The Effects of Preserving the Diaphragm on Early and Late Outcome of Lung-Sparing Radical Surgery for Malignant Pleural Mesothelioma †
Abstract
1. Introduction
2. Method
2.1. Patient Selection
2.2. Data Collection
2.3. Diaphragm-Sparing Macroscopic Complete Resection: Operative Technique
2.4. Ethics
2.5. Statistical Analysis
3. Results
3.1. Pre-Operative Patient Characteristics
3.2. Impact of Diaphragm Preservation on Perioperative Clinical Outcome
3.3. Survival
4. Discussion
4.1. Summary of Results
4.2. Impact of Diaphragm Preservation on Perioperative Clinical Outcome
4.3. Limitations
4.4. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AL | Air Leak |
| CRS | Care Record Service |
| ECOG | Eastern Cooperative Oncology Group |
| EPD | Extended Pleurectomy/Decortication |
| FEV1 | Forced Expiratory Volume in the First Second |
| FVC | Forced Vital Capacity |
| HDU | High-Dependency Unit |
| IASLC | International Association for the Study of Lung Cancer |
| IMIG | International Mesothelioma Interest Group |
| ITU | Intensive Care Unit |
| LOS | Length of Stay |
| MCR | Macroscopic Complete Resection |
| MPM | Malignant Pleural Mesothelioma |
| NHS | National Health Service |
| OS | Overall Survival |
| PD | Pleurectomy/Decortication |
| PAL | Prolonged Air Leak |
| QoL | Quality of Life |
| SCTS | Society for Cardiothoracic Surgery in Great Britain and Ireland |
| SD | Standard Deviation |
References
- Sugarbaker, D.J. Macroscopic complete resection: The goal of primary surgery in multimodality therapy for pleural mesothelioma. J. Thorac. Oncol. 2006, 1, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Burt, B.M.; Cameron, R.B.; Mollberg, N.M.; Kosinski, A.S.; Schipper, P.H.; Shrager, J.B.; Vigneswaran, W.T. Malignant pleural mesothelioma and the Society of Thoracic Surgeons Database: An analysis of surgical morbidity and mortality. J. Thorac. Cardiovasc. Surg. 2014, 148, 30–35. [Google Scholar] [CrossRef]
- Rice, D.; Rusch, V.; Pass, H.; Asamura, H.; Nakano, T.; Edwards, J.; Giroux, D.J.; Hasegawa, S.; Kernstine, K.H.; Waller, D.; et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: A consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J. Thorac. Oncol. 2011, 6, 1304–1312. [Google Scholar] [PubMed]
- Sharkey, A.J.; Bilancia, R.; Tenconi, S.; Nakas, A.; Waller, D.A. The management of the diaphragm during radical surgery for malignant pleural mesothelioma. Eur. J. Cardio-Thorac. Surg. 2016, 50, 311–316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ethics, M.O. Ministry of Ethics.Co.UK—An Online Resource for Learning Medical Ethics and Law. Available online: http://www.ministryofethics.co.uk/ (accessed on 18 October 2022).
