The Feasibility of Robot-Assisted Chin Osteotomy on Skull Models: Comparison with Surgical Guides Technique
Abstract
1. Introduction
2. Materials and Methods
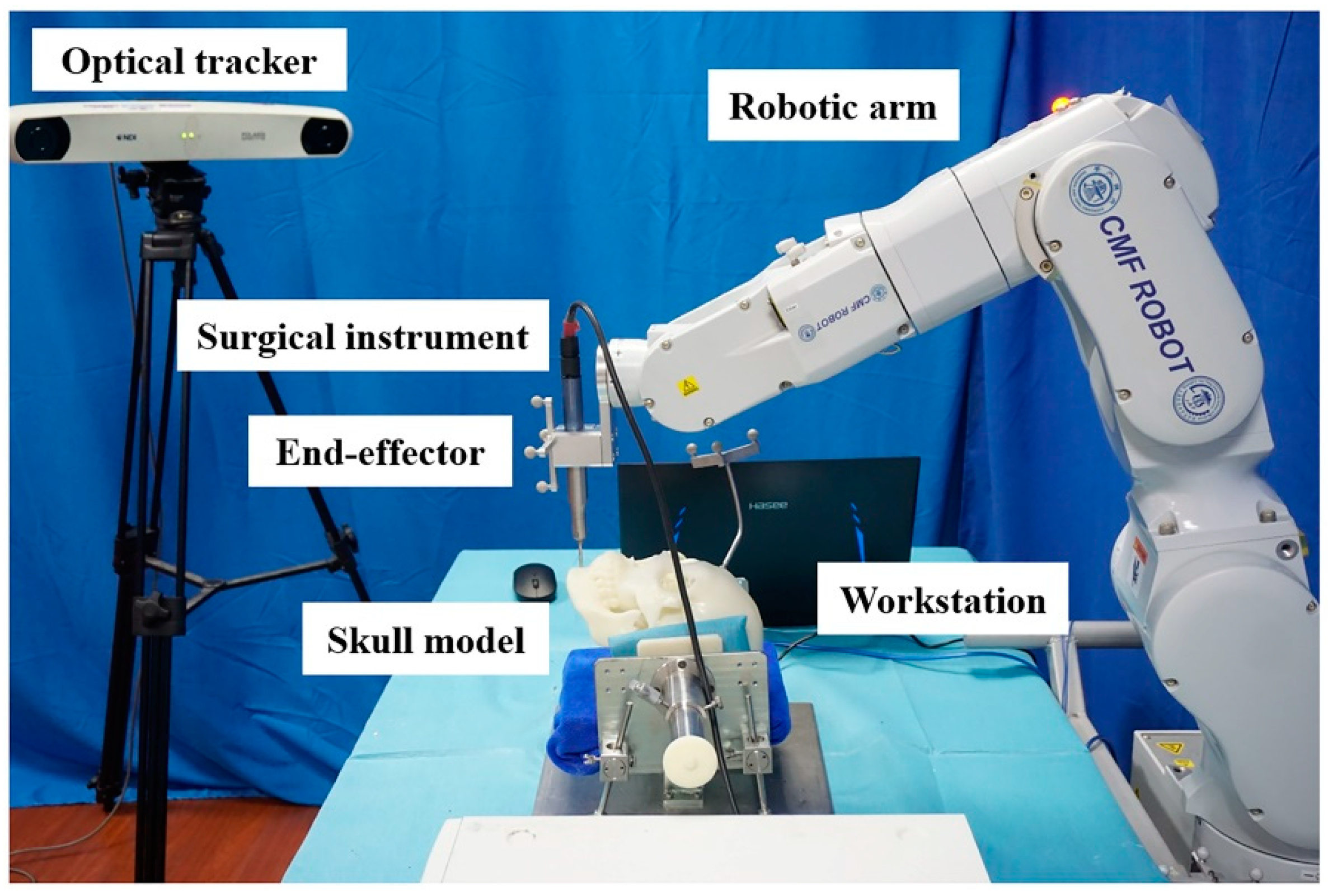
2.1. Establishment of the CMF ROBOT System
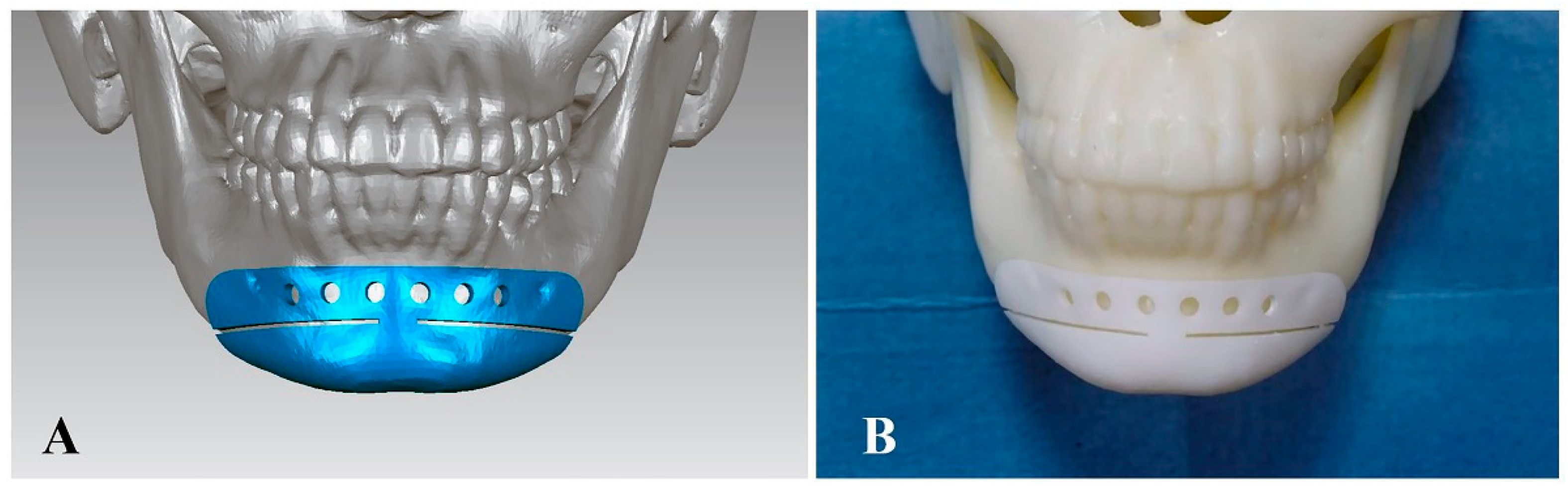
2.2. Preoperative Design and Skull Model Fabrication
2.3. Procedures of Chin Osteotomy
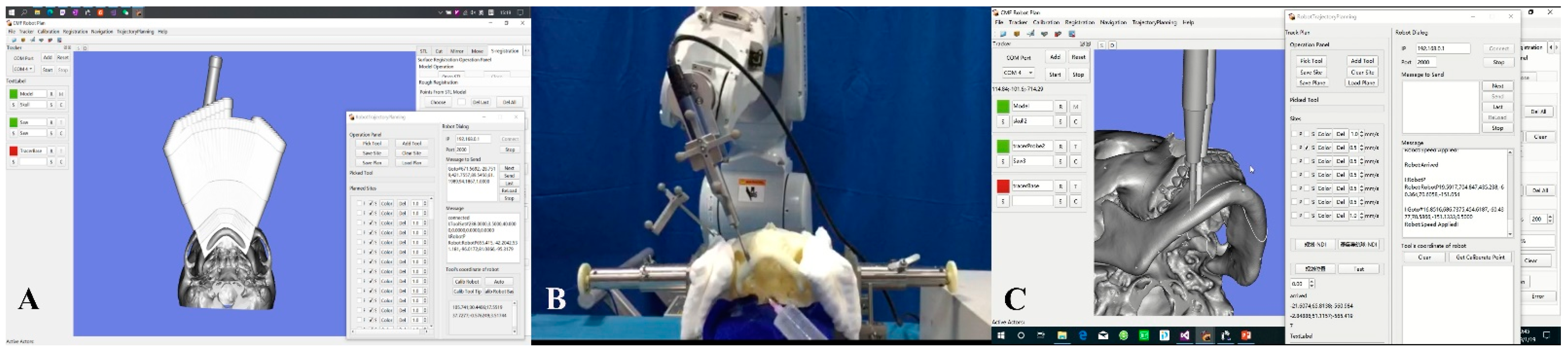
2.3.1. Experimental Group: Robot-Assisted Chin Osteotomy
2.3.2. Control Group: Surgical Guide-Assisted Osteotomy
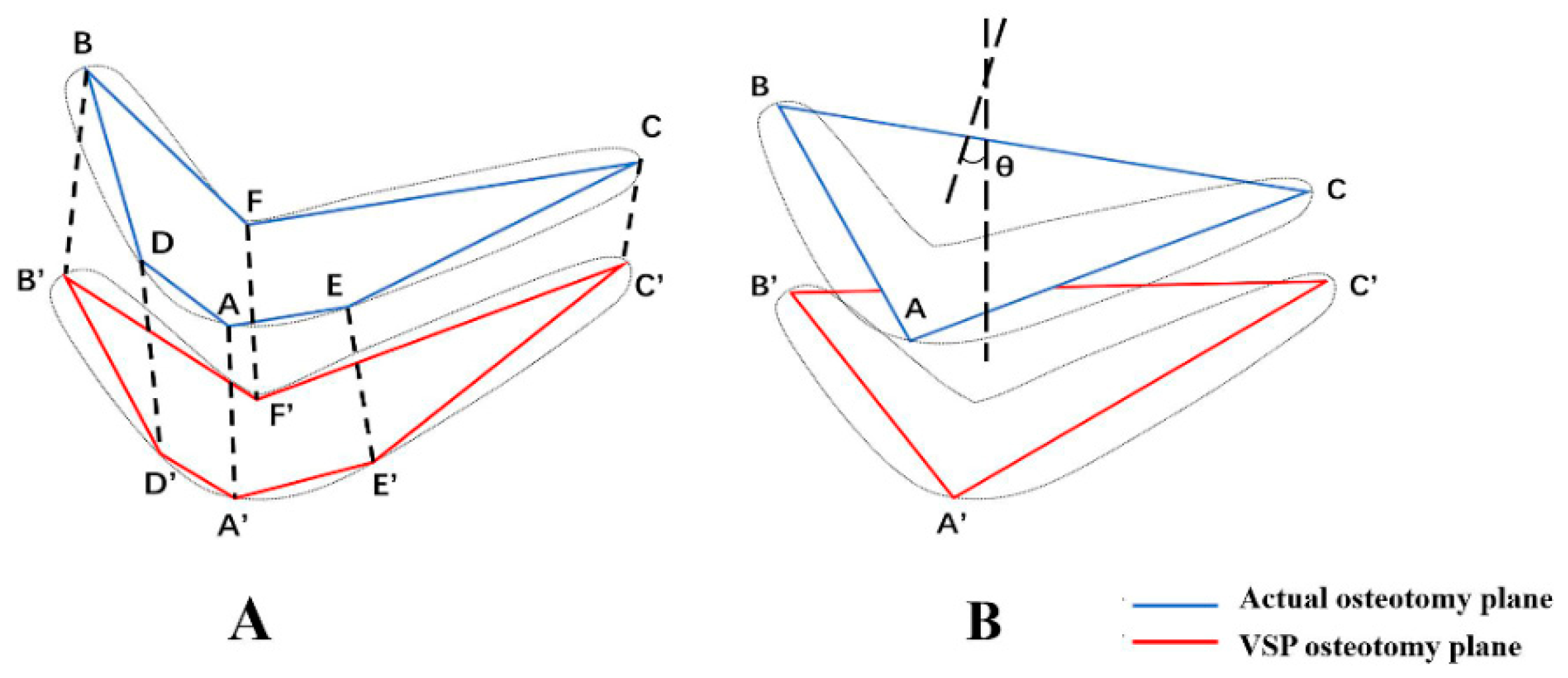
2.4. Assessment Methods
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, J.; Ip, H.H.; Samman, N.; Wang, D.; Kot, C.S.; Yeung, R.W.; Tideman, H. Computer-assisted three-dimensional surgical planning and simulation: 3D virtual osteotomy. Int. J. Oral Maxillofac. Surg. 2000, 29, 11–17. [Google Scholar] [CrossRef]
- Farrell, B.B.; Franco, P.B.; Tucker, M.R. Virtual surgical planning in orthognathic surgery. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Van Hemelen, G.; Van Genechten, M.; Renier, L.; Desmedt, M.; Verbruggen, E.; Nadjmi, N. Three-dimensional virtual planning in orthognathic surgery enhances the accuracy of soft tissue prediction. J. Craniomaxillofac. Surg. 2015, 43, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, L.; Sun, H.; Yuan, J.; Shen, S.G.; Wang, X. A novel method of computer aided orthognathic surgery using individual CAD/CAM templates: A combination of osteotomy and repositioning guides. Br. J. Oral Maxillofac. Surg. 2013, 51, e239–e244. [Google Scholar] [CrossRef]
