Pumpless Extracorporeal Hemadsorption Technique (pEHAT): A Proof-of-Concept Animal Study
Abstract
:1. Background
2. Methods
2.1. Animal Preparation
2.2. Instrumentation
- CytoSorb® HA (CytoSorbents Inc., Monmouth Junction, NJ, USA).
- Oxiris® HA (Baxter, Meyzieu, France).
2.3. Data Analysis
3. Results
3.1. Systemic Hemodynamics
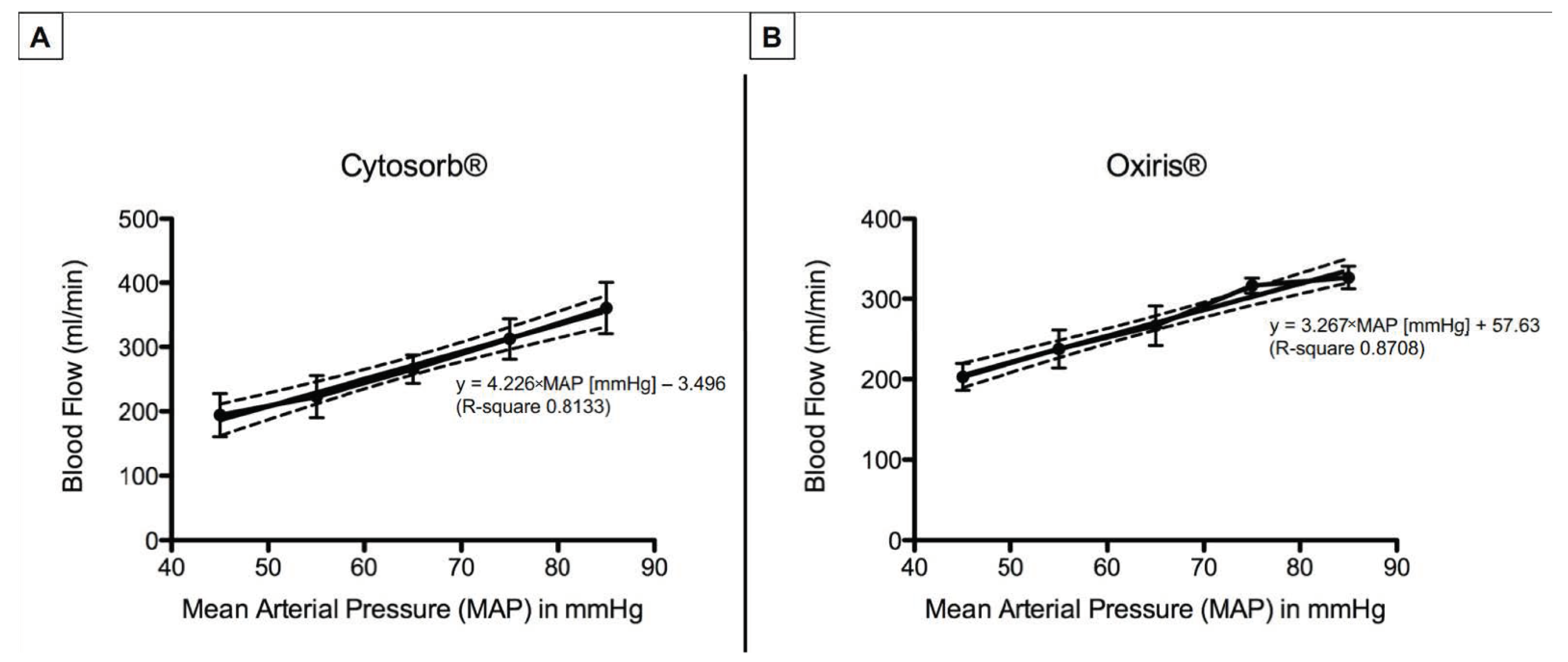
3.2. Hemadsorber Blood Flow
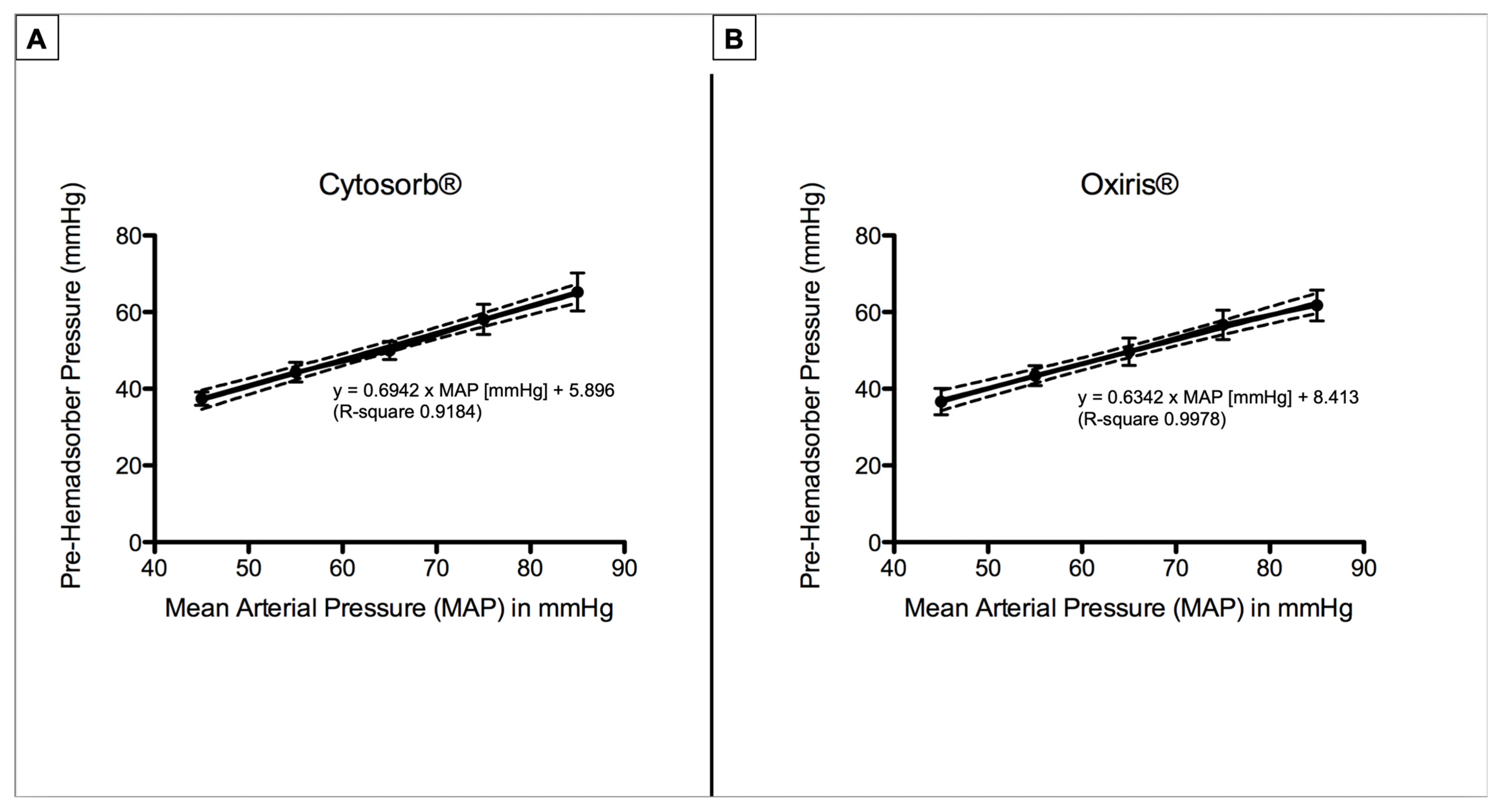
3.3. Pre- and Post-Hemadsorber Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CO | Cardiac output |
| CRRT | Continuous renal replacement therapy |
| CVVH | Continuous veno-venous hemofiltration |
| CVP | Central venous pressure |
| ECMO | Extracorporeal membrane oxygenation |
| HA | Extracorporeal hemadsorption |
| HD | Hemodialysis |
| HP | Hemoperfusion |
| MAP | Mean atrial pressure |
| PAP | Pulmonary arterial pressure |
| PEI | Polyethyleneimine |
References
- Kovacs, J. Hemoadsorption in Critical Care—It is a Useful or a Harmful Technique? J. Crit. Care Med. 2020, 6, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Ankawi, G.; Xie, Y.; Yang, B.; Xie, Y.; Xie, P.; Ronco, C. What Have We Learned about the Use of Cytosorb Adsorption Columns? Blood Purif. 2019, 48, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhou, Y.; Ma, J.; Xia, P.; Qin, Y.; Li, X. Is there a role for blood purification therapies targeting cytokine storm syndrome in critically severe COVID-19 patients? Ren. Fail. 2020, 42, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Schadler, D.; Pausch, C.; Heise, D.; Meier-Hellmann, A.; Brederlau, J.; Weiler, N.; Marx, G.; Putensen, C.; Spies, C.; Jörres, A.; et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS ONE 2017, 12, e0187015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogelmann, K.; Jarczak, D.; Scheller, M.; Druner, M. Hemoadsorption by CytoSorb in septic patients: A case series. Crit. Care 2017, 21, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwer, W.P.; Duran, S.; Kuijper, M.; Ince, C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: A propensity-score-weighted retrospective study. Crit. Care 2019, 23, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieder, M.; Wengenmayer, T.; Staudacher, D.; Duerschmied, D.; Supady, A. