Periodontal Therapy in Bariatric Surgery Patients with Periodontitis: Randomized Control Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients’ Inclusion Criteria for the Studies
2.3. Sample Size Calculation
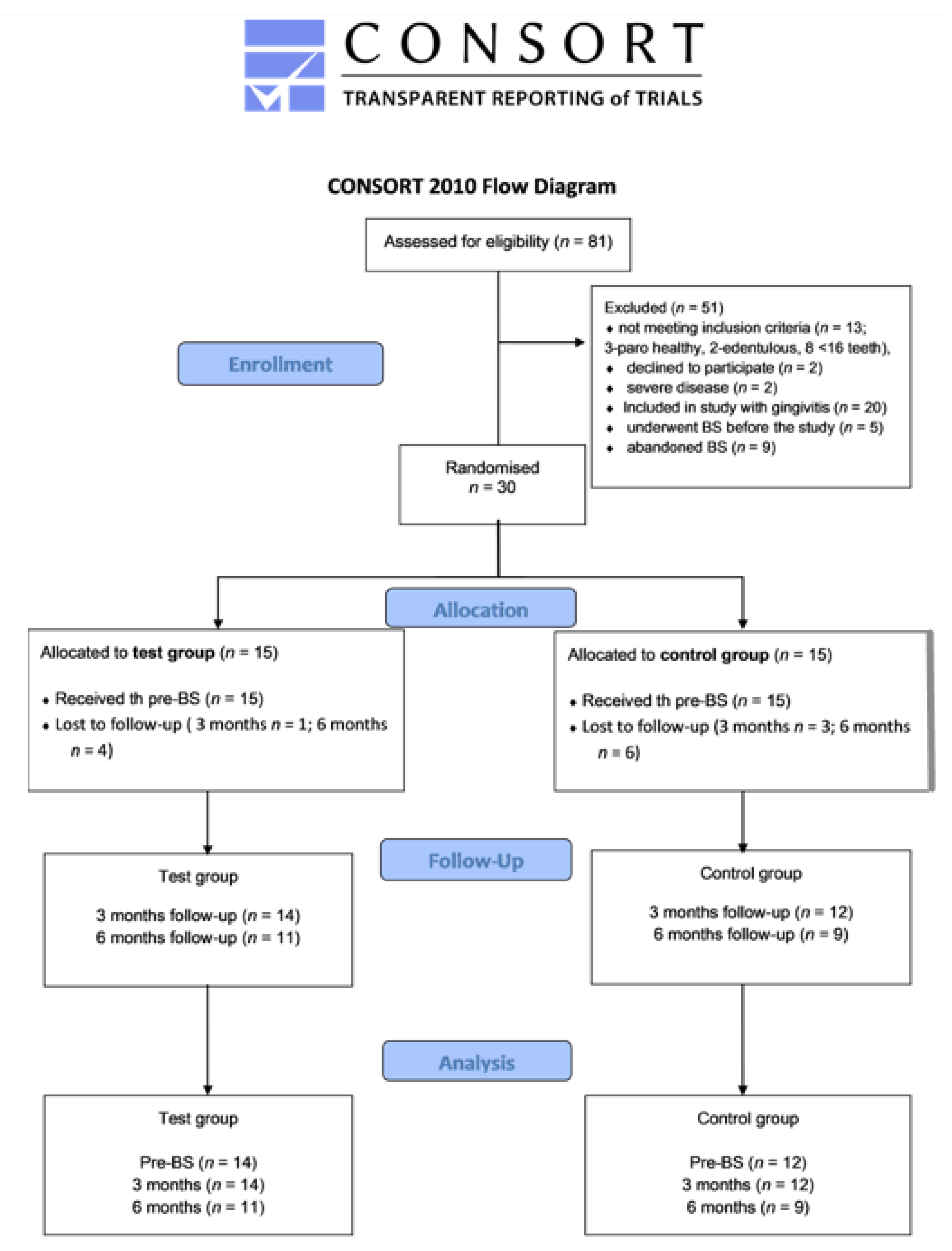
2.4. Patient Recruitment
2.5. Dental and Periodontal Clinical Examinations
2.6. Systemic Parameters
2.7. Periodontal Therapy in Test and Control Groups
2.8. Statistical Analysis
3. Results
3.1. Patients’ Baseline Characteristics
3.2. Changes in Test and Control Group after Bariatric Surgery
3.2.1. Change in Periodontal Parameters after Bariatric Surgery
3.2.2. Change in Obesity-Related Comorbidities after Bariatric Surgery
3.2.3. Systemic Biochemical Serum Parameters before and after Bariatric Surgery
3.2.4. Dietary Regime after Bariatric Surgery and Related Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, J.O. Understanding and Addressing the Epidemic of Obesity: An Energy Balance Perspective. Endocr. Rev. 2006, 27, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Khavjou, O.A.; Thompson, H.; Trogdon, J.G.; Pan, L.; Sherry, B.; Dietz, W. Obesity and Severe Obesity Forecasts through 2030. Am. J. Prev. Med. 2012, 42, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity & Inflammation: The Linking Mechanism & the Complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, R.W. Inflammation in Obesity-Related Diseases. Surgery 2009, 145, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Tsuritani, I.; Honda, R.; Noborisaka, Y.; Ishida, M.; Ishizaki, M.; Yamada, Y. Impact of Obesity on Musculoskeletal Pain and Difficulty of Daily Movements in Japanese Middle-Aged Women. Maturitas 2002, 42, 23–30. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Picca, A.; Tosato, M.; Martone, A.M.; Ortolani, E.; Sisto, A.; D’angelo, E.; Serafini, E.; Desideri, G.; et al. Body Mass Index Is Strongly Associated with Hypertension: Results from the Longevity Check-Up 7+ Study. Nutrients 2018, 10, 1976. [Google Scholar] [CrossRef]
- Ylöstalo, P.; Suominen-Taipale, L.; Reunanen, A.; Knuuttila, M. Association between Body Weight and Periodontal Infection. J. Clin. Periodontol. 2008, 35, 297–304. [Google Scholar] [CrossRef]
- Lehmann-Kalata, A.P.; Surdacka, A.; Ciężka-Hsiao, E.; Swora-Cwynar, E.; Grzymisławski, M. Clinical Parameters of Oral Cavity, Physical and Microbiological Properties of Saliva in Patients with Obesity. Dent. Med. Probl. 2015, 52, 415–423. [Google Scholar] [CrossRef]
- Frédéric, L.J.; Michel, B.; Selena, T. Oral Microbes, Biofilms and Their Role in Periodontal and Peri-Implant Diseases. Materials 2018, 11, 1802. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Polymicrobial Communities in Periodontal Disease: Their Quasi-Organismal Nature and Dialogue with the Host. Periodontol. 2000 2021, 86, 210–230. [Google Scholar] [CrossRef]
- Muhammad, A.N. Prevalence of Periodontal Disease, Its Association with Systemic Diseases and Prevention. Int. J. Health Sci. 2017, 1, 72–80. [Google Scholar] [CrossRef]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal Manifestations of Systemic Diseases and Developmental and Acquired Conditions: Consensus Report of Workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S219–S229. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The Oralome and Its Dysbiosis: New Insights into Oral Microbiome-Host Interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S. Relationship of Bacteria to the Etiology of Periodontal Disease. J. Dent. Res. 1970, 49, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Zhou, X.; Naqvi, A.; Francis, M.; Foyle, D.; Nares, S.; Diekwisch, T.G.H. MicroRNAs and Immunity in Periodontal Health and Disease. Int. J. Oral Sci. 2018, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Saini, S.; Sugandha, R. Periodontal Disease: The Sixth Complication of Diabetes. J. Fam. Community Med. 2011, 18, 31. [Google Scholar] [CrossRef]
- Koo, H.S.; Hong, S.M. Prevalence and Risk Factors for Periodontitis among Patients with Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 375–381. [Google Scholar] [CrossRef]
- Jenabian, N.; Dabbagh Sattari, F.; Salar, N.; Bijani, A.; Ghasemi, N. The Relation between Periodontitis and Anemia Associated Parameters. J. Dentomaxillofacial Radiol. Pathol. Surg. 2013, 2, 26–33. [Google Scholar]
- Alshwaiyat, N.M.; Ahmad, A.; Wan Hassan, W.M.R.W.; Al-jamal, H.A.N. Association between Obesity and Iron Deficiency (Review). Exp. Ther. Med. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Sundararajan, S.; Muthukumar, S.; Rao, S.R. Relationship between Depression and Chronic Periodontitis. J. Indian Soc. Periodontol. 2015, 19, 294. [Google Scholar] [CrossRef]
- Pallier, A.; Karimova, A.; Boillot, A.; Colon, P.; Ringuenet, D.; Bouchard, P.; Rangé, H. Dental and Periodontal Health in Adults with Eating Disorders: A Case-Control Study. J. Dent. 2019, 84, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Brode, C.S.; Mitchell, J.E. Problematic Eating Behaviors and Eating Disorders Associated with Bariatric Surgery. Psychiatr. Clin. N. Am. 2019, 42, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.J.; Trak-Fellermeier, M.A.; Joshipura, K.; Dye, B.A. Dietary Fiber Intake Is Inversely Associated with Periodontal Disease among US Adults. J. Nutr. 2016, 146, 2530–2536. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Imai, T.; Kato, Y.; Ando, F.; Shimokata, H. Relationship between Number of Metabolic Syndrome Components and Dietary Factors in Middle-Aged and Elderly Japanese Subjects. Hypertens. Res. 2010, 33, 548–554. [Google Scholar] [CrossRef]