- Yu, H.; Shen, S.G.; Wang, X.; Zhang, L.; Zhang, S. The indication and application of computer-assisted navigation in oral and maxillofacial surgery-Shanghai’s experience based on 104 cases. J. Craniomaxillofac. Surg. 2013, 41, 770–774. [Google Scholar] [CrossRef]
- Lee, G.K.; Moshrefi, S.; Fuertes, V.; Veeravagu, L.; Nazerali, R.; Lin, S.J. What is your reality? Virtual, augmented, and mixed reality in plastic surgery training, education, and practice. Plast. Reconstr. Surg. 2021, 147, 505–511. [Google Scholar] [CrossRef]
- Liu, K.; Gao, Y.; Abdelrehem, A.; Zhang, L.; Chen, X.; Xie, L.; Wang, X. Augmented reality navigation method for recontouring surgery of craniofacial fibrous dysplasia. Sci. Rep. 2021, 11, 10043. [Google Scholar] [CrossRef]
- De Ceulaer, J.; De Clercq, C.; Swennen, G.R. Robotic surgery in oral and maxillofacial, craniofacial and head and neck surgery: A systematic review of the literature. Int. J. Oral Maxillofac. Surg. 2012, 41, 1311–1324. [Google Scholar] [CrossRef]
- Liu, H.H.; Li, L.J.; Shi, B.; Xu, C.W.; Luo, E. Robotic surgical systems in maxillofacial surgery: A review. Int. J. Oral Sci. 2017, 9, 63–73. [Google Scholar] [CrossRef]
- Wu, J.; Hui, W.; Chen, S.; Niu, J.; Lin, Y.; Luan, N.; Zhang, S.; Shen, S.G.F. Error analysis of robot-assisted orthognathic surgery. J. Craniofac. Surg. 2020, 31, 2324–2328. [Google Scholar] [CrossRef]
- Lin, H.H.; Lonic, D.; Lo, L.J. 3D printing in orthognathic surgery—A literature review. J. Formos. Med. Assoc. 2018, 117, 547–558. [Google Scholar] [CrossRef]