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation. Crit. Care 2020, 24, 435. [Google Scholar] [CrossRef] [PubMed]
- Rieder, M.; Duerschmied, D.; Zahn, T.; Lang, C.; Benk, C.; Lother, A.; Paul, B.; Christoph, B.; Tobias, W.; Dawid, S.; et al. Cytokine Adsorption in Severe Acute Respiratory Failure Requiring Veno-Venous Extracorporeal Membrane Oxygenation. Asaio J. 2021, 67, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, M.O.; Reyher, C.; Kalenka, A.; Rolfes, C.; Lotz, C.; Muellenbach, R.M. Pumpless Extracorporeal Hemadsorption Technique: A New Method for Early Cytokine Elimination? Blood Purif. 2021, 50, 968–970. [Google Scholar] [CrossRef] [PubMed]
- Napp, L.C.; Ziegeler, S.; Kindgen-Milles, D. Rationale of Hemoadsorption during Extracorporeal Membrane Oxygenation Support. Blood Purif. 2019, 48, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Monard, C.; Rimmelé, T.; Ronco, C. Extracorporeal Blood Purification Therapies for Sepsis. Blood Purif. 2019, 47 (Suppl. S3), 1–14. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.baxter.com/oxiris-critical-care (accessed on 12 October 2022).
- Available online: https://cytosorb-therapy.com/en/the-adsorber/ (accessed on 12 October 2022).
- Friesecke, S.; Stecher, S.S.; Gross, S.; Felix, S.B.; Nierhaus, A. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: A prospective single-center study. J. Artif. Organs 2017, 20, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Kaech, S.M.; Ahmed, R. Memory CD8+ T cell differentiation: Initial antigen encounter triggers a developmental program in naïve cells. Nat. Immunol. 