Study Protocol for a Multicenter, Randomized Controlled Trial to Improve Upper Extremity Hemiparesis in Chronic Stroke Patients by One-to-One Training (NEURO®) with Repetitive Transcranial Magnetic Stimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Aims
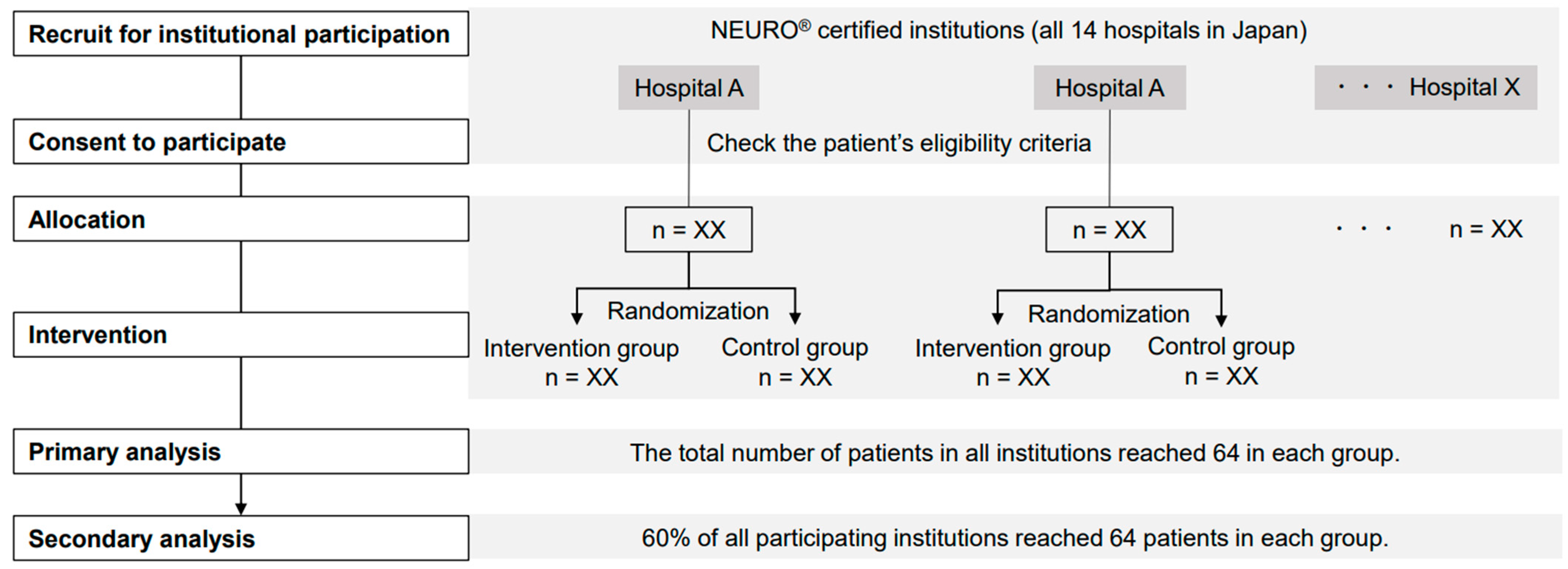
2.2. Study Design
2.3. Participants and Settings
2.4. Participant Characteristics
2.5. Sample Size
2.6. Randomization
2.7. Sequence Generation
2.8. Allocation Concealment Mechanism
2.9. Blinding
2.10. Interventions
2.10.1. rTMS
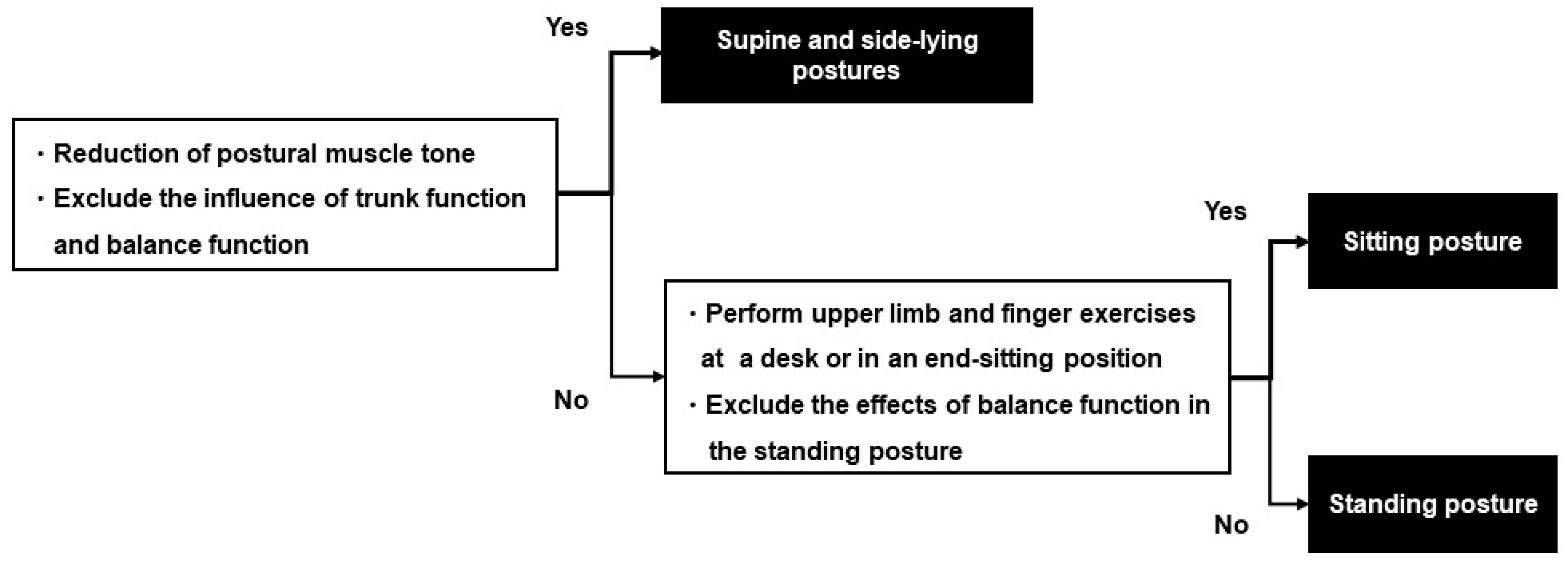
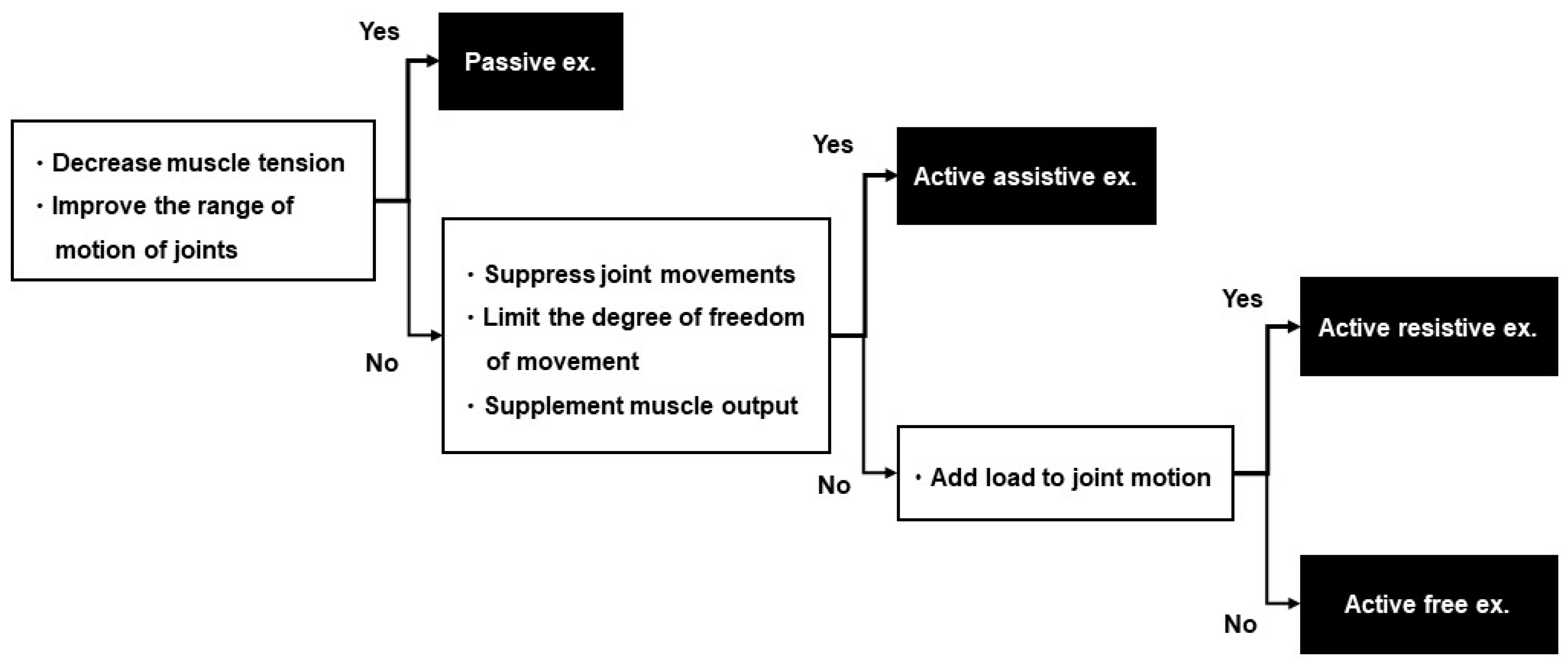
2.10.2. Rehabilitation
2.11. Intervention for the Control Group
2.12. Treatment for the Intervention Group
2.13. Outcome Evaluation
2.14. Statistical Analysis
2.15. Ethical Considerations and Declarations
2.16. Status and Timeline of the Study
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Palstam, A.; Sjödin, A.; Sunnerhagen, K.S. Participation and autonomy five years after stroke: A longitudinal observational study. PLoS ONE 2019, 14, e0219513. [Google Scholar] [CrossRef]
- van Mierlo, M.L.; van Heugten, C.M.; Post, M.W.; Hajós, T.R.; Kappelle, L.J.; Visser-Meily, J.M. Quality of life during the first two years post stroke: The Restore4Stroke cohort study. Cerebrovasc. Dis. 2016, 41, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, W.; Abo, M.; Kobayashi, K.; Momosaki, R.; Yokoi, A.; Fukuda, A.; Ishikawa, A.; Ito, H.; Tominaga, A. Low-frequency repetitive transcranial magnetic stimulation and intensive occupational therapy for poststroke patients with upper limb hemiparesis: Preliminary study of a 15-day protocol. Int. J. Rehabil. Res. 2010, 33, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, W.; Abo, M.; Shimizu, M.; Sasanuma, J.; Okamoto, T.; Yokoi, A.; Taguchi, K.; Mitani, S.; Harashima, H.; Urushidani, N.; et al. A multi-center study on low-frequency rTMS combined with intensive occupational therapy for upper limb hemiparesis in post-stroke patients. J. Neuroeng. Rehabil. 2012, 9, 4. [Google Scholar] [CrossRef]