- Lorincz, B.B.; Jowett, N.; Knecht, R. Decision management in transoral robotic surgery: Indications, individual patient selection, and role in the multidisciplinary treatment for head and neck cancer from a European perspective. Head Neck 2016, 38, e2190–e2196. [Google Scholar] [CrossRef]
- Kang, S.W.; Lee, S.H.; Ryu, H.R.; Lee, K.Y.; Jeong, J.J.; Nam, K.H.; Chung, W.Y.; Park, C.S. Initial experience with robot-assisted modified radical neck dissection for the management of thyroid carcinoma with lateral neck node metastasis. Surgery 2010, 148, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Mozer, P.S. Accuracy and deviation analysis of static and robotic guided implant surgery: A case study. Int. J. Oral Maxillofac. Implants 2020, 35, e86–e90. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, F.; Fan, S.; Chow, J.K. Robotics in dental implantology. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Zhang, S.; Luan, N.; Lin, Y.; Shen, S.G.; Bautista, J.S. A novel system for navigation-and robot-assisted craniofacial surgery: Establishment of the principle prototype. J. Craniofac. Surg. 2015, 26, e746–e749. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.Y.; Lee, S.J.; Yoo, J.Y.; Han, J.J.; Hwang, S.J.; Huh, K.H.; Lee, S.S.; Heo, M.S.; Choi, S.C.; Yi, W.J. Autonomous bone reposition around anatomical landmark for robot-assisted orthognathic surgery. J. Craniomaxillofac. Surg. 2017, 45, 1980–1988. [Google Scholar] [CrossRef]
- Han, J.J.; Woo, S.Y.; Yi, W.J.; Hwang, S.J. Robot-assisted maxillary positioning in orthognathic surgery: A feasibility and accuracy evaluation. J. Clin. Med. 2021, 10, 2596. [Google Scholar] [CrossRef]