- Alberga, A.S.; Edache, I.Y.; Forhan, M.; Russell-Mayhew, S. Weight Bias and Health Care Utilization: A Scoping Review. Prim. Health Care Res. Dev. 2019, 20, e116. [Google Scholar] [CrossRef]
- Schwenke, M.; Luppa, M.; Pabst, A.; Welzel, F.D.; Löbner, M.; Luck-Sikorski, C.; Kersting, A.; Blüher, M.; Riedel-Heller, S.G. Attitudes and Treatment Practice of General Practitioners towards Patients with Obesity in Primary Care. BMC Fam. Pract. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A New Classification Scheme for Periodontal and Peri-Implant Diseases and Conditions – Introduction and Key Changes from the 1999 Classification. J. Clin. Periodontol. 2018, 45, S1–S8. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Sanz, M. Implementation of the New Classification of Periodontal Diseases: Decision-Making Algorithms for Clinical Practice and Education. J. Clin. Periodontol. 2019, 46, 398–405. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Kumari, M.; Kalra, N.; Priyanka, N. Correlation of MCP-4 and High-Sensitivity C-Reactive Protein as a Marker of Inflammation in Obesity and Chronic Periodontitis. Cytokine 2013, 61, 772–777. [Google Scholar] [CrossRef]
- Pradeep, A.; Priyanka, N.; Prasad, M.; Kalra, N.; Kumari, M. Association of Progranulin and High Sensitivity CRP Concentrations in Gingival Crevicular Fluid and Serum in Chronic Periodontitis Subjects with and without Obesity. Dis. Markers 2012, 33, 207–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nijakowski, K.; Lehmann, A.; Rutkowski, R.; Korybalska, K.; Witowski, J.; Surdacka, A. Poor Oral Hygiene and High Levels of Inflammatory Cytokines in Saliva Predict the Risk of Overweight and Obesity. Int. J. Environ. Res. Public Health 2020, 17, 6310. [Google Scholar] [CrossRef] [PubMed]
- Pataro, A.L.; Costa, F.O.; Cortelli, S.C.; Cortelli, J.R.; Abreu, M.H.N.G.; Costa, J.E. Association between Severity of Body Mass Index and Periodontal Condition in Women. Clin. Oral Investig. 2012, 16, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, B.C.; Praveen, K.; Chandrashekar, B.R.; Rani, R.M.V.; Bhalla, A. Periodontal Infections: A Risk Factor for Various Systemic Diseases. Natl. Med. J. India 2011, 24, 214–219. [Google Scholar] [CrossRef]
- Lamster, I.B.; Pagan, M. Periodontal Disease and the Metabolic Syndrome. Int. Dent. J. 2017, 67, 67–77. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Kopra, E.; Pietiäinen, M.; Lehto, M.; Zaric, S.; Paju, S.; Salminen, A. Periodontitis and Cardiometabolic Disorders: The Role of Lipopolysaccharide and Endotoxemia. Periodontol. 2000 2022, 89, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, I.Z.; Debrey, S.; Oladubu, M.; Ugarte, R. Markers of Systemic Bacterial Exposure in Periodontal Disease and Cardiovascular Disease Risk: A Systematic Review and Meta-Analysis. J. Periodontol. 2007, 78, 2289–2302. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Bissett, S.M. Periodontitis and Diabetes. Br. Dent. J. 2019, 227, 577–584. [Google Scholar] [CrossRef]
- Perunovic, N.D.; Rakic, M.M.; Nikolic, L.I.; Jankovic, S.M.; Aleksic, Z.M.; Plecas, D.V.; Madianos, P.N.; Cakic, S.S. The Association Between Periodontal Inflammation and Labor Triggers (Elevated Cytokine Levels) in Preterm Birth: A Cross-Sectional Study. J. Periodontol. 2016, 87, 248–256. [Google Scholar] [CrossRef]
- Martinez-Herrera, M.; Silvestre-Rangil, J.; Silvestre, F.J. Association between Obesity and Periodontal Disease. A Systematic Review of Epidemiological Studies and Controlled Clinical Trials. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e708–e715. [Google Scholar] [CrossRef]
- Čolak, D.; Pintar, T.; Cmok Kučič, A.; Salobir, J.; Gašpirc, B.; Gašperšič, R. Periodontal and Hepatic Parameters in Obese Patients Undergoing Bariatric Surgery. Oral Health Prev. Dent. 2022, 20, 295–304. [Google Scholar] [CrossRef]
- Giuffrè, M.; Campigotto, M.; Campisciano, G.; Comar, M.; Crocè, L.S. A Story of Liver and Gut Microbes: How Does the Intestinal Flora Affect Liver Disease? A Review of the Literature. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G889–G906. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Premnath, A.; Arunima, P.R.; Kassim, R.M. Critical Appraisal of Bidirectional Relationship between Periodontitis and Hyperlipidemia. J. Int. Soc. Prev. Community Dent. 2019, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Suvan, J.; Deschner, J. The Association of Periodontal Diseases with Metabolic Syndrome and Obesity. Periodontology 2000, 83, 125–153. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Yamazaki, K. Can Oral Bacteria Affect the Microbiome of the Gut? J. Oral Microbiol. 2019, 11, 1586422. [Google Scholar] [CrossRef] [PubMed]
- Page, R.C. The Pathobiology of Periodontal Diseases May Affect Systemic Diseases: Inversion of a Paradigm. Ann. Periodontol. 1998, 3, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Yamazaki, Y.; Mita, A.; Takada, K.; Seto, M.; Nishinoue, N.; Sasaki, Y.; Motohashi, M.; Maeno, M. A Cohort Study on the Association between Periodontal Disease and the Development of Metabolic Syndrome. J. Periodontol. 2010, 81, 512–519. [Google Scholar] [CrossRef]