- Abo, M.; Kakuda, W.; Momosaki, R.; Harashima, H.; Kojima, M.; Watanabe, S.; Sato, T.; Yokoi, A.; Umemori, T.; Sasanuma, J. Randomized, multicenter, comparative study of NEURO versus CIMT in poststroke patients with upper limb hemiparesis: The NEURO-VERIFY study. Int. J. Stroke 2014, 9, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Hoogendam, J.M.; Ramakers, G.M.; Di Lazzaro, V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010, 3, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Goldsworthy, M.R.; Hordacre, B.; Rothwell, J.C.; Ridding, M.C. Effects of rTMS on the brain: Is there value in variability? Cortex 2021, 139, 43–59. [Google Scholar] [CrossRef]
- Sasaki, N.; Abo, M.; Hara, T.; Yamada, N.; Niimi, M.; Kakuda, W. High-frequency rTMS on leg motor area in the early phase of stroke. Acta Neurol. Belg. 2017, 117, 189–194. [Google Scholar] [CrossRef]
- Ueda, R.; Yamada, N.; Abo, M.; Ruwan, P.W.; Senoo, A. MRI evaluation of motor function recovery by rTMS and intensive occupational therapy and changes in the activity of motor cortex. Int. J. Neurosci. 2020, 130, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, D.; Zhao, Y.Y.; Hai, H.; Ma, Y.W. Effects of high-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex on motor recovery in severe hemiplegic stroke: A randomized clinical trial. Brain Stimul. 2020, 13, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kudo, Y.; Sugawara, E.; Nakamizo, T.; Amari, K.; Takahashi, K.; Tanaka, O.; Endo, M.; Hayakawa, Y.; Johkura, K. Comparative study of ipsilesional and contralesional repetitive transcranial magnetic stimulations for acute infarction. J. Neurol. Sci. 2018, 384, 10–14. [Google Scholar] [CrossRef]
