Clinical Outcomes and Prolonged SARS-CoV-2 Viral Shedding in ICU Patients with Severe COVID-19 Infection and Nosocomial Bacterial Pneumonia: A Retrospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design, Setting, and Patients
2.2. Data Collection and Measurements
2.3. HAP and VAP Definitions
2.4. SARS-CoV-2 RT-PCR
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COVID-19 | coronavirus disease 2019 |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| HAP | hospital-acquired pneumonia |
| VAP | ventilator-associated pneumonia |
| ICU | intensive care unit |
| RT-PCR | real-time reverse-transcriptase polymerase chain reaction |
| VGHTPE | Taipei Veterans General Hospital |
| HIV | human immunodeficiency virus |
| IRB | Institutional Review Board |
| MV | mechanical ventilation |
| ECMO | extracorporeal membrane oxygenation |
| mg | milligrams |
| SOFA score | sequential organ failure assessment score |
| ELISA | enzyme-linked immunosorbent assay |
| DNA | deoxyribonucleic acid |
| IQRs | interquartile ranges |
| ORs | odds ratios |
| CI | confidence interval |
| LDH | lactate dehydrogenase |
| ARDS | acute respiratory distress syndrome |
Appendix A


References
- Rynda-Apple, A.; Robinson, K.M.; Alcorn, J.F. Influenza and Bacterial Superinfection: Illuminating the Immunologic Mechanisms of Disease. Infect. Immun. 2015, 83, 3764–3770. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Dowling, R.B.; Jackson, A.D. The biology of bacterial colonization and invasion of the respiratory mucosa. Eur. Respir. J. 1996, 9, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Jeannoël, M.; Lina, G.; Rasigade, J.P.; Lina, B.; Morfin, F.; Casalegno, J.S. Microorganisms associated with respiratory syncytial virus pneumonia in the adult population. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Klompas, M.; Luyt, C.-E. Ventilator-associated pneumonia in adults: A narrative review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef]
- Markowicz, P.; Wolff, M.; Djedaini, K.; Cohen, Y.; Chastre, J.; Delclaux, C.; Merrer, J.; Herman, B.; Veber, B.; Fontaine, A.; et al. Multicenter prospective study of ventilator-associated pneumonia during acute respiratory distress syndrome. Incidence, prognosis, and risk factors. ARDS Study Group. Am. J. Respir. Crit. Care Med. 2000, 161, 1942–1948. [Google Scholar] [CrossRef]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients with COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef]
- Waller, J.V.; Kaur, P.; Tucker, A.; Lin, K.K.; Diaz, M.J.; Henry, T.S.; Hope, M. Diagnostic Tools for Coronavirus Disease (COVID-19): Comparing CT and RT-PCR Viral Nucleic Acid Testing. AJR Am. J. Roentgenol. 2020, 215, 834–838. [Google Scholar] [CrossRef]
- Diao, B.; Wen, K.; Zhang, J.; Chen, J.; Han, C.; Chen, Y.; Wang, S.; Deng, G.; Zhou, H.; Wu, Y. Accuracy of a nucleocapsid protein antigen rapid test in the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 2021, 27, 289.e1–289.e4. [Google Scholar] [CrossRef]