- Bengtsson, V.W.; Persson, G.R.; Berglund, J.S.; Renvert, S. Periodontitis Related to Cardiovascular Events and Mortality: A Long-Time Longitudinal Study. Clin. Oral Investig. 2021, 25, 4085–4095. [Google Scholar] [CrossRef]
- Montero, E.; López, M.; Vidal, H.; Martínez, M.; Marrero, J.; Herrera, D.; Zapatero, A.; Sanz, M. Impact of Periodontal Therapy on Systemic Markers of Inflammation in Patients with Metabolic Syndrome: A Randomized Clinical Trial. Diabetes Obes. Metab. 2020, 22, 2120–2132. [Google Scholar] [CrossRef]
- Czesnikiewicz-Guzik, M.; Osmenda, G.; Siedlinski, M.; Nosalski, R.; Pelka, P.; Nowakowski, D.; Wilk, G.; Mikolajczyk, T.P.; Schramm-Luc, A.; Furtak, A.; et al. Causal Association between Periodontitis and Hypertension: Evidence from Mendelian Randomization and a Randomized Controlled Trial of Non-Surgical Periodontal Therapy. Eur. Heart J. 2019, 40, 3459–3470. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Taylor, J.J.; Jaedicke, K.M.; De Jager, M.; Bikker, J.W.; Selten, W.; Bissett, S.M.; Whall, K.M.; van de Merwe, R.; Areibi, A.; et al. Treatment of Periodontitis Reduces Systemic Inflammation in Type 2 Diabetes. J. Clin. Periodontol. 2020, 47, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Mercanoglu, F.; Oflaz, H.; Öz, O.; Gökbuget, A.Y.; Genchellac, H.; Sezer, M.; Nişanci, Y.; Umman, S. Endothelial Dysfunction in Patients With Chronic Periodontitis and Its Improvement After Initial Periodontal Therapy. J. Periodontol. 2004, 75, 1694–1700. [Google Scholar] [CrossRef]
- Cao, R.; Li, Q.; Wu, Q.; Yao, M.; Chen, Y.; Zhou, H. Effect of Non-Surgical Periodontal Therapy on Glycemic Control of Type 2 Diabetes Mellitus: A Systematic Review and Bayesian Network Meta-Analysis. BMC Oral Health 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.W.; Li, X.X.; Xu, H.Z.; Gong, Y.Q.; Yang, Y. Effects of Periodontal Therapy on Serum Lipid Profile and Proinflammatory Cytokines in Patients with Hyperlipidemia: A Randomized Controlled Trial. Clin. Oral Investig. 2016, 20, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Suvan, J.; Masi, S.; Harrington, Z.; Santini, E.; Raggi, F.; D’Aiuto, F.; Solini, A. Effect of Treatment of Periodontitis on Incretin Axis in Obese and Nonobese Individuals: A Cohort Study. J. Clin. Endocrinol. Metab. 2021, 106, E74–E82. [Google Scholar] [CrossRef] [PubMed]
- MarquezZampiva, M.M.; de Paula, H.; Cheah, C.W.; Neto, T.P.T.; Nakamura, K.K.; Ueda, J.; Nassar, P.O.; Nassar, C.A. Clinical Evaluation of Obesity In Patients with Type 2 Diabetes Mellitus after Periodontal Treatment: A Comparative Study. J. Int. Acad. Periodontol. 2019, 21, 132–138. [Google Scholar]
- Gerber, F.A.; Sahrmann, P.; Schmidlin, O.A.; Heumann, C.; Beer, J.H.; Schmidlin, P.R. Influence of Obesity on the Outcome of Non-Surgical Periodontal Therapy—A Systematic Review. BMC Oral Health 2016, 16. [Google Scholar] [CrossRef]
- Silva-Boghossian, C.M.; Dezonne, R.S. What Are the Clinical and Systemic Results of Periodontitis Treatment in Obese Individuals? Curr. Oral Heal. Reports 2021, 8, 48–65. [Google Scholar] [CrossRef]
- Martinez-Herrera, M.; López-Domènech, S.; Silvestre, F.J.; Silvestre-Rangil, J.; Bañuls, C.; Hernández-Mijares, A.; Rocha, M. Dietary Therapy and Non-Surgical Periodontal Treatment in Obese Patients with Chronic Periodontitis. J. Clin. Periodontol. 2018, 45, 1448–1457. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures—2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology. Endocr. Pract. 2019, 25, 1346–1359. [Google Scholar] [CrossRef]
- Picot, J.; Jones, J.; Colquitt, J.L.; Gospodarevskaya, E.; Loveman, E.; Baxter, L.; Clegg, A.J. The Clinical Effectiveness and Cost-Effectiveness of Bariatric (Weight Loss) Surgery for Obesity: A Systematic Review and Economic Evaluation. Health Technol. Assess. 2009, 13, 1–357. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Cohen, R.V. Bariatric/Metabolic Surgery to Treat Type 2 Diabetes in Patients With a BMI 35 kg/m2. Diabetes Care 2016, 39, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Kow, L.; Brown, W.; Welbourn, R.; Dixon, J.; Kinsman, R.; Walton, P. 5th IFSO Global Registry Report; Dendrite Clinical Systems Ltd.: Reading, UK, 2019; ISBN 978-1-9160207-3-3. [Google Scholar]
- Fouse, T.; Brethauer, S. Resolution of Comorbidities and Impact on Longevity Following Bariatric and Metabolic Surgery. Surg. Clin. N. Am. 2016, 96, 717–732. [Google Scholar] [CrossRef]
- Porcelli, I.C.S.; Roma, C.C.; Nunes, M.C.P.; Maciel, S.M.; Pascotto, R.C. Effects of Bariatric Surgery on the Oral Health of Patients. Int. J. Dent. Oral Health. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Castilho, A.V.S.S.; Foratori-Junior, G.A.; de Carvalho Sales-Peres, S.H. Bariatric Surgery Impact on Gastroesophageal Reflux and Dental Wear: A Systematic Review. Arq. Bras. Cir. Dig. 2019, 32, e1466. [Google Scholar] [CrossRef]
- de Moura-Grec, P.G.; Ceneviva, R.; de Souza Leite, C.V.; de Brito, G.B.; Marsicano, J.A.; Brienze, S.L.A.; Yamashita, J.M.; de Carvalho Sales-Peres, S.H.; Marsicano, J.A.; Ceneviva, R.; et al. Impact of Bariatric Surgery on Oral Health Conditions: 6-Months Cohort Study. Int. Dent. J. 2014, 64, 144–149. [Google Scholar] [CrossRef]
- Čolak, D.; Gašperšič, R.; Cmok Kučič, A.; Pintar, T.; Gašpirc, B.; Cmok Kučič, A.; Pintar, T.; Gašpirc, B. The Effect of Bariatric Surgery on Periodontal Health: Systematic Review and Meta-Analyses. Arch. Med. Sci. 2021, 17, 1118–1127. [Google Scholar] [CrossRef]