- Lapidot, M.; Mazzola, E.; Freyaldenhoven, S.; De León, L.E.; Jaklitsch, M.T.; Bueno, R. Postoperative Empyema after Pleurectomy Decortication for Malignant Pleural Mesothelioma. Ann. Thorac. Surg. 2021, 114, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, J.S.; Culligan, M.J.; Tsao, A.S.; Rusch, V.; Sepesi, B.; Pass, H.I.; Bueno, R.; Burt, B.; Sugarbaker, D.J.; de Perrot, M.; et al. A Proposed System Toward Standardizing Surgical-Based Treatments for Malignant Pleural Mesothelioma, from the Joint National Cancer Institute-International Association for the Study of Lung Cancer-Mesothelioma Applied Research Foundation Taskforce. J. Thorac. Oncol. 2019, 14, 1343–1353. [Google Scholar] [CrossRef]
- Bölükbas, S.; Eberlein, M.; Schirren, J. Prospective study on functional results after lung-sparing radical pleurectomy in the management of malignant pleural mesothelioma. J. Thorac. Oncol. 2012, 7, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, J.S.; Culligan, M.J.; Tsao, A.S.; Rusch, V.; Adusumilli, P.S.; Sepesi, B.; Pass, H.I.; Bueno, R.; Burt, B.; Sugarbaker, D.J.; et al. Reply to Waller et al. Standardizing Surgical Treatment for Mesothelioma. J. Thorac. Oncol. 2020, 15, e75–e77. [Google Scholar] [CrossRef] [PubMed]
- Lang-Lazdunski, L.; Bille, A.; Lal, R.; Cane, P.; McLean, E.; Landau, D.; Steele, J.; Spicer, J. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J. Thorac. Oncol. 2012, 7, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Waller, D.A.; Bilancia, R.; Bille, A.; Tenconi, S. Standardizing Surgical Treatment of Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2020, 15, e73–e74. [Google Scholar] [CrossRef] [PubMed]

| Parameter | EPD (n = 18) | PD (n = 28) | Total (n = 46) | p | |
|---|---|---|---|---|---|
| Age [years] | Mean (SD) | 68.78 (6.08) | 65.71 (8.73) | 66.91 (7.88) | p = 0.305 |
| Median (quartiles) | 69 (64.75–73) | 67 (60–71.25) | 68.5 (62–72) | ||
| Range | 69 (57–78) | 67 (45–78) | 68.5 (45–78) | ||
| Sex | Male | 18 (100.00%) | 22 (78.57%) | 40 (86.96%) | p = 0.068 |
| Female | 0 (0.00%) | 6 (21.43%) | 6 (13.04%) | ||
| Comorbidity | No | 5 (27.78%) | 11 (39.29%) | 16 (34.78%) | p = 0.629 |
| Yes | 13 (72.22%) | 17 (60.71%) | 30 (65.22%) | ||
| ECOG Performance Status | ECOG 0 | 5 (27.78%) | 19 (67.86%) | 24 (52.17%) | p = 0.019 * |
| ECOG 1 | 13 (72.22%) | 9 (32.14%) | 22 (47.83%) | ||