2001, 2, 415–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankawi, G.; Neri, M.; Zhang, J.; Breglia, A.; Ricci, Z.; Ronco, C. Extracorporeal techniques for the treatment of critically ill patients with sepsis beyond conventional blood purification therapy: The promises and the pitfalls. Crit. Care 2018, 22, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muellenbach, R.M.; Kredel, M.; Kuestermann, J.; Klingelhoefer, M.; Schuster, F.; Wunder, C.; Kranke, P.; Roewer, N.; Brederlau, J. Combining “open-lung” ventilation and arteriovenous extracorporeal lung assist: Influence of different tidal volumes on gas exchange in experimental lung failure. Med. Sci. Monit. 2009, 15, Br213–Br220. [Google Scholar] [PubMed]
- Muellenbach, R.M.; Kredel, M.; Wilhelm, J.; Küstermann, J.; Fink, L.; Siebenliest, G.; Klosterhalfen, B.; Foerster, C.Y.; Kranke, P.; Wunder, C.; et al. High-frequency oscillation combined with arteriovenous extracorporeal lung assist reduces lung injury. Exp. Lung Res. 2010, 36, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Muellenbach, R.M.; Wunder, C.; Nuechter, D.C.; Smul, T.; Trautner, H.; Kredel, M.; Roewer, N.; Brederlau, J. Early treatment with arteriovenous extracorporeal lung assist and high-frequency oscillatory ventilation in a case of severe acute respiratory distress syndrome. Acta Anaesthesiol. Scand. 2007, 51, 766–769. [Google Scholar] [CrossRef] [PubMed]

| Baseline | MAP45 | MAP55 | MAP65 | MAP75 | MAP85 | p-Value | |
|---|---|---|---|---|---|---|---|
| HR (L/min) | |||||||
| CytoSorb® | 92 ± 6.2 | 82.7 ± 7.4 | 82.3 ± 4.4 | 84.3 ± 4.1 | 91.5 ± 7.5 | 103 ± 6.4 | * 0.0011 |
| Oxiris® | 95.8 ± 20.9 | 91.8 ± 19.9 | 92.9 ± 13.7 | 90.9 ± 9.3 | 86.1 ± 8.3 | 89.9 ± 18.6 | 0.73 |
| CI (L/min/m2) | |||||||
| CytoSorb® | 4.3 ± 0.2 | 3.7 ± 0.8 | 3,7 ± 0.8 | 3.9 ± 0.8 | 4.1 ± 0.5 | 4.4 ± 0.5 | * 0.04 |
| Oxiris® | 4.4 ± 1 | 4.6 ± 0.7 | 4.2 ± 0.6 | 4.1 ± 1.5 | 3.8 ± 0.4 | 4.8 ± 0.9 | 0.41 |
| PAP (mmHg) | |||||||
| CytoSorb® | 24 ± 2.3 | 19.9 ± 5.3 | 24.8 ± 1.6 | 23.8 ± 1.5 | 25.3 ± 1.5 | 26 ± 2.2 | 0.03 |
| Oxiris® | 24.6 ± 1.3 | 17.5 ± 3.2 | 20.8 ± 2.0 | 21.7 ± 1.1 | 23.3 ± 2.8 | 26.7 ± 1.1 | * <0.0001 |
| CVP (mmHg) | |||||||