- de Carvalho Sales-Peres, S.H.; de Carvalho Sales-Peres, M.; Ceneviva, R.; Bernabé, E. Weight Loss after Bariatric Surgery and Periodontal Changes: A 12-Month Prospective Study. Surg. Obes. Relat. Dis. 2017, 13, 637–642. [Google Scholar] [CrossRef]
- De Carvalho Sales-Peres, S.H.; de Moura-Grec, P.G.; Yamashita, J.M.; Torres, E.A.; Dionísio, T.J.; De Souza Leite, C.V.; Sales-Peres, A.; Ceneviva, R.; de Carvalho Sales-Peres, S.H.; de Moura-Grec, P.G.; et al. Periodontal Status and Pathogenic Bacteria after Gastric Bypass: A Cohort Study. J. Clin. Periodontol. 2015, 42, 530–536. [Google Scholar] [CrossRef]
- Pataro, A.L.; Cortelli, S.C.; Abreu, M.H.N.G.; Cortelli, J.R.; Franco, G.C.N.; Aquino, D.R.; Cota, L.O.M.; Costa, F.O.; Pataro, A.L.; Cortelli, S.C.; et al. Frequency of Periodontal Pathogens and Helicobacter Pylori in the Mouths and Stomachs of Obese Individuals Submitted to Bariatric Surgery: A Cross-Sectional Study. J. Appl. Oral Sci. 2016, 24, 229–238. [Google Scholar] [CrossRef]
- Vargas, J.A.; Bonato, R.C.S.; Orenha, E.S.; Sales-Peres, S.H. Assessment of Alveolar Bone Pattern in Obese and Nonobese Women, before and after Bariatric Surgery: A Prospective Cohort Study. Arq. Bras. Cir. Dig. 2020, 33, e1501. [Google Scholar] [CrossRef] [PubMed]
- Ulker, I.; Yildiran, H. The Effects of Bariatric Surgery on Gut Microbiota in Patients with Obesity: A Review of the Literature. Biosci. Microbiota Food Health 2019, 38, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.; Pratt, J. Metabolic and Bariatric Surgery: Nutrition and Dental Considerations. J. Am. Dent. Assoc. 2015, 146, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Heifetz-Li, J.; Roknian, V.A.; Abdelsamie, S.; Mulligan, J.P. Clinical Considerations for the Implant-Seeking Post-Bariatric Surgery Patient: A Mini-Review. Oral Heal. Dent. Sci. 2021, 5, 1–6. [Google Scholar] [CrossRef]
- Franco, R.; Barlattani, J.; Perrone, M.A.; Basili, M.; Miranda, M.; Costacurta, M.; Gualtieri, P.; Pujia, A.; Merra, G.; Bollero, P. Obesity, Bariatric Surgery and Periodontal Disease: A Literature Update. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5036–5045. [Google Scholar] [CrossRef]
- Aznar, F.D.; Aznar, F.D.; Lauris, J.R.; Chaim, E.A.; Cazzo, E.; de Carvalho Sales-Peres, S.H. Dental Wear and Tooth Loss in Morbid Obese Patients after Bariatric Surgery. Arq. Bras. Cir. Dig. 2019, 32, e1458. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Almas, K. The Role of Nutrition in Periodontal Health: An Update. Nutrients 2016, 8, 530. [Google Scholar] [CrossRef]
- Lertpimonchai, A.; Rattanasiri, S.; Arj-Ong Vallibhakara, S.; Attia, J.; Thakkinstian, A. The Association between Oral Hygiene and Periodontitis: A Systematic Review and Meta-Analysis. Int. Dent. J. 2017, 67, 332–343. [Google Scholar] [CrossRef]
- Colles, S.L.; Dixon, J.B.; O’Brien, P.E. Grazing and Loss of Control Related to Eating: Two High-Risk Factors Following Bariatric Surgery. Obesity 2008, 16, 615–622. [Google Scholar] [CrossRef]
- Konings, G.; Drukker, M.; Mulkens, S.; Severeijns, R.; van Os, J.; Ponds, R. Postsurgical Compliance and Eating Behavior 5 Years After Surgery. Bariatr. Surg. Pract. Patient Care 2020, 15, 148–154. [Google Scholar] [CrossRef]
- Sarwer, D.B.; Dilks, R.J.; West-Smith, L. Dietary Intake and Eating Behavior after Bariatric Surgery: Threats to Weight Loss Maintenance and Strategies for Success. Surg. Obes. Relat. Dis. 2011, 7, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Krall, E.; Hayes, C.; Garcia, R. How Dentition Status and Masticatory Function Affect Nutrient Intake. J. Am. Dent. Assoc. 1998, 129, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Shimazaki, Y.; Nonoyama, T.; Tadokoro, Y. Number of Teeth, Oral Self-Care, Eating Speed, and Metabolic Syndrome in an Aged Japanese Population. J. Epidemiol. 2019, 29, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Taghat, N.; Werling, M.; Östberg, A.L. Oral Health–Related Quality of Life After Gastric Bypass Surgery. Obes. Surg. 2020, 30, 224–232. [Google Scholar] [CrossRef]
- Versteegden, D.P.A.; Van himbeeck, M.J.J.; Nienhuijs, S.W. Improvement in Quality of Life after Bariatric Surgery: Sleeve versus Bypass. Surg. Obes. Relat. Dis. 2018, 14, 170–174. [Google Scholar] [CrossRef]
- Meusel, D.R.D.Z.; Ramacciato, J.C.; Motta, R.H.L.; Brito Júnior, R.B.; Flório, F.M. Impact of the Severity of Chronic Periodontal Disease on Quality of Life. J. Oral Sci. 2015, 57, 87–94. [Google Scholar] [CrossRef]
- Centrella, L.M.; Boyd, L.D. Oral Health of Postbariatric Surgery Recipients. Bariatr. Surg. Pract. Patient Care 2020, 15, 106–109. [Google Scholar] [CrossRef]
- Čolak, D.; Cmok Kučič, A.; Pintar, T.; Gašpirc, B.; Gašperšič, R. Periodontal and Systemic Health of Morbidly Obese Patients Eligible for Bariatric Surgery: A Cross-Sectional Study. BMC Oral Health 2022, 22, 1–11. [Google Scholar] [CrossRef]
- Jaiswal, G.R.; Jain, V.K.; Dhodapkar, S.V.; Kumathalli, K.I.; Kumar, R.; Nemawat, A.; Jain, A. Impact of Bariatric Surgery and Diet Modification on Periodontal Status: A Six Month Cohort Study. J. Clin. Diagnostic Res. 2015, 9, ZC43–ZC45. [Google Scholar] [CrossRef]
- The Effects of Non-Surgical Periodontal Therapy in Patients Indicated for Bariatric Surgery-Full Text View-ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04653714 (accessed on 18 October 2022).