- Al Bayat, S.; Mundodan, J.; Hasnain, S.; Sallam, M.; Khogali, H.; Ali, D.; Alateeg, S.; Osama, M.; Elberdiny, A.; Al-Romaihi, H.; et al. Can the cycle threshold (Ct) value of RT-PCR test for SARS-CoV2 predict infectivity among close contacts? J. Infect. Public Health 2021, 14, 1201–1205. [Google Scholar] [CrossRef]
- Long, H.; Zhao, J.; Zeng, H.L.; Lu, Q.B.; Fang, L.Q.; Wang, Q.; Wu, Q.-M.; Liu, W. Prolonged viral shedding of SARS-CoV-2 and related factors in symptomatic COVID-19 patients: A prospective study. BMC Infect. Dis. 2021, 21, 1282. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Bitker, L.; Dhelft, F.; Chauvelot, L.; Frobert, E.; Folliet, L.; Mezidi, M.; Trouillet-Assant, S.; Belot, A.; Lina, B.; Wallet, F.; et al. Protracted viral shedding and viral load are associated with ICU mortality in COVID-19 patients with acute respiratory failure. Ann. Intensive Care 2020, 10, 167. [Google Scholar] [CrossRef]
- Jang, S.; Rhee, J.Y.; Wi, Y.M.; Jung, B.K. Viral kinetics of SARS-CoV-2 over the preclinical, clinical, and postclinical period. Int. J. Infect. Dis. 2021, 102, 561–565. [Google Scholar] [CrossRef]
- Young, B.E.; Ong, S.W.X.; Ng, L.F.P.; Anderson, D.E.; Chia, W.N.; Chia, P.Y.; Ang, L.W.; Mak, T.-M.; Kalimuddin, S.; Chai, L.Y.A.; et al. Viral Dynamics and Immune Correlates of Coronavirus Disease 2019 (COVID-19) Severity. Clin. Infect. Dis. 2021, 73, e2932–e2942. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.R.; Shen, H.C.; Sun, C.Y.; Chen, W.C.; Chen, Y.M.; Feng, J.Y.; Yang, K.Y. COVID-19-associated pulmonary aspergillosis is associated with increased in-hospital mortality and prolonged SARS-CoV-2 viral shedding. J. Formos. Med. Assoc. 2022; in press. [Google Scholar]
- Meduri, G.U.; Golden, E.; Freire, A.X.; Taylor, E.; Zaman, M.; Carson, S.J.; Gibson, M.; Umberger, R. Methylprednisolone infusion in early severe ARDS: Results of a randomized controlled trial. Chest 2007, 131, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Dhasmana, D.J.; RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.A.O.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients with Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef]
- Ritter, L.A.; Britton, N.; Heil, E.L.; Teeter, W.A.; Murthi, S.B.; Chow, J.H.; Ricotta, E.; Chertow, D.S.; Grazioli, A.; Levine, A.R. The Impact of Corticosteroids on Secondary Infection and Mortality in Critically Ill COVID-19 Patients. J. Intensive Care Med. 2021, 36, 1201–1208. [Google Scholar] [CrossRef]
- Ripa, M.; Galli, L.; Poli, A.; Oltolini, C.; Spagnuolo, V.; Mastrangelo, A.; Muccini, C.; Monti, G.; De Luca, G.; Landoni, G.; et al. Secondary infections in patients hospitalized with COVID-19: Incidence and predictive factors. Clin. Microbiol. Infect. 2021, 27, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Cogliati Dezza, F.; Oliva, A.; Cancelli, F.; Savelloni, G.; Valeri, S.; Mauro, V.; Calabretto, M.; Russo, G.; Venditti, M.; Turrizian, O.; et al. Determinants of prolonged viral RNA shedding in hospitalized patients with SARS-CoV-2 infection. Diagn. Microbiol. Infect. Dis. 2021, 100, 115347. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Chan, P.K.; Hui, D.S.; Rainer, T.H.; Wong, E.; Choi, K.W.; Lui, G.C.Y.; Wong, B.C.K.; Wong, R.Y.W.; Lam, W.-Y.; et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J. Infect. Dis. 2009, 200, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, V.; Guffanti, M.; Galli, L.; Poli, A.; Querini, P.R.; Ripa, M.; Clementi, M.; Scarpellini, P.; Lazzarin, A.; Tresoldi, M.; et al. Viral clearance after early corticosteroid treatment in patients with moderate or severe COVID-19. Sci. Rep. 2020, 10, 21291. [Google Scholar] [CrossRef]
- Bosch, A.A.; Biesbroek, G.; Trzcinski, K.; Sanders, E.A.; Bogaert, D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013, 9, e1003057. [Google Scholar] [CrossRef]
- Barman, T.K.; Metzger, D.W. Disease Tolerance during Viral-Bacterial Co-Infections. Viruses 2021, 13, 2362. [Google Scholar] [CrossRef]
- Bhatt, P.J.; Shiau, S.; Brunetti, L.; Xie, Y.; Solanki, K.; Khalid, S.; Mohayya, S.; Au, P.H.; Pham, C.; Uprety, P.; et al. Risk Factors and Outcomes of Hospitalized Patients with Severe Coronavirus Disease 2019 (COVID-19) and Secondary Bloodstream Infections: A Multicenter Case-Control Study. Clin. Infect. Dis. 2021, 72, e995–e1003. [Google Scholar] [CrossRef]


| HAP/VAP (n = 30) | Non-HAP/VAP (n = 42) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 67.50 (60.50–74.00) | 65.00 (55.00–74.00) | 0.591 |
| Male | 23 (76.7%) | 24 (57.1%) | 0.086 |
| Time from symptoms onset to hospital admission, days | 5.50 (2.00–9.00) | 4.00 (2.00–8.25) | 0.382 |
| Time from symptoms onset to secondary pneumonia diagnosis, days | 21.50 (16.50–29.25) | NA | |
| Underlying disease | |||
| Cardiovascular disease | 2 (6.7%) | 4 (9.5%) | 0.665 |
| Diabetes mellitus | 13 (43.3%) | 12 (28.6%) | 0.195 |
| Cirrhosis | 4 (13.3%) | 7 (16.7%) | 0.698 |
| Chronic kidney disease | 4 (13.3%) | 6 (14.3%) | 0.908 |
| Hemodialysis | 1 (3.3%) | 2 (4.8%) | 0.765 |
| Malignancy | 4 (13.3%) | 7 (16.7%) | 0.698 |
| Laboratory tests at ICU admission | |||
| White blood cells, 109/L | 6413.00 (5050.00–12,375.00) | 5700.00 (3400.00–8975.00) | 0.089 |
| Lymphocytes, 109/L | 689.70 (427.70–877.75) | 607.50 (442.13–925.05) | 0.918 |
| Albumin, g/dL | 3.30 (3.10–3.62) | 3.55 (3.30–4.08) | 0.057 |
| C-reactive protein, mg/dL | 8.03 (3.42–14.33) | 8.45 (3.00–10.03) | 0.668 |
| Procalcitonin, ng/mL | 0.18 (0.11–0.82) | 0.13 (0.07–0.33) | 0.028 |
| LDH, U/L | 522.50 (398.00–679.00) | 361.50 (283.75–555.00) | 0.014 |
| Lactate, mg/dL | 18.40 (14.33–28.33) | 15.20 (10.30–18.88) | 0.022 |
| D-dimer, ug/mL | 1.45 (0.63–6.57) | 0.80 (0.42–1.40) | 0.028 |
| Fibrinogen, mg/dL | 410.05 (271.15–523.50) | 440.20 (369.30–537.50) | 0.395 |
| Treatment | |||
| Cumulative dose of dexamethasone a | 153.00 (66.00–186.50) | 60.00 (22.50–159.25) | 0.011 |
| Dexamethasone dose > 60 mg | 24 (80.0%) | 20 (47.6%) | 0.005 |
| Dexamethasone dose > 150 mg | 15 (50.0%) | 12 (28.6%) | 0.064 |
