive Sleep Apnea. Clin. Exp. Otorhinolaryngol. 2020, 13, 225–233. [Google Scholar] [CrossRef]
- Mashaqi, S.; Patel, S.I.; Combs, D.; Estep, L.; Helmick, S.; Machamer, J.; Parthasarathy, S. The Hypoglossal Nerve Stimulation as a Novel Therapy for Treating Obstructive Sleep Apnea—A Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 1642. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Strasberg, S.M.; Linehan, D.C.; Hawkins, W.G. The accordion severity grading system of surgical complications. Ann. Surg. 2009, 250, 177–186. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Lloyd, R.M.; Marcus, C.L.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0.2; American Academy of Sleep Medicine: Darien, IL, USA, 2013. [Google Scholar]
- Sher, A.E.; Schechtman, K.B.; Piccirillo, J.F. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 1996, 19, 156–177. [Google Scholar] [CrossRef] [PubMed]
- Elshaug, A.G.; Moss, J.R.; Southcott, A.M.; Hiller, J.E. Redefining success in airway surgery for obstructive sleep apnea: A meta analysis and synthesis of the evidence. Sleep 2007, 30, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Memon, M.; Kay, J.; Wood, T.J.; Tushinski, D.M.; Khanna, V. Preoperative Patient Factors Affecting Length of Stay following Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. J. Arthroplast. 2019, 34, 2124–2165. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Machin, D.; Bryant, T.N.; Gardner, M.J. Statistics with Confidence: Confidence Intervals and Statistical Guidelines, 2nd ed.; BMJ Books: London, UK, 2000; p. 254. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettega, G.; Pepin, J.L.; Veale, D.; Deschaux, C.; Raphael, B.; Levy, P. Obstructive sleep apnea syndrome. fifty-one consecutive patients treated by maxillofacial surgery. Am. J. Respir. Crit. Care Med. 2000, 162, 641–649. [Google Scholar] [CrossRef]
- Bianchi, A.; Betti, E.; Tarsitano, A.; Morselli-Labate, A.M.; Lancellotti, L.; Marchetti, C. Volumetric three-dimensional computed tomographic evaluation of the upper airway in patients with obstructive sleep apnoea syndrome treated by maxillomandibular advancement. Br. J. Oral Maxillofac. Surg. 2014, 52, 831–837. [Google Scholar] [CrossRef]
- Boyd, S.B.; Walters, A.S.; Waite, P.; Harding, S.M.; Song, Y. Long-Term Effectiveness and Safety of Maxillomandibular Advancement for Treatment of Obstructive Sleep Apnea. J. Clin. Sleep Med. 2015, 11, 699–708. [Google Scholar] [CrossRef]
- Conradt, R.; Hochban, W.; Brandenburg, U.; Heitmann, J.; Peter, J.H. Long-term follow-up after surgical treatment of obstructive sleep apnoea by maxillomandibular advancement. Eur. Respir. J. 1997, 10, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Gerbino, G.; Bianchi, F.A.; Verzé, L.; Ramieri, G. Soft tissue changes after maxillo-mandibular advancement in OSAS patients: A three-dimensional study. J. Cranio-Maxillofac. Surg. 2014, 42, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Goh, Y.H.; Lim, K.A. Modified maxillomandibular advancement for the treatment of obstructive sleep apnea: A preliminary report. Laryngoscope 2003, 113, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Goodday, R.H.; Bourque, S.E.; Edwards, P.B. Objective and Subjective Outcomes Following Maxillomandibular Advancement Surgery for Treatment of Patients With Extremely Severe Obstructive Sleep Apnea (Apnea-Hypopnea Index >100). J. Oral Maxillofac. Surg. 