- Boyle, M.; Mahawar, K. One Anastomosis Gastric Bypass Performed with a 150-Cm Biliopancreatic Limb Delivers Weight Loss Outcomes Similar to Those with a 200-Cm Biliopancreatic Limb at 18–24 Months. Obes. Surg. 2020, 30, 1258–1264. [Google Scholar] [CrossRef]
- Brolin, R.E. Long Limb Roux En Y Gastric Bypass Revisited. Surg. Clin. N. Am. 2005, 85, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Dias Gonçalves, T.E.; Feres, M.; Zimmermann, G.S.; Faveri, M.; Figueiredo, L.C.; Braga, P.G.; Duarte, P.M. Effects of Scaling and Root Planing on Clinical Response and Serum Levels of Adipocytokines in Patients with Obesity and Chronic Periodontitis. J. Periodontol. 2015, 86, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Dupont, W.D.; Plummer, W.D. Power and Sample Size Calculations. A Review and Computer Program. Control. Clin. Trials 1990, 11, 116–128. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and Proposals for Recording Gingivitis and Plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar] [PubMed]
- Pudgar, P.; Povšič, K.; Čuk, K.; Seme, K.; Petelin, M.; Gašperšič, R. Probiotic Strains of Lactobacillus Brevis and Lactobacillus Plantarum as Adjunct to Non-Surgical Periodontal Therapy: 3-Month Results of a Randomized Controlled Clinical Trial. Clin. Oral Investig. 2021, 25, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L. Periodontal Health and Gingival Diseases and Conditions on an Intact and a Reduced Periodontium: Consensus Report of Workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S74–S84. [Google Scholar] [CrossRef]
- Slade, G.D. Derivation and Validation of a Short-Form Oral Health Impact Profile. Community Dent. Oral Epidemiol. 1997, 25, 284–290. [Google Scholar] [CrossRef]
- Rener-Sitar, K.; Asja Celebić, N.P.; Papić, M.; Sapundzhiev, D.; Kansky, A.; Marion, L.; Kopac, I.; Zaletel-Kragelj, L. The Slovenian Version of the Oral Health Impact Profile Questionnaire (OHIP-SVN): Translation and Psychometric Properties. Coll. Antropol. 2009, 33, 1177–1183. [Google Scholar]
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int. J. Sports Med. 2021, 42, 199–214. [Google Scholar] [CrossRef]
- Canning, K.L.; Brown, R.E.; Wharton, S.; Sharma, A.M.; Kuk, J.L. Edmonton Obesity Staging System Prevalence and Association with Weight Loss in a Publicly Funded Referral-Based Obesity Clinic. J. Obes. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Singh, S.P.; Barik, R.K. NonInvasive Biomarkers in Nonalcoholic Fatty Liver Disease: Are We There Yet? J. Clin. Exp. Hepatol. 2020, 10, 88–98. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Faghihimani, E.; Adibi, P. Nonalcoholic Fatty Liver Disease: Diagnostic Biomarkers. World J. Gastrointest. Pathophysiol. 2017, 8, 11–26. [Google Scholar] [CrossRef]
- Eddelbuettel, D.; François, R. Rcpp: Seamless R and C++ Integration. J. Stat. Softw. 2011, 40, 1–18. [Google Scholar] [CrossRef]
- Microsoft Corporation. Microsoft Excel. 2018. Available online: https://office.microsoft.com/excel (accessed on 18 October 2022).