| Tocilizumab | 20 (66.7%) | 20 (47.6%) | 0.109 |
| Remdesivir | 24 (80.0%) | 30 (71.4%) | 0.408 |
| Enoxaparin | 27 (90.0%) | 16 (38.1%) | <0.001 |
| Severity scores | |||
| SOFA at admission | 7.50 (5.00–10.00) | 4.00 (1.00–8.00) | 0.002 |
| HAP/VAP (n = 30) | Non-HAP/VAP (n = 42) | p-Value | |
|---|---|---|---|
| Clinical course and outcomes | |||
| Mechanical ventilator | 28 (93.3%) | 20 (47.6%) | <0.001 |
| ECMO | 5 (16.7%) | 0 (0.0%) | 0.006 |
| Prone position | 9 (30.0%) | 8 (19.0%) | 0.281 |
| Renal replacement therapy | 2 (6.7%) | 4 (9.5%) | 0.665 |
| GI bleeding events | 14 (46.7%) | 4 (9.5%) | <0.001 |
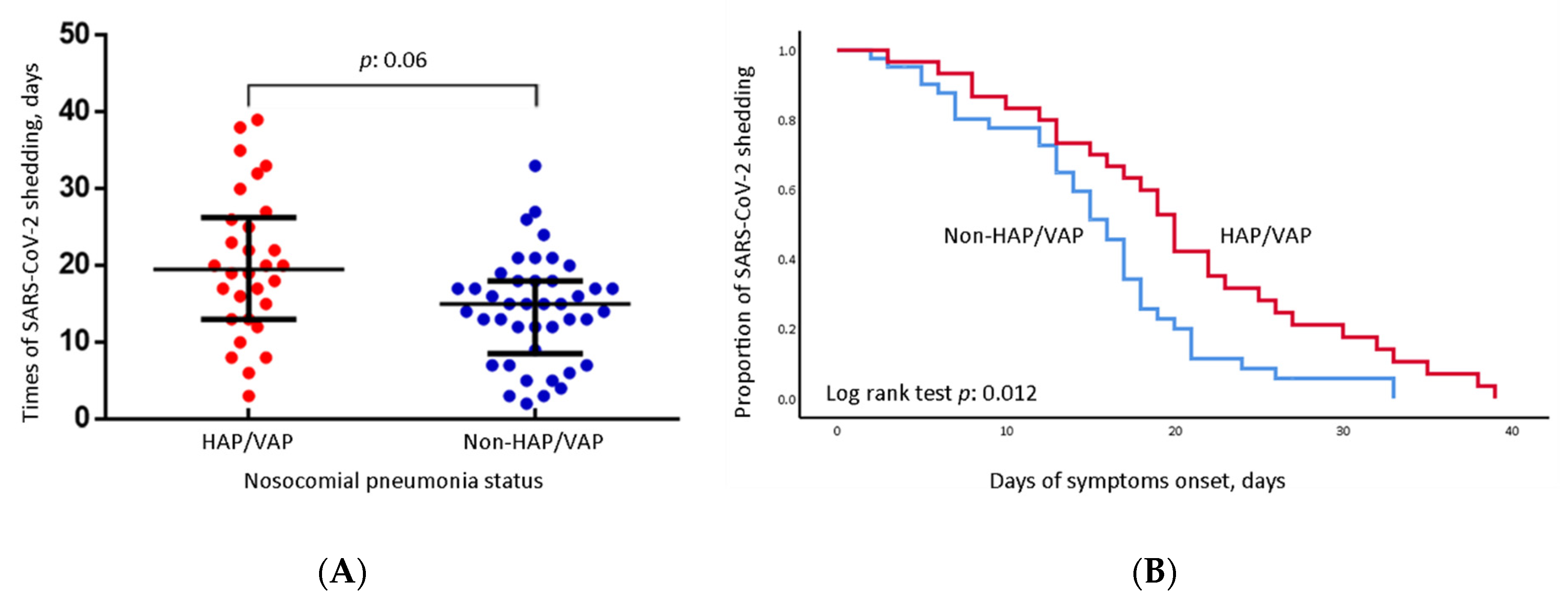
| SARS-CoV-2 shedding days | 19.50 (13.00–26.25) | 15.00(8.50–18.00) | 0.006 |
| ICU stay, days | 39.50 (17.00–63.75) | 10.00 (6.00–18.00) | <0.001 |
| Ventilator free days at Day 28 | 0.00 (0.00–0.00) | 10.50 (0.00–13.00) | 0.006 |
| ICU mortality | 7 (23.3%) | 6 (14.3%) | 0.325 |
| In-hospital mortality | 7 (23.3%) | 7 (16.7%) | 0.481 |
| Univariate a | Multivariate a | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Male | 0.406 | 0.143–1.152 | 0.090 | |||
| White blood cell, 109/L | 1.000 | 1.000–1.000 | 0.094 | |||
| Albumin, g/dL | 0.857 | 0.429–1.711 | 0.662 | |||
| Procalcitonin, ng/mL | 1.199 | 0.844–1.703 | 0.311 | |||
| LDH, U/L | 1.003 | 1.000–1.005 | 0.029 | |||
| Lactate, mg/dL | 1.004 | 0.984–1.024 | 0.720 | |||
| D-dimer, ug/mL | 1.038 | 0.984–1.094 | 0.168 | |||
| Cumulative dose of dexamethasone b | 1.007 | 1.001–1.013 | 0.017 | |||
| SOFA at admission | 1.239 | 1.069–1.436 | 0.004 | 1.339 | 1.115–1.608 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.-Y.; Feng, J.-Y.; Huang, J.-R.; Shen, H.-C.; Chen, Y.-M.; Chen, W.-C.; Yang, K.-Y. Clinical Outcomes and Prolonged SARS-CoV-2 Viral Shedding in ICU Patients with Severe COVID-19 Infection and Nosocomial Bacterial Pneumonia: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 6796. https://doi.org/10.3390/jcm11226796
Sun C-Y, Feng J-Y, Huang J-R, Shen H-C, Chen Y-M, Chen W-C, Yang K-Y. Clinical Outcomes and Prolonged SARS-CoV-2 Viral Shedding in ICU Patients with Severe COVID-19 Infection and Nosocomial Bacterial Pneumonia: A Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(22):6796. https://doi.org/10.3390/jcm11226796
Chicago/Turabian StyleSun, Chuan-Yen, Jia-Yih Feng, Jhong-Ru Huang, Hisao-Chin Shen, Yuh-Min Chen, Wei-Chih Chen, and Kuang-Yao Yang. 2022. "Clinical Outcomes and Prolonged SARS-CoV-2 Viral Shedding in ICU Patients with Severe COVID-19 Infection and Nosocomial Bacterial Pneumonia: A Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 22: 6796. https://doi.org/10.3390/jcm11226796
APA StyleSun, C.-Y., Feng, J.-Y., Huang, J.-R., Shen, H.-C., Chen, Y.-M., Chen, W.-C., & Yang, K.-Y. (2022). Clinical Outcomes and Prolonged SARS-CoV-2 Viral Shedding in ICU Patients with Severe COVID-19 Infection and Nosocomial Bacterial Pneumonia: A Retrospective Cohort Study. Journal of Clinical Medicine, 11(22), 6796. https://doi.org/10.3390/jcm11226796