| CytoSorb® | 13.1± 1.2 | 13.3 ± 2.9 | 15.5 ± 2.6 | 14.8 ± 2.6 | 13.4 ± 1.2 | 13.4 ± 0.5 | 0.45 |
| Oxiris® | 11.1 ± 2.2 | 7.3 ±1.7 | 9. 7 ± 2.1 | 10.9 ± 4.1 | 10.7 ± 2.1 | 12.5 ± 1.7 | * 0.03 |
| Baseline | MAP45 | MAP55 | MAP65 | MAP75 | MAP85 | p-Value | |
|---|---|---|---|---|---|---|---|
| Blood Flow (mL/min) | |||||||
| CytoSorb® | 0 | 194.2 ± 33.5 | 222.9 ± 32.6 | 265.7 ± 22.2 | 312.5 ± 31.6 | 360.7 ± 39.9 | <0.0001 |
| Oxiris® | 0 | 202.8 ± 16.8 | 237.4 ± 23.7 | 266.5 ± 24.5 | 316.4 ± 9.5 | 326.7 ± 14.1 | <0.0001 |
| Pre-Hemadsorber Pressure (mmHg) | |||||||
| CytoSorb® | 0 | 37.4 ± 1.7 | 44.3 ± 2.6 | 50 ± 2.4 | 58.1 ± 3.9 | 65.3 ± 5 | <0.0001 |
| Oxiris® | 0 | 36.7 ± 3.4 | 43.4 ± 2.6 | 49.7 ± 3.5 | 56.7 ± 3.8 | 61.8 ± 4.0 | <0.0001 |
| Post-Hemadsorber Pressure (mmHg) | |||||||
| CytoSorb® | 0 | 14.5 ± 3.7 | 18.9 ± 1.8 | 18.9 ± 0.6 | 20 ± 1.4 | 22 ± 2 | <0.0001 |
| Oxiris® | 0 | 11.8 ± 1.7 | 12.8 ± 2.4 | 15.8 ± 3.2 | 15.3 ± 3.8 | 18.8 ± 4.8 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiedler, M.O.; Muellenbach, R.M.; Rolfes, C.; Lotz, C.; Nickel, F.; Müller-Stich, B.P.; Supady, A.; Lepper, P.M.; Weigand, M.A.; Meybohm, P.; et al. Pumpless Extracorporeal Hemadsorption Technique (pEHAT): A Proof-of-Concept Animal Study. J. Clin. Med. 2022, 11, 6815. https://doi.org/10.3390/jcm11226815
Fiedler MO, Muellenbach RM, Rolfes C, Lotz C, Nickel F, Müller-Stich BP, Supady A, Lepper PM, Weigand MA, Meybohm P, et al. Pumpless Extracorporeal Hemadsorption Technique (pEHAT): A Proof-of-Concept Animal Study. Journal of Clinical Medicine. 2022; 11(22):6815. https://doi.org/10.3390/jcm11226815
Chicago/Turabian StyleFiedler, Mascha O., Ralf M. Muellenbach, Caroline Rolfes, Christopher Lotz, Felix Nickel, Beat P. Müller-Stich, Alexander Supady, Philipp M. Lepper, Markus A. Weigand, Patrick Meybohm, and et al. 2022. "Pumpless Extracorporeal Hemadsorption Technique (pEHAT): A Proof-of-Concept Animal Study" Journal of Clinical Medicine 11, no. 22: 6815. https://doi.org/10.3390/jcm11226815