- de Souza Porcelli, I.C.; Corsi, N.M.; Fracasso, M.D.L.C.; Pascotto, R.C.; Cardelli, A.A.M.; Poli-Frederico, R.C.; Nasser, D.; Maciel, S.M. Oral Health Promotion in Patients With Morbid Obesity After Gastroplasty: A Randomized Clinical Trial. Arq. Bras. Cir. Dig. 2019, 32, e1437. [Google Scholar] [CrossRef]
- Basher, S.S.; Saub, R.; Vaithilingam, R.D.; Safii, S.H.; Daher, A.M.; Al-Bayaty, F.H.; Baharuddin, N.A. Impact of Non-Surgical Periodontal Therapy on OHRQoL in an Obese Population, a Randomised Control Trial. Health Qual. Life Outcomes 2017, 15, 225. [Google Scholar] [CrossRef]
- Graziani, F.; Music, L.; Bozic, D.; Tsakos, G. Is Periodontitis and Its Treatment Capable of Changing the Quality of Life of a Patient? Br. Dent. J. 2019, 227, 621–625. [Google Scholar] [CrossRef]
- Bagewitz, I.C.; Söderfeldt, B.; Palmqvist, S.; Nilner, K. Oral prostheses and oral health-related quality of life: A survey study of an adult Swedish population. Int. J. Prosthodont. 2007, 20, 132–142. [Google Scholar] [CrossRef][Green Version]
- Kamal, Y.; O’Toole, S.; Bernabé, E. Obesity and Tooth Wear among American Adults: The Role of Sugar-Sweetened Acidic Drinks. Clin. Oral Investig. 2020, 24, 1379–1385. [Google Scholar] [CrossRef]
- Anbarserri, N.M.; Ismail, K.M.; Anbarserri, H.; Alanazi, D.; AlSaffan, A.D.; Baseer, M.A.; Shaheen, R. Impact of Severity of Tooth Loss on Oral-Health-Related Quality of Life among Dental Patients. J. Fam. Med. Prim. Care 2020, 9, 187–191. [Google Scholar] [CrossRef]
- Arboleda, S.; Pianeta, R.; Vargas, M.; Lafaurie, G.I.; Aldana-Parra, F.; Chaux, C.F. Impact of Bariatric Surgery on Periodontal Status in an Obese Cohort at One Year of Follow-up. Med. Int. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- Aljehani, Y.A. Risk Factors of Periodontal Disease: Review of the Literature. Int. J. Dent. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Maciejewski, M.L.; Arterburn, D.E.; Van Scoyoc, L.; Smith, V.A.; Yancy, W.S.; Weidenbacher, H.J.; Livingston, E.H.; Olsen, M.K. Bariatric Surgery and Long-Term Durability of Weight Loss. JAMA Surg. 2016, 151, 1046–1055. [Google Scholar] [CrossRef]
- Chang, W.W.; Hawkins, D.N.; Brockmeyer, J.R.; Faler, B.J.; Hoppe, S.W.; Prasad, B.M. Factors Influencing Long-Term Weight Loss after Bariatric Surgery. Surg. Obes. Relat. Dis. 2019, 15, 456–461. [Google Scholar] [CrossRef]
- Escalante-García, M.; López-Rosales, F.; Hernández-Escalante, V.M.; Martínez-Díaz, G.; Torres-Escalante, J.L.; Castro-Sansores, C.J. Remission of Metabolic Syndrome with Bariatric Surgery. Med. Interna México 2018, 34, 678–682. [Google Scholar] [CrossRef]
- Villarreal-Calderon, J.R.; Cuellar-Tamez, R.; Castillo, E.C.; Luna-Ceron, E.; García-Rivas, G.; Elizondo-Montemayor, L. Metabolic Shift Precedes the Resolution of Inflammation in a Cohort of Patients Undergoing Bariatric and Metabolic Surgery. Sci. Rep. 2021, 11, 12127. [Google Scholar] [CrossRef]
- Nora, M.; Guimarães, M.; Almeida, R.; Martins, P.; Gonçalves, G.; Santos, M.; Morais, T.; Freitas, C.; Monteiro, M.P. Excess Body Mass Index Loss Predicts Metabolic Syndrome Remission after Gastric Bypass. Diabetol. Metab. Syndr. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Gurav, A.N. Periodontitis and Insulin Resistance: Casual or Causal Relationship? Diabetes Metab. J. 2012, 36, 404–411. [Google Scholar] [CrossRef]
- Montero, E.; Iniesta, M.; Rodrigo, M.; Marín, M.J.; Figuero, E.; Herrera, D.; Sanz, M. Clinical and Microbiological Effects of the Adjunctive Use of Probiotics in the Treatment of Gingivitis: A Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2017, 44, 708–716. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.N.; Zhen, Z.; Pelekos, G.; Wu, M.Z.; Chen, Y.; Tonetti, M.; Tse, H.F.; Yiu, K.H.; Jin, L. A Randomized Controlled Trial of the Effects of Non-Surgical Periodontal Therapy on Cardiac Function Assessed by Echocardiography in Type 2 Diabetic Patients. J. Clin. Periodontol. 2020, 47, 726–736. [Google Scholar] [CrossRef]
- Seinost, G.; Wimmer, G.; Skerget, M.; Thaller, E.; Brodmann, M.; Gasser, R.; Bratschko, R.O.; Pilger, E. Periodontal Treatment Improves Endothelial Dysfunction in Patients with Severe Periodontitis. Am. Heart J. 2005, 149, 1050–1054. [Google Scholar] [CrossRef]
- Kuin, C.; den Ouden, F.; Brandts, H.; Deden, L.; Hazebroek, E.; van Borren, M.; de Boer, H. Treatment of Severe Protein Malnutrition After Bariatric Surgery. Obes. Surg. 2019, 29, 3095–3102. [Google Scholar] [CrossRef]
- Dursun, E.; Akalin, F.A.; Genc, T.; Cinar, N.; Erel, O.; Yildiz, B.O. Oxidative Stress and Periodontal Disease in Obesity. Medicine 2016, 95, e3136. [Google Scholar] [CrossRef]
- Thanakun, S.; Izumi, Y. Effect of Periodontitis on Adiponectin, C-Reactive Protein, and Immunoglobulin G Against Porphyromonas Gingivalis in Thai People With Overweight or Obese Status. J. Periodontol. 2015, 87, 566–576. [Google Scholar] [CrossRef]