- Lin, L.; Xu, C.; Shi, Y.; Zhou, C.; Zhu, M.; Chai, G.; Xie, L. Preliminary clinical experience of robot-assisted surgery in treatment with genioplasty. Sci. Rep. 2021, 11, 6365. [Google Scholar] [CrossRef]
- Lin, L.; Shi, Y.; Tan, A.; Bogari, M.; Zhu, M.; Xin, Y.; Xu, H.; Zhang, Y.; Xie, L.; Chai, G. Mandibular angle split osteotomy based on a novel augmented reality navigation using specialized robot-assisted arms-a feasibility study. J. Craniomaxillofac. Surg. 2016, 44, 215–223. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, M.; Shi, Y.; Lin, L.; Chai, G.; Zhang, Y.; Xie, L. Robot-assisted surgery for mandibular angle split osteotomy using augmented reality: Preliminary results on clinical animal experiment. Aesthetic Plast. Surg. 2017, 41, 1228–1236. [Google Scholar] [CrossRef]
- Sun, M.; Chai, Y.; Chai, G.; Zheng, X. Fully automatic robot-assisted surgery for mandibular angle split osteotomy. J. Craniofac. Surg. 2020, 31, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.H.; Weimer, K.; Raczkowsky, J.; Zhang, Y.; Kunze, M.; Cody, D.; Selber, J.C.; Hanasono, M.M.; Skoracki, R.J. Pre-programmed robotic osteotomies for fibula free flap mandible reconstruction: A preclinical investigation. Microsurgery 2016, 36, 246–249. [Google Scholar] [CrossRef]
- Zhu, J.H.; Deng, J.; Liu, X.J.; Wang, J.; Guo, Y.X.; Guo, C.B. Prospects of robot-assisted mandibular reconstruction with fibula flap: Comparison with a computer-assisted navigation system and freehand technique. J. Reconstr. Microsurg. 2016, 32, 661–669. [Google Scholar] [CrossRef]