2016, 74, 583–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, Y.J.; Liao, Y.F.; Chen, N.H.; Chen, Y.R. Changes in the calibre of the upper airway and the surrounding structures after maxillomandibular advancement for obstructive sleep apnoea. Br. J. Oral Maxillofac. Surg. 2014, 52, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Kastoer, C.; Op de Beeck, S.; Dom, M.; Neirinckx, T.; Verbraecken, J.; Braem, M.J.; Van de Heyning, P.H.; Nadjmi, N.; Vanderveken, O.M. Drug-Induced Sleep Endoscopy Upper Airway Collapse Patterns and Maxillomandibular Advancement. Laryngoscope 2020, 130, E268–E274. [Google Scholar] [CrossRef]
- Li, K.K.; Guilleminault, C.; Riley, R.W.; Powell, N.B. Obstructive sleep apnea and maxillomandibular advancement: An assessment of airway changes using radiographic and nasopharyngoscopic examinations. J. Oral Maxillofac. Surg. 2002, 60, 526–530; discussion 531. [Google Scholar] [CrossRef]
- Li, K.K.; Powell, N.B.; Riley, R.W.; Troell, R.; Guilleminault, C. Long Term Results of Maxillomandibular Advancement Surgery. Sleep Breath. 2000, 4, 137–140. [Google Scholar] [CrossRef]
- Li, K.K.; Riley, R.W.; Powell, N.B.; Troell, R.; Guilleminault, C. Overview of phase II surgery for obstructive sleep apnea syndrome. Ear Nose Throat J. 1999, 78, 851, 854–857. [Google Scholar] [CrossRef] [Green Version]
- Li, K.K.; Troell, R.J.; Riley, R.W.; Powell, N.B.; Koester, U.; Guilleminault, C. Uvulopalatopharyngoplasty, maxillomandibular advancement, and the velopharynx. Laryngoscope 2001, 111, 1075–1078. [Google Scholar] [CrossRef]
- Liao, Y.F.; Chiu, Y.T.; Lin, C.H.; Chen, Y.A.; Chen, N.H.; Chen, Y.R. Modified maxillomandibular advancement for obstructive sleep apnoea: Towards a better outcome for Asians. Int. J. Oral Maxillofac. Surg. 2015, 44, 189–194. [Google Scholar] [CrossRef]
- Lin, C.H.; Chin, W.C.; Huang, Y.S.; Wang, P.F.; Li, K.K.; Pirelli, P.; Chen, Y.H.; Guilleminault, C. Objective and subjective long term outcome of maxillomandibular advancement in obstructive sleep apnea. Sleep Med. 2020, 74, 289–296. [Google Scholar] [CrossRef]
- Rubio-Bueno, P.; Landete, P.; Ardanza, B.; Vazquez, L.; Soriano, J.B.; Wix, R.; Capote, A.; Zamora, E.; Ancochea, J.; Naval-Gías, L. Maxillomandibular advancement as the initial treatment of obstructive sleep apnoea: Is the mandibular occlusal plane the key? Int. J. Oral Maxillofac. Surg. 2017, 46, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Veys, B.; Pottel, L.; Mollemans, W.; Abeloos, J.; Swennen, G.; Neyt, N. Three-dimensional volumetric changes in the upper airway after maxillomandibular advancement in obstructive sleep apnoea patients and the impact on quality of life. Int. J. Oral Maxillofac. Surg. 2017, 46, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Vicini, C.; Dallan, I.; Campanini, A.; De Vito, A.; Barbanti, F.; Giorgiomarrano, G.; Bosi, M.; Plazzi, G.; Provini, F.; Lugaresi, E. Surgery vs ventilation in adult severe obstructive sleep apnea syndrome. Am. J. Otolaryngol. 2010, 31, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, A.; Tamisier, R.; Orset, E.; Pepin, J.L.; Bettega, G. Maxillomandibular advancement for obstructive sleep apnea syndrome treatment: Long-term results. J. Cranio-Maxillofac. Surg. 2017, 45, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, Y.; Wang, P.; Xiang, Z.; Ye, B.; Li, J. The inverted-L ramus osteotomy versus sagittal split ramus osteotomy in maxillomandibular advancement for the treatment of obstructive sleep apnea patients: A retrospective study. J. Cranio-Maxillofac. Surg. 2019, 47, 1839–1847. [Google Scholar] [CrossRef]