- Balogh, B.; Somodi, S.; Tanyi, M.; Miszti, C.; Márton, I.; Kelentey, B. Follow-up Study of Microflora Changes in Crevicular Gingival Fluid in Obese Subjects After Bariatric Surgery. Obes. Surg. 2020, 30, 5157–5161. [Google Scholar] [CrossRef]
- Novljan, U.; Pintar, T. Small Intestinal Bacterial Overgrowth in Patients with Roux-En-Y Gastric Bypass and One-Anastomosis Gastric Bypass. Obes. Surg. 2022, 32, 4102–4109. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Maiorani, C.; Preda, C.; Chiesa, A.; Esposito, F.; Pascadopoli, M.; Scribante, A. Management of Gingival Bleeding in Periodontal Patients with Domiciliary Use of Toothpastes Containing Hyaluronic Acid, Lactoferrin, or Paraprobiotics: A Randomized Controlled Clinical Trial. Appl. Sci. 2021, 11, 8586. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home Oral Care of Periodontal Patients Using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: A Split-Mouth Randomized Clinical Trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in Non-Surgical Periodontal Therapy: Clinical and Microbiological Aspects in a 6-Month Follow-Up Domiciliary Protocol for Oral Hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Gallo, S.; Alovisi, M.; Lovati, E.; Mutti, E.; Scribante, A. Domiciliary Management of Periodontal Indexes and Glycosylated Hemoglobin (HbA1c) in Type 1 Diabetic Patients with Paraprobiotic-Based Toothpaste and Mousse: Randomized Clinical Trial. Appl. Sci. 2022, 12, 8610. [Google Scholar] [CrossRef]
- Weaver, C.M. Nutrition and Bone Health. Oral Dis. 2017, 23, 412–415. [Google Scholar] [CrossRef]
| Sociodemographic Characteristics | Test Group n = 14 | Control Group n = 12 | p Value |
|---|---|---|---|
| Age (years) | 51.8 (7.6) | 49.4 (11.1) | 0.6 |
| Woman | 71% 10/14 | 75% 9/12 | 1 |
| High level of education | 7/14 (50% (23–77%)) | 5/12 (42% (15–72%)) | 0.7 |
| Behavior habits | |||
| Smokers | 5/14 (36% (13–65%)) | 3/12 (25% (5–57%)) | 0.7 |
| Alcohol use | 1/14 (7% (0–34%)) | 1/12 (8% (0–38%)) | 1 |
| Regular physical activity | 5/14 (36% (13–65%)) | 6/12 (50% (23–71%)) | 0.7 |
| Regular dental visits | 9/14 (64% (35–87%)) | 7/12 (58% (28–85%)) | 1 |
| Proper daily oral hygiene | 6/14 (43% (18–71%)) | 4/12 (33% (10–65%)) | 0.7 |
| Primary reason for bariatric surgery to improve health | 6/14 (43% (18–71%)) | 8/12 (67% (35–90%)) | 0.3 |
| Obesity related parameters | Test group n = 14 | Control group n = 12 | |
| BMI (kg/m2) | 46.3 (5.4) | 49.6 (7) | 0.2 |
| Waist circumference (cm) | 131 (15) | 135 (8.6) | 0.4 |
| EOSS | |||
| 1 | 2/14 (14% (2–43%)) | 1/12 (7% (0–34%)) | 1 |
| 2 | 2/14 (14% (2–43%)) | 4/12 (29% (8–58%)) | |
| 3 | 9/14 (64% (35–87%)) | 6/12 (43% (18–71%)) | |
| 4 | 1/14 (7% (0–34%)) | 1/12 (7% (0–34%)) | |
| Periodontal and dental parameters | Test group n = 14 | Control group n = 12 | |
| Number of teeth missing | 4 (3–9.5) | 6.5 (2.5–10) | 0.8 |
| Denture present | 2/14 (14% (2–43%)) | 2/12 (17% (2–48%) | 1 |
| Number of crowns | 4 (0–5) | 1 (0–5.5) | 0.9 |
| Number of pontics | 0 (0–1) | 0 (0–1) | 0.6 |
| Periodontitis stage | |||
| I | 4/14 (29% (8–58%)) | 3/12 (21% (5–51%)) | 1 |
| II | 4/14 (29% (8–58%)) | 4/12 (29% (8–58%)) | |
| III | 4/14 (29% (8–58%)) | 3/12 (21% (5–51%)) | |
| IV | 2/14 (14% (2–43%)) | 2/12 (14% (2–43%)) | |
| Periodontitis grade | |||
| A | 2/14 (14% (2–43%)) | 2/12 (14% (2–43%)) | 1 |
| B | 7/14 (50% (23–77%)) | 5/12 (36% (13–65%)) | |
| C | 5/14 (36% (13–65%)) | 5/12 (36% (13–65%)) |
| Obesity Parameter | Pre-BS TG (n = 14) | Pre-BS CG (n = 12) | 3 m TG (n = 14) | 3 m CG (n = 12) | 6 m TG (n = 11) | 6 m CG (n = 9) | p-Value, ANOVA |
|---|---|---|---|---|---|---|---|
| BMI kg/m2 | 46.3 (5.4) | 49.6 (7) | 36 (5.6) # | 39 (5) # | 32 (6) # | 35 (7) # | <0.001 |
| Periodontal Parameters | Pre-BS TG (n = 14) | Pre-BS CG (n = 12) | 3 m TG (n = 14) | 3 m CG (n = 12) | 6 m TG (n = 11) | 6 m CG (n = 9) | p-Value, ANOVA |
|---|---|---|---|---|---|---|---|
| Bop % | 32.5 (26.5–42.8) | 37 (24.8–65.5) | 30.5 (20–41) | 52 (33–72) | 30 (20–33) * | 55 (51–70) | 0.015 |
| FMPI % | 37 (28–45) | 37 (21.5–77) | 25 (16–32) # | 38 (30–65) | 25 (8–28) * # | 60 (32–70) | 0.0022 |
| PPD mm | 3.2 (2.7–3.3) | 3.1 (2.9–3.5) | 2.8 (2.4–3.1) * | 3.4 (3.1–3.7) | 2.8 (2.4–3) * | 3.4 (3.2–3.8) | 0.0015 |
| %PPD > 4 mm | 19.5 (18–23.5) | 24 (14–27) | 4.5 (3.3–9.5) * # | 29 (11.5–33) | 5 (1.5–10.5) * # | 33 (22–36) | <0.001 |
| CAL mm | 1.3 (1–2) | 1.2 (0.7–2) | 1.3 (0.7–2.3) | 1.4 (1–2.2) | 1.5 (0.7–2.5) | 1.9 (0.7–2.9) | 0.9 |
| REC mm | 1.5 (1.2–1.5) | 1.2 (1–1.9) | 1.3 (1.2–1.5) | 1 (1–2) | 1.3 (1.1–1.5) | 1.5 (1–2) | 0.9 |
| OHIP-14 | 6 (3–9.5) | 7 (6–12) | 3.5 (2–5.75) | 6.5 (4–9.5) | 4 (2–9) | 15 (4–17) | 0.44 |
| Good oral hygiene practices | 43% (17–81%) | 33% (10–65%) | 79% (49–95%) | 58% (28–85%) | 82% (48–98%) | 67% (30–93%) | 0.09 (Fisher’s exact test) |