- Augello, M.; Baetscher, C.; Segesser, M.; Zeilhofer, H.F.; Cattin, P.; Juergens, P. Performing partial mandibular resection, fibula free flap reconstruction and midfacial osteotomies with a cold ablation and robot-guided Er:YAG laser osteotome (CARLO®)—A study on applicability and effectiveness in human cadavers. J. Craniomaxillofac. Surg. 2018, 46, 1850–1855. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Fan, J.; Tao, B.; Wang, S.; Mo, J.; Wu, Y.; Liang, Q.; Chen, X. An image-guided hybrid robot system for dental implant surgery. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Qin, C.; Fan, S.; Yu, D.; Wu, Y.; Qin, J.; Chen, X. Pilot study of a surgical robot system for zygomatic implant placement. Med. Eng. Phys. 2020, 75, 72–78. [Google Scholar] [CrossRef]
- Ma, Q.; Kobayashi, E.; Wang, J.; Hara, K.; Suenaga, H.; Sakuma, I.; Masamune, K. Development and preliminary evaluation of an autonomous surgical system for oral and maxillofacial surgery. Int. J. Med. Robot. 2019, 15, e1997. [Google Scholar] [CrossRef]
- Xu, C.; Lin, L.; Zhou, C.; Xie, L. A compact surgical robot system for craniomaxillofacial surgery and its preliminary study. J. Craniofac. Surg. 2021, 32, 101–107. [Google Scholar] [CrossRef]
- Sun, M.; Lin, L.; Chen, X.; Xu, C.; Zin, M.A.; Han, W.; Chai, G. Robot-assisted mandibular angle osteotomy using electromagnetic navigation. Ann. Transl. Med. 2021, 9, 567. [Google Scholar] [CrossRef]
- Bell, S.W.; Anthony, I.; Jones, B.; MacLean, A.; Rowe, P.; Blyth, M. Improved Accuracy of component positioning with robotic-assisted unicompartmental knee arthroplasty: Data from a prospective, randomized controlled study. J. Bone Joint Surg. Am. 2016, 20, 627–635. [Google Scholar] [CrossRef]