- Du, J.; Yang, F.; Hu, J.; Hu, J.; Xu, Q.; Cong, N.; Zhang, Q.; Liu, L.; Mantini, D.; Zhang, Z.; et al. Effects of high- and low-frequency repetitive transcranial magnetic stimulation on motor recovery in early stroke patients: Evidence from a randomized controlled trial with clinical, neurophysiological and functional imaging assessments. NeuroImage Clin. 2019, 21, 101620. [Google Scholar] [CrossRef] [PubMed]
- Vabalaite, B.; Petruseviciene, L.; Savickas, R.; Kubilius, R.; Ignatavicius, P.; Lendraitiene, E. Effects of high-frequency (HF) repetitive transcranial magnetic stimulation (rTMS) on upper extremity motor function in stroke patients: A systematic review. Medicina 2021, 57, 1215. [Google Scholar] [CrossRef]
- Sasaki, N.; Kakuda, W.; Abo, M. Bilateral high- and low-frequency rTMS in acute stroke patients with hemiparesis: A comparative study with unilateral high-frequency rTMS. Brain Inj. 2014, 28, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Legg, L.A.; Lewis, S.R.; Schofield-Robinson, O.J.; Drummond, A.; Langhorne, P. Occupational therapy for adults with problems in activities of daily living after stroke. Cochrane Database Syst. Rev. 2017, 7, CD003585. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Kim, H. How assistive devices affect activities of daily living and cognitive functions of people with brain injury: A meta-analysis. Disabil. Rehabil. Assist. Technol. 2018, 13, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Fudickar, S.; Kiselev, J.; Frenken, T.; Wegel, S.; Dimitrowska, S.; Steinhagen-Thiessen, E.; Hein, A. Validation of the ambient TUG chair with light barriers and force sensors in a clinical trial. Assist. Technol. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Han, C.E.; Arbib, M.A.; Schweighofer, N. Stroke rehabilitation reaches a threshold. PLoS Comput. Biol. 2008, 4, e1000133. [Google Scholar] [CrossRef] [PubMed]