| Smoking | Never | 11 (61.11%) | 12 (42.86%) | 23 (50.00%) | p = 0.395 |
| Ex | 7 (38.89%) | 14 (50.00%) | 21 (45.65%) | ||
| Current | 0 (0.00%) | 2 (7.14%) | 2 (4.35%) | ||
| Tumour Maximum Thickness [mm] | Mean (SD) | 19.69 (17.9) | 11.36 (8.24) | 14.21 (12.81) | p = 0.073 |
| Median (quartiles) | 15 (8–25) | 9 (5–14) | 9.5 (5–18) | ||
| Range | 15 (5–64) | 9 (3–32) | 9.5 (3–64) | ||
| Unavailable | 5 | 3 | 8 | ||
| Parameter | EPD (n = 18) | PD (n = 28) | Total (n = 46) | p | |
|---|---|---|---|---|---|
| Laterality | Left | 13 (72.22%) | 7 (25.00%) | 20 (43.48%) | p = 0.004 * |
| Right | 5 (27.78%) | 21 (75.00%) | 26 (56.52%) | ||
| Histology | Epithelioid | 14 (77.78%) | 24 (85.71%) | 38 (82.61%) | p = 0.693 |
| Non-Epithelioid | 4 (22.22%) | 4 (14.29%) | 8 (17.39%) | ||
| T staging | T1 | 2 (11.11%) | 13 (46.43%) | 15 (32.61%) | p = 0.03 * |
| T2 | 5 (27.78%) | 8 (28.57%) | 13 (28.26%) | ||
| T3 | 10 (55.56%) | 6 (21.43%) | 16 (34.78%) | ||
| T4 | 1 (5.56%) | 1 (3.57%) | 2 (4.35%) | ||
| N staging | N0 | 12 (66.67%) | 20 (71.43%) | 32 (69.57%) | p = 0.989 |
| N1 | 6 (33.33%) | 8 (28.57%) | 14 (30.43%) | ||
| Regional lymph node status | Negative | 10 (55.56%) | 21 (75.00%) | 31 (67.39%) | p = 0.293 |
| Positive | 8 (44.44%) | 7 (25.00%) | 15 (32.61%) | ||
| Parameter | EPD (n = 18) | PD (n = 28) | Total (n = 46) | p | |
|---|---|---|---|---|---|
| Operation time [min] | Mean (SD) | 234.12 (56.55) | 190.82 (41.1) | 207.18 (51.48) | p = 0.007 * |
| Median (quartiles) | 220 (201–238) | 188 (164–213) | 202 (173–222) | ||
| Range | 220 (173–385) | 188 (112–271) | 202 (112–385) | ||
| Intra-Operative complications | No | 17 (94.44%) | 28 (100.00%) | 45 (97.83%) | p = 0.391 |
| Yes | 1 (5.56%) | 0 (0.00%) | 1 (2.17%) | ||
| Inotrope duration [days] | Mean (SD) | 1.39 (1.2) | 0.61 (0.63) | 0.91 (0.96) | p = 0.009 * |
| Median (quartiles) | 1 (1–2) | 1 (0–1) | 1 (0–1) | ||
| Range | 1 (0–5) | 1 (0–2) | 1 (0–5) | ||
| Exclusion | 0 | 0 | 0 | ||
| Post-Operative ITU/HDU stay [days] | Mean (SD) | 3.56 (1.31) | 3.08 (1.83) | 3.26 (1.65) | p = 0.177 |
| Median (quartiles) | 3 (3–4.25) | 3 (2–3.75) | 3 (2–4) | ||
| Range | 3 (2–6) | 3 (1–8) | 3 (1–8) | ||
| Exclusion | 2 | 2 | 4 | ||
| Duration of air leak [days] | Mean (SD) | 10.56 (4.62) | 5.92 (3.14) | 7.69 (4.36) | p = 0.001 * |
| Median (quartiles) | 10 (7–14) | 5.5 (4–7) | 7 (4–10.75) | ||