- Ellis, E., III; Kittidumkerng, W. Analysis of treatment forisolated zygomaticomaxillary complex fractures. J. Oral Maxillofac. Surg. 1996, 54, 386–400. [Google Scholar] [CrossRef]
- Mazzoni, S.; Badiali, G.; Lancellotti, L.; Babbi, L.; Bianchi, A.; Marchetti, C. Simulation-guided navigation: A new approach to improve intraoperative three-dimensional reproducibility during orthognathic surgery. J. Craniofac. Surg. 2010, 21, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Bobek, S.L. Applications of navigation for orthognathic surgery. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.W.; Dard, M.; Zeilhofer, H.F.; Cattin, P.C.; Juergens, P. Comparing the bone healing after cold ablation robot-guided Er:YAG laser osteotomy and piezoelectric osteotomy—A pilot study in a minipig mandible. Lasers Surg. Med. 2021, 53, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Ureel, M.; Wilken, T.; Müller, A.A.; Schicho, K.; Millesi, G.; Juergens, P. First-in-man application of a cold ablation robot guided laser osteotome in midface osteotomies. J. Craniomaxillofac. Surg. 2021, 49, 531–537. [Google Scholar] [CrossRef]


| Experimental Group | Control Group | |||
|---|---|---|---|---|
| No | Distance Error (mm) | Direction Error (°) | Distance Error (mm) | Direction Error (°) |
| 1 | 1.14 | 9.7 | 1.09 | 8.3 |
| 2 | 1.37 | 8.6 | 1.21 | 8.6 |
| 3 | 1.71 | 9.5 | 1.65 | 7.9 |
| 4 | 1.56 | 8.3 | 1.66 | 9.0 |
| 5 | 1.59 | 7.3 | 1.67 | 8.3 |
| 6 | 1.91 | 6.8 | 1.83 | 8.8 |
| 7 | 1.93 | 6.5 | 1.88 | 9.2 |
| 8 | 1.67 | 6.8 | 1.54 | 8.7 |
| 9 | 1.43 | 9.2 | 1.57 | 8.2 |
| 10 | 1.70 | 7.6 | 1.57 | 9.1 |