- Peurala, S.H.; Kantanen, M.P.; Sjögren, T.; Paltamaa, J.; Karhula, M.; Heinonen, A. Effectiveness of constraint-induced movement therapy on activity and participation after stroke: A systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 2012, 26, 209–223. [Google Scholar] [CrossRef]
- Rice, D.B.; McIntyre, A.; Mirkowski, M.; Janzen, S.; Viana, R.; Britt, E.; Teasell, R. Patient-centered goal setting in a hospital-based outpatient stroke rehabilitation Center. PM R. 2017, 9, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.C.; Lewthwaite, R.; Rocktashel, J.; Winstein, C.J. Self-efficacy and reach performance in individuals with mild motor impairment due to stroke. Neurorehabil. Neural Repair. 2019, 33, 319–328. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Yamada, N.; Hada, T.; Abo, M. Prediction of motor recovery in the upper extremity for repetitive transcranial magnetic stimulation and occupational therapy goal setting in patients with chronic stroke: A retrospective analysis of prospectively collected data. Front. Neurol. 2020, 11, 581186. [Google Scholar] [CrossRef] [PubMed]
- Tatsuno, H.; Hamaguchi, T.; Sasanuma, J.; Kakita, K.; Okamoto, T.; Shimizu, M.; Nakaya, N.; Abo, M. Does a combination treatment of repetitive transcranial magnetic stimulation and occupational therapy improve upper limb muscle paralysis equally in patients with chronic stroke caused by cerebral hemorrhage and infarction?: A retrospective cohort study. Medicine 2021, 100, e26339. [Google Scholar] [CrossRef] [PubMed]
- Introducing NEURO®. Available online: https://www.j-sts.jp/about_neuro.html (accessed on 12 January 2022).