| Obesity-Related Parameters/Diseases | Pre-BS TG (n = 14) | Pre-BS CG (n = 12) | 3 m TG (n = 14) | 3 m CG (n = 12) | 6 m TG (n = 11) | 6 m CG (n = 9) | p-Value, Fisher’s Exact Test |
|---|---|---|---|---|---|---|---|
| MS | 12/14 (86% (57–98%)) | 9/12 (75% (43–95%)) | 6/14 (43% (18–71%)) * | 8/12 (67% (35–90%)) | 3/11 (27% (6–61%)) # | 5/9 (56% (21–86%)) | 0.036 |
| Diabetes | 7/14 (50% (23–77%)) | 9/12 (75% (43–95%)) | 2/14 (14% (2–43%)) | 5/12 (42% (15–72%)) | 1/11 (9% (0–41%)) # | 3/9 (33% (7–70%)) # | 0.008 |
| High cholesterol | 12/14 (86% (57–98%)) | 10/12 (83% (52–98%)) | 6/14 (43% (18–71%)) * | 7/12 (58% (28–85%)) | 4/11 (36% (11–69%)) # | 6/9 (67% (30–93%)) | 0.039 |
| High triglyceride | 7/14 (50% (23–77%)) | 7/12 (58% (28–85%)) | 4/14 (29% (8–58%)) | 6/12 (50% (21–79%)) | 2/11 (18% (2–52%)) | 2/9 (22% (3–60%)) | 0.21 |
| Hypertension | 11/14 (79% (49–95%)) | 7/12 (58% (28–85%)) | 7/14 (50% (23–77%)) | 6/12 (50% (21–79%)) | 3/11 (27% (6–61%)) # | 4/9 (44% (14–79%)) | 0.21 |
| Sleep apnea | 11/14 (79% (49–95%)) | 7/12 (58% (28–85%)) | 5/14 (36% (13–65%)) | 3/12 (25% (5–57%)) | 1/11 (9% (0–41%)) # | 1/9 (11% (0–48%)) # | 0.0014 |
| Joint and muscle problems | 10/14 (71% (43–92%)) | 7/12 (58% (28–85%)) | 6/14 (43% (18–71%)) | 7/12 (58% (28–85%)) | 4/11 (36% (11–69%)) # | 4/9 (44% (14–79%)) | 0.31 |
| Depression | 2/14 (14% (2–43%)) | 2/12 (17% (2–48%)) | 1/14 (7% (0–34%)) | 3/12 (25% (5–57%)) | 2/11 (18% (2–52%)) | 2/9 (22% (3–60%)) | 0.87 |
| GERD | 8/14 (57% (29–82%)) | 7/12 (58% (28–85%)) | 6/14 (43% (18–71%)) | 5/12 (42% (15–72%)) | 3/11 (27% (6–61%)) | 3/9 (33% (7–70%)) | 0.62 |
| Habits | Pre-BS TG (n = 14) | Pre-BS CG (n = 12) | 3 m TG (n = 14) | 3 m CG (n = 12) | 6 m TG (n = 11) | 6 m CG (n = 9) | p-value |
| Smoking | 5/14 (36% (13–65%)) | 3/12 (25% (5–57%)) | 1/14 (7% (0–34%)) | 1/12 (8% (0–38%)) | 1/11 (9% (0–41%)) | 2/9 (22% (3–60%)) | 0.36 |
| Alcohol consumption | 1/14 (7% (0–34%)) | 1/12 (8% (0–38%)) | 0/14 (0% (0–23%)) | 0/12 (0% (0–26%)) | 0/11 (0% (0–28%)) | 0/9 (0 (0–34%)) | 0.92 |
| Regular physical activity | 5/14 (36% (13–65%)) | 6/12 (50% (23–71%)) | 8/14 (57% (29–82%)) | 9/12 (58% (28–85%)) | 9/11 (82% (48–98%)) | 6/9 (67% (30–93%)) | 0.25 |
| Biomarker | Pre-BS TG (n = 11) | Pre-BS CG (n = 9) | 6 m TG (n = 11) | p Value Pre-BS vs. 6 m TG | 6 m CG (n = 9) | p-Value, Pre-BS vs. 6 m CG | p Value, TG vs. CG at 6 m |
|---|---|---|---|---|---|---|---|
| CRP (mg/L) | 12 (8–12.5) | 16 (11–21) | 3 (2.3–6.3) | 0.018 * | 4.5 (2.8–9.3) | 0.028 * | 0.46 |
| AST (ukat/L) | 0.4 (0.36–0.7) | 0.41 (0.28–0.47) | 0.47 (0.38–0.76) | 0.9 | 0.4 (0.37–0.48) | 0.62 | 0.38 |
| ALT (ukat/L) | 0.63 (0.47–0.79) | 0.54 (0.42–0.63) | 0.5 (0.35–0.60) | 0.29 | 0.55 (0.42–0.73) | 0.74 | 0.74 |
| GGT (ukat/L) | 0.34 (0.29–0.59) | 0.35 (0.29–0.38) | 0.27 (0.22–0.37) | 0.3 | 0.29 (0.23–0.35) | 0.6 | 0.85 |
| HDL (mmol/L) | 1.1 (1.1–1.4) | 1.2 (1–1.3) | 1.5 (1.2–1.7) | 0.4 | 1.1 (1–1.6) | 0.72 | 0.72 |
| LDL (mmol/L) | 3.6 (3–4) | 3 (2.9–3.7) | 2.2 (1.8–3.6) | 0.31 | 2.7 (2.4–3.3) | 0.72 | 0.44 |
| Triglyceride (mmol/L) | 1.5 (1.2–1.7) | 1.9 (1.5–2.3) | 1.2 (1.1–1.4) | 0.31 | 1 (0.9–1.5) | 0.1 | 0.44 |
| Albumin (g/L) | 45 (45–47) | 46 (42.3–48) | 42 (37–44) | 0.005 * | 44 (41–46) | 0.32 | 0.13 |
| Glucose (mmol/L) | 5.3 (4.6–5.5) | 6.6 (5.1–7.7) | 4.7 (4.4–5) | 0.09 | 5.2 (4.6–5.5) | 0.23 | 0.19 |
| HBA1c (%) | 5.7 (5.3–6) | 6.1 (5.1–6.8) | 5.4 (5.3–5.5) | 0.22 | 5.3 (5–5.7) | 0.54 | 0.69 |
| 3 Months Post BS | Data (n = 26) |
|---|---|
| 5–6 meals a day | 25/26 (96% (79–100%)) |
| Liquid diet after BS (weeks) | 4.4 (1) |
| Soft food diet (weeks) | 3 (1.8) |
| Solid food diet on 3-m control (yes %) | 25/26 (96% (79–100%)) |
| Taking supplements (yes %) | 25/26 (96% (79–100%)) |
| Vomiting at least 1xweek | 10/26 (38% (17–59%)) |
| How long did patients vomit | 3.5 (1.2) weeks after BS |
| Proton pump inhibitors | 26/26 (100% (86–100%)) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čolak, D.; Cmok Kučič, A.; Pintar, T.; Gašperšič, R. Periodontal Therapy in Bariatric Surgery Patients with Periodontitis: Randomized Control Clinical Trial. J. Clin. Med. 2022, 11, 6837. https://doi.org/10.3390/jcm11226837
Čolak D, Cmok Kučič A, Pintar T, Gašperšič R. Periodontal Therapy in Bariatric Surgery Patients with Periodontitis: Randomized Control Clinical Trial. Journal of Clinical Medicine. 2022; 11(22):6837. https://doi.org/10.3390/jcm11226837
Chicago/Turabian StyleČolak, Dejana, Alja Cmok Kučič, Tadeja Pintar, and Rok Gašperšič. 2022. "Periodontal Therapy in Bariatric Surgery Patients with Periodontitis: Randomized Control Clinical Trial" Journal of Clinical Medicine 11, no. 22: 6837. https://doi.org/10.3390/jcm11226837
APA StyleČolak, D., Cmok Kučič, A., Pintar, T., & Gašperšič, R. (2022). Periodontal Therapy in Bariatric Surgery Patients with Periodontitis: Randomized Control Clinical Trial. Journal of Clinical Medicine, 11(22), 6837. https://doi.org/10.3390/jcm11226837