- Heiser, C.; Edenharter, G.; Bas, M.; Wirth, M.; Hofauer, B. Palatoglossus coupling in selective upper airway stimulation. Laryngoscope 2017, 127, E378–E383. [Google Scholar] [CrossRef]
- Philip, P.; Heiser, C.; Bioulac, S.; Altena, E.; Penchet, G.; Cuny, E.; Hofauer, B.; Monteyrol, P.J.; Micoulaud-Franchi, J.A. Hypoglossal nerve stimulation on sleep and level of alertness in OSA: A preliminary study. Neurology 2018, 91, e615–e619. [Google Scholar] [CrossRef]
- Steffen, A.; Abrams, N.; Suurna, M.V.; Wollenberg, B.; Hasselbacher, K. Upper-Airway Stimulation Before, After, or Without Uvulopalatopharyngoplasty: A Two-Year Perspective. Laryngoscope 2019, 129, 514–518. [Google Scholar] [CrossRef]
- Steffen, A.; Sommer, U.J.; Maurer, J.T.; Abrams, N.; Hofauer, B.; Heiser, C. Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea. Sleep Breath. 2020, 24, 979–984. [Google Scholar] [CrossRef]
- Van de Heyning, P.H.; Badr, M.S.; Baskin, J.Z.; Cramer Bornemann, M.A.; De Backer, W.A.; Dotan, Y.; Hohenhorst, W.; Knaack, L.; Lin, H.S.; Maurer, J.T.; et al. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope 2012, 122, 1626–1633. [Google Scholar] [CrossRef]
- Vanderveken, O.M.; Maurer, J.T.; Hohenhorst, W.; Hamans, E.; Lin, H.S.; Vroegop, A.V.; Anders, C.; de Vries, N.; Van de Heyning, P.H. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J. Clin. Sleep Med. 2013, 9, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Suurna, M.V.; Steffen, A.; Boon, M.; Chio, E.; Copper, M.; Patil, R.D.; Green, K.; Hanson, R.; Heiser, C.; Huntley, C.; et al. Impact of Body Mass Index and Discomfort on Upper Airway Stimulation: ADHERE Registry 2020 Update. Laryngoscope 2021, 131, 2616–2624. [Google Scholar] [CrossRef] [PubMed]
- Bachour, A.; Bäck, L.; Pietarinen, P. No changes in nocturnal respiration with hypoglossal neurostimulation therapy for obstructive sleep apnoea. Clin. Respir. J. 2021, 15, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Heiser, C.; Steffen, A.; Boon, M.; Hofauer, B.; Doghramji, K.; Maurer, J.T.; Sommer, J.U.; Soose, R.; Strollo, P.J., Jr.; Schwab, R.; et al. Post-approval upper airway stimulation predictors of treatment effectiveness in the ADHERE registry. Eur. Respir. J. 2019, 53, 1801405. [Google Scholar] [CrossRef]
- Safiruddin, F.; Vanderveken, O.M.; de Vries, N.; Maurer, J.T.; Lee, K.; Ni, Q.; Strohl, K.P. Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions. Eur. Respir. J. 2015, 45, 129–138. [Google Scholar] [CrossRef]
- Li, K.K.; Riley, R.W.; Powell, N.B.; Gervacio, L.; Troell, R.J.; Guilleminault, C. Obstructive sleep apnea surgery: Patient perspective and polysomnographic results. Otolaryngol. Head Neck Surg. 2000, 123, 572–575. [Google Scholar] [CrossRef]
- Butterfield, K.J.; Marks, P.L.; McLean, L.; Newton, J. Quality of life assessment after maxillomandibular advancement surgery for obstructive sleep apnea. J. Oral Maxillofac. Surg. 2016, 74, 1228–1237. [Google Scholar] [CrossRef]
- Beranger, T.; Garreau, E.; Ferri, J.; Raoul, G. Morphological impact on patients of maxillomandibular advancement surgery for the treatment of obstructive sleep apnea-hypopnea syndrome. Int. Orthod. 2017, 15, 40–53. [Google Scholar] [CrossRef]