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, M.L.; Velozo, C.A.; Richards, L.G.; Duncan, P.W.; Studenski, S.; Lai, S.M. Dimensionality and construct validity of the Fugl-Meyer Assessment of the upper extremity. Arch. Phys. Med. Rehabil. 2007, 88, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Hijikata, N.; Kawakami, M.; Ishii, R.; Tsuzuki, K.; Nakamura, T.; Okuyama, K.; Liu, M. Item difficulty of Fugl-Meyer assessment for upper extremity in persons with chronic stroke with moderate-to-severe upper limb impairment. Front. Neurol. 2020, 11, 577855. [Google Scholar] [CrossRef] [PubMed]
- Tauchi, Y.; Kyougoku, M.; Takahashi, K.; Okita, Y.; Takebayashi, T. Dimensionality and item-difficulty hierarchy of the Fugl-Meyer assessment of the upper extremity among Japanese patients who have experienced stroke. Top. Stroke Rehabil. 2021, 29, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Massie, C.L.; Fritz, S.; Malcolm, M.P. Elbow extension predicts motor impairment and performance after stroke. Rehabil. Res. Pract. 2011, 2011, 381978. [Google Scholar] [CrossRef] [PubMed]
- McPherson, J.G.; Chen, A.; Ellis, M.D.; Yao, J.; Heckman, C.J.; Dewald, J.P.A. Progressive recruitment of contralesional cortico-reticulospinal pathways drives motor impairment post stroke. J. Physiol. 2018, 596, 1211–1225. [Google Scholar] [CrossRef]
- Lotze, M.; Ladda, A.M.; Stephan, K.M. Cerebral plasticity as the basis for upper limb recovery following brain damage. Neurosci. Biobehav. Rev. 2019, 99, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Brunnstrom, S. Motor testing procedures in hemiplegia: Based on sequential recovery stages. Phys. Ther. 1966, 46, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Doering, T.J.; Resch, K.L.; Steuernagel, B.; Brix, J.; Schneider, B.; Fischer, G.C. Passive and active exercises increase cerebral blood flow velocity in young, healthy individuals. Am. J. Phys. Med. Rehabil. 1998, 77, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [PubMed]
- Hoonhorst, M.H.; Nijland, R.H.; van den Berg, J.S.; Emmelot, C.H.; Kollen, B.J.; Kwakkel, G. How do Fugl-Meyer arm motor scores relate to dexterity according to the action research arm test at 6 months poststroke? Arch. Phys. Med. Rehabil. 2015, 96, 1845–1849. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D. A quantitative test of upper extremity function. J. Chronic Dis. 1965, 18, 479–491. [Google Scholar] [CrossRef]
- Lyle, R.C. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int. J. Rehabil. Res. 1981, 4, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.L.; Catlin, P.A.; Ellis, M.; Archer, A.L.; Morgan, B.; Piacentino, A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke 2001, 32, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Kakuda, W.; Taguchi, K.; Uruma, G.; Abo, M. The reliability and validity of a new subjective assessment scale for poststroke upper limb hemiparesis, the Jikei assessment scale for motor impairtment in daily living. Tokyo Jikei Med. J. 2010, 125, 159–167. [Google Scholar]
- van der Lee, J.H.; Beckerman, H.; Knol, D.L.; de Vet, H.C.; Bouter, L.M. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke 2004, 35, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Uswatte, G.; Taub, E.; Morris, D.; Vignolo, M.; McCulloch, K. Reliability and validity of the upper-extremity Motor Activity Log-14 for measuring real-world arm use. Stroke 2005, 36, 2493–2496. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Hsu, M.J.; Sheu, C.F.; Wu, T.S.; Lin, R.T.; Chen, C.H.; Hsieh, C.L. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Phys. Ther. 2009, 89, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The fugl-Meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Pickersgill, J.W.; Turco, C.V.; Ramdeo, K.; Rehsi, R.S.; Foglia, S.D.; Nelson, A.J. The combined influences of exercise, diet and sleep on neuroplasticity. Front. Psychol. 2022, 13, 831819. [Google Scholar] [CrossRef] [PubMed]



| Intervention Group | Control Group | Total | ||
|---|---|---|---|---|
| Number of patients | N = XX | N = XX | N = XX | |
| Age | XX | XX | XX | |