| 11 | 1.12 | 7.4 | 1.43 | 9.2 |
| 12 | 1.76 | 8.2 | 1.56 | 8.1 |
| Mean ± SD 1 | 1.57 ± 0.26 | 7.99 ± 1.10 | 1.55 ± 0.23 | 8.61 ± 1.05 |
| Paired Groups | Measurement | Mean | SD 1 | SEM 2 | 95% CI 3 | p Value | |
|---|---|---|---|---|---|---|---|
| Lower Limit | Superior Limit | ||||||
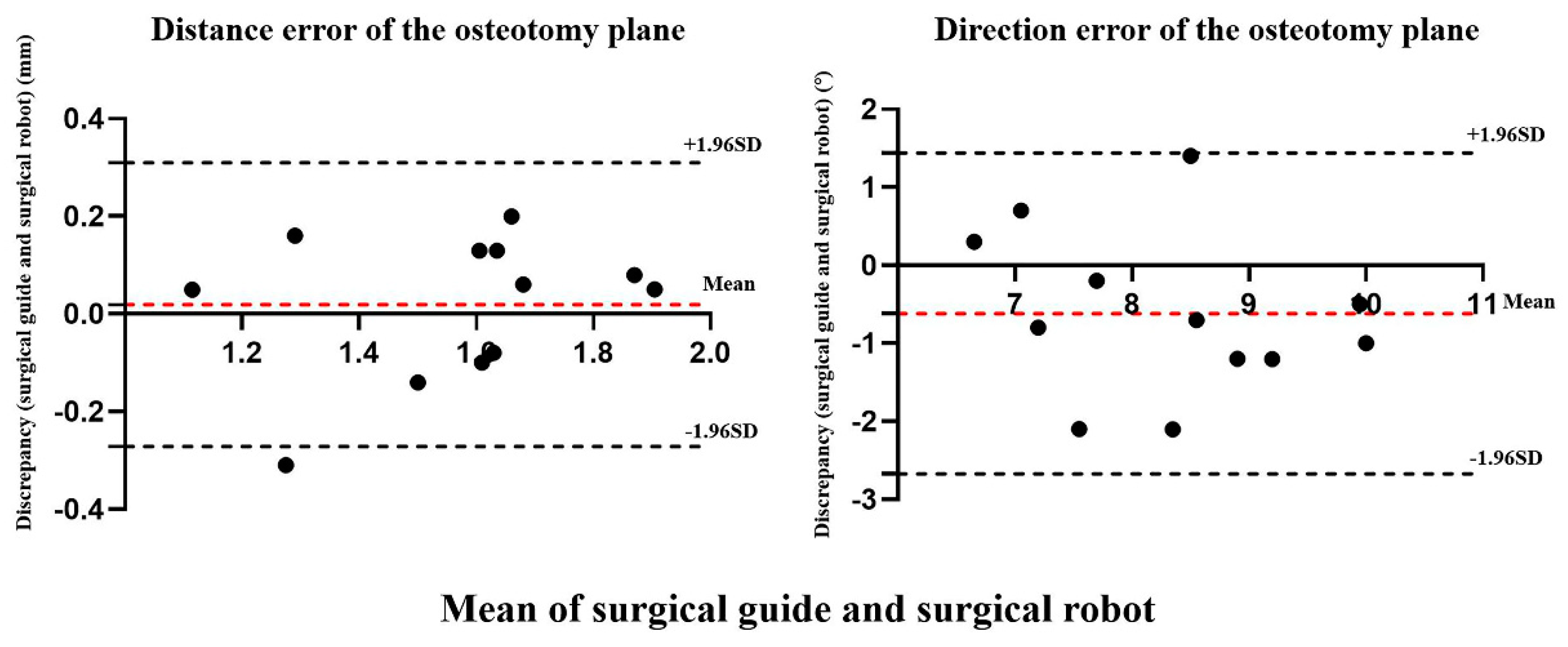
| Experimental group | Distance error (mm) | 0.02 | 0.15 | 0.04 | −0.08 | 0.11 | 0.66 |
| Control group | Direction error (°) | −0.62 | 1.05 | −0.30 | −1.28 | 0.05 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Hui, W.; Huang, J.; Luan, N.; Lin, Y.; Zhang, Y.; Zhang, S. The Feasibility of Robot-Assisted Chin Osteotomy on Skull Models: Comparison with Surgical Guides Technique. J. Clin. Med. 2022, 11, 6807. https://doi.org/10.3390/jcm11226807
Wu J, Hui W, Huang J, Luan N, Lin Y, Zhang Y, Zhang S. The Feasibility of Robot-Assisted Chin Osteotomy on Skull Models: Comparison with Surgical Guides Technique. Journal of Clinical Medicine. 2022; 11(22):6807. https://doi.org/10.3390/jcm11226807
Chicago/Turabian StyleWu, Jinyang, Wenyu Hui, Jianhua Huang, Nan Luan, Yanping Lin, Yong Zhang, and Shilei Zhang. 2022. "The Feasibility of Robot-Assisted Chin Osteotomy on Skull Models: Comparison with Surgical Guides Technique" Journal of Clinical Medicine 11, no. 22: 6807. https://doi.org/10.3390/jcm11226807
APA StyleWu, J., Hui, W., Huang, J., Luan, N., Lin, Y., Zhang, Y., & Zhang, S. (2022). The Feasibility of Robot-Assisted Chin Osteotomy on Skull Models: Comparison with Surgical Guides Technique. Journal of Clinical Medicine, 11(22), 6807. https://doi.org/10.3390/jcm11226807