- Cillo, J.E.; Robertson, N.; Dattilo, D.J. Maxillomandibular Advancement for Obstructive Sleep Apnea Is Associated With Very Long-Term Overall Sleep-Related Quality-of-Life Improvement. J. Oral Maxillofac. Surg. 2020, 78, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Mehra, R.; Steffen, A.; Heiser, C.; Hofauer, B.; Withrow, K.; Doghramji, K.; Boon, M.; Huntley, C.; Soose, R.J.; Stevens, S.; et al. Upper Airway Stimulation versus Untreated Comparators in Positive Airway Pressure Treatment-Refractory Obstructive Sleep Apnea. Ann. Am. Thorac. Soc. 2020, 17, 1610–1619. [Google Scholar] [CrossRef]
- Pottel, L.; Neyt, N.; Hertegonne, K.; Pevernagie, D.; Veys, B.; Abeloos, J.; De Clercq, C. Long-term quality of life outcomes of maxillomandibular advancement osteotomy in patients with obstructive sleep apnoea syndrome. Int. J. Oral Maxillofac. Surg. 2019, 48, 332–340. [Google Scholar] [CrossRef]
- Riley, R.W.; Powell, N.B.; Li, K.K.; Troell, R.J.; Guilleminault, C. Surgery and obstructive sleep apnea: Long-term clinical outcomes. Otolaryngol. Head Neck Surg. 2000, 122, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Hofauer, B.; Steffen, A.; Knopf, A.; Hasselbacher, K.; Heiser, C. Patient experience with upper airway stimulation in the treatment of obstructive sleep apnea. Sleep Breath. 2019, 23, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Peacock, Z.S.; Lee, C.C.; Klein, K.P.; Kaban, L.B. Orthognathic surgery in patients over 40 years of age: Indications and special considerations. J. Oral Maxillofac. Surg. 2014, 72, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Passeri, L.A.; Choi, J.G.; Kaban, L.B.; Lahey, E.T. Morbidity and Mortality Rates After Maxillomandibular Advancement for Treatment of Obstructive Sleep Apnea. J. Oral Maxillofac. Surg. 2016, 74, 2033–2043. [Google Scholar] [CrossRef] [Green Version]
- Van Sickels, J.E.; Hatch, J.P.; Dolce, C.; Bays, R.A.; Rugh, J.D. Effects of age, amount of advancement, and genioplasty on neurosensory disturbance after a bilateral sagittal split osteotomy. J. Oral Maxillofac. Surg. 2002, 60, 1012–1017. [Google Scholar] [CrossRef]
- Zhou, N.; Ho, J.P.T.F.; Huang, Z.; Spijker, R.; de Vries, N.; Aarab, G.; Lobbezoo, F.; Ravesloot, M.J.L.; de Lange, J. Maxillomandibular advancement versus multilevel surgery for treatment of obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 57, 101471. [Google Scholar] [CrossRef]
- Withrow, K.; Evans, S.; Harwick, J.; Kezirian, E.; Strollo, P. Upper Airway Stimulation Response in Older Adults with Moderate to Severe Obstructive Sleep Apnea. Otolaryngol. Head Neck Surg. 2019, 161, 714–719. [Google Scholar] [CrossRef]
- Murphey, A.W.; Baker, A.B.; Soose, R.J.; Padyha, T.A.; Nguyen, S.A.; Xiao, C.C.; Gillespie, M.B. Upper airway stimulation for obstructive sleep apnea: The surgical learning curve. Laryngoscope 2016, 126, 501–506. [Google Scholar] [CrossRef]
- Pietzsch, J.B.; Liu, S.; Garner, A.M.; Kezirian, E.J.; Strollo, P.J. Long-Term Cost-Effectiveness of Upper Airway Stimulation for the Treatment of Obstructive Sleep Apnea: A Model-Based Projection Based on the STAR Trial. Sleep 2015, 38, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Pietzsch, J.B.; Richter, A.-K.; Randerath, W.; Steffen, A.; Liu, S.; Geisler, B.P.; Wasem, J.; Biermann-Stallwitz, J. Clinical and economic benefits of upper airway stimulation for obstructive sleep apnea in a European setting. Respiration 2019, 98, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Awad, M.; Riley, R.; Capasso, R. The Role of the Revised Stanford Protocol in Today’s Precision Medicine. Sleep Med. Clin. 2019, 14, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Riley, R.W. Continuing the Original Stanford Sleep Surgery Protocol from Upper Airway Reconstruction to Upper Airway Stimulation: Our First Successful Case. J. Oral Maxillofac. Surg. 2017, 75, 1514–1518. [Google Scholar] [CrossRef]