| Range | 10 (4–20) | 5.5 (2–13) | 7 (2–20) | ||
| Exclusion | 2 | 2 | 4 | ||
| Total length of stay [days] | Mean (SD) | 12.67 (5.38) | 8.92 (3.83) | 10.29 (4.76) | p = 0.034 * |
| Median (quartiles) | 13 (8–16) | 8 (7–9.75) | 9 (7–13) | ||
| Range | 13 (5–22) | 8 (4–23) | 9 (4–23) | ||
| Exclusion | 3 | 2 | 5 | ||
| Arrythmia | No | 9 (50.00%) | 18 (64.29%) | 27 (58.70%) | p = 0.513 |
| Yes | 9 (50.00%) | 10 (35.71%) | 19 (41.30%) | ||
| Chyle leak | No | 17 (94.44%) | 26 (92.86%) | 43 (93.48%) | p = 1 |
| Yes | 1 (5.56%) | 2 (7.14%) | 3 (6.52%) | ||
| Respiratory failure | No | 15 (83.33%) | 28 (100.00%) | 43 (93.48%) | p = 0.054 |
| Yes | 3 (16.67%) | 0 (0.00%) | 3 (6.52%) | ||
| Chest infection | No | 14 (77.78%) | 20 (71.43%) | 34 (73.91%) | p = 0.739 |
| Yes | 4 (22.22%) | 8 (28.57%) | 12 (26.09%) | ||
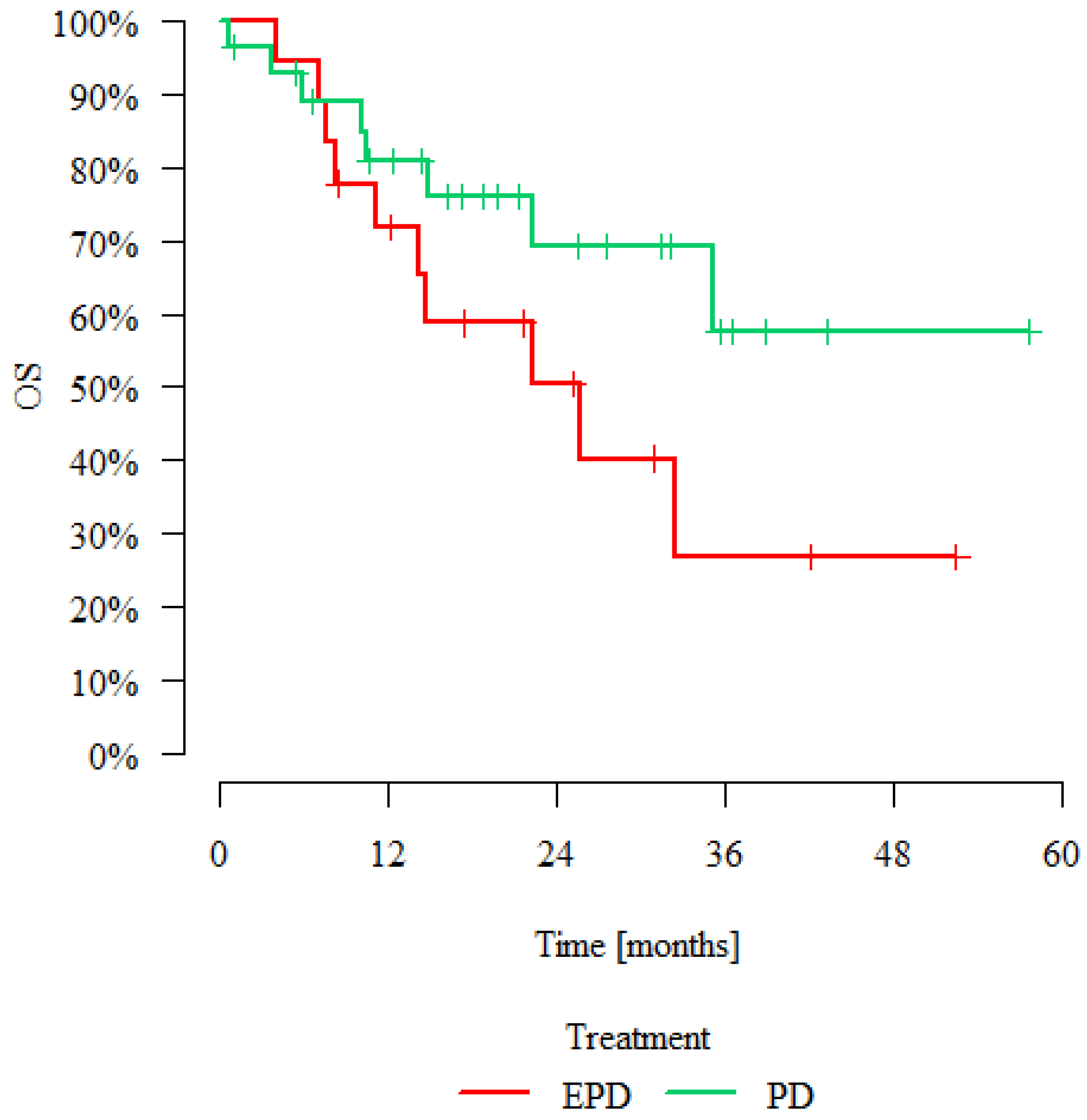
| Treatment | Patients | Deaths | Overall Survival | p | |||
|---|---|---|---|---|---|---|---|
| 12 Months | 24 Months | 36 Months | Median [Months] | ||||
| EPD | 18 | 10 | 71.79% | 50.35% | 26.85% | 25.66 | p = 0.123 |
| PD | 28 | 8 | 80.78% | 69.12% | 57.60% | >max obs. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Ventura, L.; Baranowski, R.; Hargrave, J.; Waller, D. The Effects of Preserving the Diaphragm on Early and Late Outcome of Lung-Sparing Radical Surgery for Malignant Pleural Mesothelioma. J. Clin. Med. 2022, 11, 6839. https://doi.org/10.3390/jcm11226839
Lee M, Ventura L, Baranowski R, Hargrave J, Waller D. The Effects of Preserving the Diaphragm on Early and Late Outcome of Lung-Sparing Radical Surgery for Malignant Pleural Mesothelioma. Journal of Clinical Medicine. 2022; 11(22):6839. https://doi.org/10.3390/jcm11226839
Chicago/Turabian StyleLee, Michelle, Luigi Ventura, Ralitsa Baranowski, Joanne Hargrave, and David Waller. 2022. "The Effects of Preserving the Diaphragm on Early and Late Outcome of Lung-Sparing Radical Surgery for Malignant Pleural Mesothelioma" Journal of Clinical Medicine 11, no. 22: 6839. https://doi.org/10.3390/jcm11226839
APA StyleLee, M., Ventura, L., Baranowski, R., Hargrave, J., & Waller, D. (2022). The Effects of Preserving the Diaphragm on Early and Late Outcome of Lung-Sparing Radical Surgery for Malignant Pleural Mesothelioma. Journal of Clinical Medicine, 11(22), 6839. https://doi.org/10.3390/jcm11226839