| Sex | Female = XX Male = XX | Female = XX Male = XX | Female = XX Male = XX | |
| Height | XX | XX | XX | |
| Weight | XX | XX | XX | |
| BMI | XX | XX | XX | |
| Affected side | Left = XX, Right = XX | Left = XX, Right = XX | Left = XX, Right = XX | |
| Dominant hand | Left = XX, Right = XX | Left = XX, Right = XX | Left = XX, Right = XX | |
| Diagnosis | CI | N = XX | N = XX | N = XX |
| ICH | N = XX | N = XX | N = XX | |
| Time from onset | XX | XX | XX | |
| FMA-UE severity | ||||
| No (<23) | N = XX | N = XX | N = XX | |
| Poor (≤23–≤31) | N = XX | N = XX | N = XX | |
| Limited (≤32–≤47) | N = XX | N = XX | N = XX | |
| Notable (≤48–≤52) | N = XX | N = XX | N = XX | |
| Full (≤53–≤66) | N = XX | N = XX | N = XX | |
| First Week | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday |
|---|---|---|---|---|---|---|---|
| Event | Admission | ||||||
| rTMS (pulse) | 2400 | 2400 | 2400 | 2400 | 2400 | 2400 | - |
| One-to-one training (time) | - | 500 | 500 | 500 | 500 | 500 | - |
| Second week | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday |
| Event | Discharge | ||||||
| rTMS (pulse) | 2400 | 2400 | 2400 | 2400 | 2400 | 2400 | |
| One-to-one training (time) | 500 | 500 | 500 | 500 | 500 | - |
| One-to-One Training | Self-Training | |
|---|---|---|
| Plan 1 | Scapular retraction/protraction Shoulder flexion to 0–180°, elbow extended Shoulder flexion to 0–90°, elbow extended Elbow extension _______________________ | Scapular retraction/protraction Shoulder flexion to 0–180°, elbow extended Shoulder flexion to 0–90°, elbow extended Elbow extension ___________________ |
| Plan 2 | Shoulder flexion to 0–180°, elbow extended Shoulder flexion to 0–90°, elbow extended Elbow extension Finger extension, elbow extended _____________________ | Scapular retraction/protraction Shoulder flexion to 0–180°, elbow extendedElbow extension Finger extension ______________________ |
| Plan 3 | Shoulder flexion to 90–180°, elbow extended Shoulder abduction to 0–180°, elbow extended Wrist flexion/extension, elbow extended Finger extension ____________________ | Shoulder flexion to 90–180°, elbow extended Forearm supination/pronation Wrist flexion/extension Finger extension ______________________ |
| Plan 4 | Shoulder abduction to 0–180°, elbow extended Forearm supination/pronation, elbow extended Wrist flexion/extension, elbow extended Finger extension, elbow extended ______________________ | Shoulder flexion to 90–180°, elbow extended Forearm supination/pronation Wrist flexion/extension Finger extension ___________________ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, D.; Hamaguchi, T.; Murata, K.; Ishikawa, A.; Nakayama, Y.; Abo, M. Study Protocol for a Multicenter, Randomized Controlled Trial to Improve Upper Extremity Hemiparesis in Chronic Stroke Patients by One-to-One Training (NEURO®) with Repetitive Transcranial Magnetic Stimulation. J. Clin. Med. 2022, 11, 6835. https://doi.org/10.3390/jcm11226835
Sakamoto D, Hamaguchi T, Murata K, Ishikawa A, Nakayama Y, Abo M. Study Protocol for a Multicenter, Randomized Controlled Trial to Improve Upper Extremity Hemiparesis in Chronic Stroke Patients by One-to-One Training (NEURO®) with Repetitive Transcranial Magnetic Stimulation. Journal of Clinical Medicine. 2022; 11(22):6835. https://doi.org/10.3390/jcm11226835
Chicago/Turabian StyleSakamoto, Daigo, Toyohiro Hamaguchi, Kai Murata, Atsushi Ishikawa, Yasuhide Nakayama, and Masahiro Abo. 2022. "Study Protocol for a Multicenter, Randomized Controlled Trial to Improve Upper Extremity Hemiparesis in Chronic Stroke Patients by One-to-One Training (NEURO®) with Repetitive Transcranial Magnetic Stimulation" Journal of Clinical Medicine 11, no. 22: 6835. https://doi.org/10.3390/jcm11226835
APA StyleSakamoto, D., Hamaguchi, T., Murata, K., Ishikawa, A., Nakayama, Y., & Abo, M. (2022). Study Protocol for a Multicenter, Randomized Controlled Trial to Improve Upper Extremity Hemiparesis in Chronic Stroke Patients by One-to-One Training (NEURO®) with Repetitive Transcranial Magnetic Stimulation. Journal of Clinical Medicine, 11(22), 6835. https://doi.org/10.3390/jcm11226835