- Sarber, K.M.; Chang, K.W.; Ishman, S.L.; Epperson, M.V.; Dhanda Patil, R. Hypoglossal Nerve Stimulator Outcomes for Patients Outside the U.S. FDA Recommendations. Laryngoscope 2020, 130, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Trăistaru, T.; Pantea, M.; Țâncu, A.M.C.; Imre, M. Elements of Diagnosis and Non-surgical Treatment of Obstructive Sleep Apnea in Adults from the Dental Medicine Perspective. In Sleep Medicine and the Evolution of Contemporary Sleep Pharmacotherapy; Larrivee, D., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Carberry, J.C.; Amatoury, J.; Eckert, D.J. Personalized Management Approach for OSA. Chest 2018, 153, 744–755. [Google Scholar] [CrossRef]
- Chan, A.S.; Sutherland, K.; Schwab, R.J.; Zeng, B.; Petocz, P.; Lee, R.W.; Darendeliler, M.A.; Cistulli, P.A. The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax 2010, 65, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Ramar, K.; Dort, L.C.; Katz, S.G.; Lettieri, C.J.; Harrod, C.G.; Thomas, S.M.; Chervin, R.D. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J. Clin. Sleep Med. 2015, 11, 773–827. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Mo, J.H.; Choi, I.J.; Lee, H.J.; Seo, B.S.; Kim, D.Y.; Yun, P.Y.; Yoon, I.Y.; Won Lee, H.; Kim, J.W. The mandibular advancement device and patient selection in the treatment of obstructive sleep apnea. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 439–444. [Google Scholar] [CrossRef]
- Vecchierini, M.F.; Attali, V.; Collet, J.M.; d’Ortho, M.P.; Goutorbe, F.; Kerbrat, J.B.; Leger, D.; Lavergne, F.; Monaca, C.; Monteyrol, P.J.; et al. Mandibular advancement device use in obstructive sleep apnea: ORCADES study 5-year follow-up data. J. Clin. Sleep Med. 2021, 17, 1695–1705. [Google Scholar] [CrossRef]
- Cook, J.A. The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials 2009, 10, 9. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019; pp. 182–183. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, N.; Ho, J.-P.T.F.; Spijker, R.; Aarab, G.; de Vries, N.; Ravesloot, M.J.L.; de Lange, J. Maxillomandibular Advancement and Upper Airway Stimulation for Treatment of Obstructive Sleep Apnea: A Systematic Review. J. Clin. Med. 2022, 11, 6782. https://doi.org/10.3390/jcm11226782
Zhou N, Ho J-PTF, Spijker R, Aarab G, de Vries N, Ravesloot MJL, de Lange J. Maxillomandibular Advancement and Upper Airway Stimulation for Treatment of Obstructive Sleep Apnea: A Systematic Review. Journal of Clinical Medicine. 2022; 11(22):6782. https://doi.org/10.3390/jcm11226782
Chicago/Turabian StyleZhou, Ning, Jean-Pierre T. F. Ho, René Spijker, Ghizlane Aarab, Nico de Vries, Madeline J. L. Ravesloot, and Jan de Lange. 2022. "Maxillomandibular Advancement and Upper Airway Stimulation for Treatment of Obstructive Sleep Apnea: A Systematic Review" Journal of Clinical Medicine 11, no. 22: 6782. https://doi.org/10.3390/jcm11226782






