Anemia and Iron Deficiency in Outpatients with Inflammatory Bowel Disease: Ubiquitous Yet Suboptimally Managed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Data Collection
2.3. Study Definitions
2.4. Questionnaire for Medical Professionals
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Prevalence of Anemia, Iron Deficiency, and Iron-Deficiency Anemia
3.3. Differences between Iron-Deficient and Iron-Sufficient Patients
3.4. Risk Factors for Anemia and Iron Deficiency
3.5. Iron Therapy in the Study Population
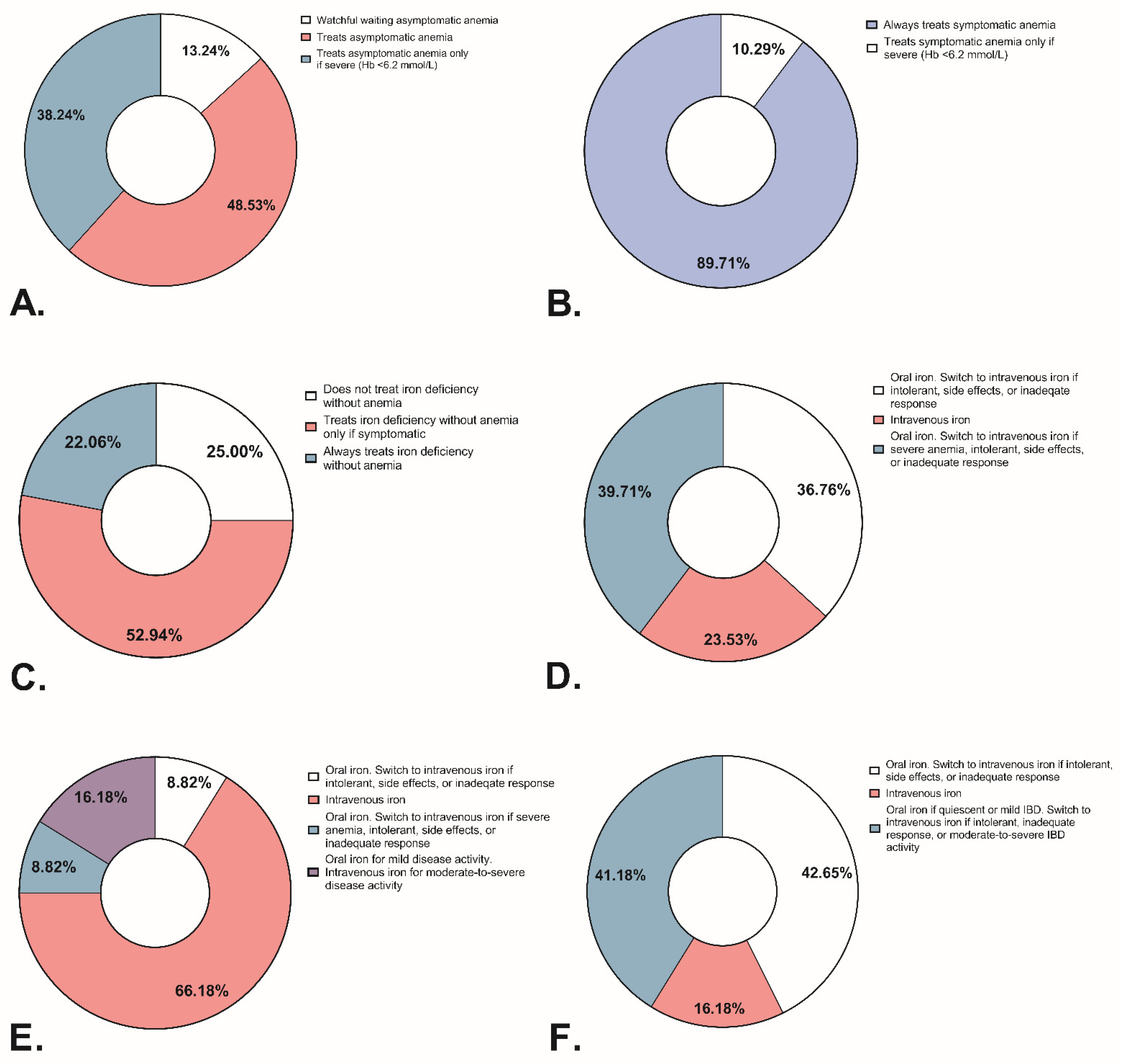
3.6. Iron Deficiency and Anemia Management: Responses from Medical Professionals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kulnigg, S.; Gasche, C. Systematic review: Managing anaemia in crohn’s disease. Aliment. Pharmacol. Ther. 2006, 24, 1507–1523. [Google Scholar] [CrossRef]
- Wilson, A.; Reyes, E.; Ofman, J. Prevalence and outcomes of anemia in inflammatory bowel disease: A systematic review of the literature. Am. J. Med. 2004, 116 (Suppl. 7A), 44s–49s. [Google Scholar] [CrossRef] [PubMed]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. 2017, 21 (Suppl. 1), S6–S20. [Google Scholar] [CrossRef] [Green Version]
- Dugan, C.; MacLean, B.; Cabolis, K.; Abeysiri, S.; Khong, A.; Sajic, M.; Richards, T.; Women’s Health research Collaborative. The misogyny of iron deficiency. Anaesthesia 2021, 76 (Suppl. 4), 56–62. [Google Scholar] [CrossRef] [PubMed]
- Gasche, C.; Lomer, M.C.E.; Cavill, I.; Weiss, G. Iron, anaemia, and inflammatory bowel diseases. Gut 2004, 53, 1190–1197. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Deguise, C.; Casellas, F.; Robles, V.; Navarro, E.; Borruel, N. Iron deficiency in the absence of anemia impairs the perception of health-related quality of life of patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2016, 22, 1450–1455. [Google Scholar] [CrossRef] [Green Version]
- Alayón, C.G.; Crespo, C.P.; Pedrosa, S.M.; Benítez, J.M.; Flores, E.I.; Rodríguez, I.S.; Medina, R.M.; García-Sánchez, V. Prevalence of iron deficiency without anaemia in inflammatory bowel disease and impact on health-related quality of life. Gastroenterol. Hepatol. 2018, 41, 22–29. [Google Scholar]
- Thuluvath, A.; Rayapati, D.; Limketkai, B.; Parian, A. Half of IBD patients without anemia have iron deficiency leading to similar rates of fatigue as iron deficiency anemia: 599. Off. J. Am. Coll. Gastroenterol.|ACG 2018, 113, S343. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Bermejo, F.; Pajares, R.; Pérez-Calle, J.-L.; Rodríguez, M.; Algaba, A.; Mancenido, N.; De La Morena, F.; Carneros, J.A.; McNicholl, A.G.; et al. Oral and intravenous iron treatment in inflammatory bowel disease: Hematological response and quality of life improvement. Inflamm. Bowel Dis. 2009, 15, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- García-López, S.; Bocos, J.M.; Gisbert, J.P.; Bajador, E.; Chaparro, M.; Castaño, C.; García-Erce, J.A.; Gomollón, F. High-dose intravenous treatment in iron deficiency anaemia in inflammatory bowel disease: Early efficacy and impact on quality of life. Blood Transfus. 2016, 14, 199–205. [Google Scholar]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J. Crohn’s Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef]
- Daude, S.; Remen, T.; Chateau, T.; Danese, S.; Gastin, I.; Baumann, C.; Gueant, J.L.; Peyrin-Biroulet, L. Comparative accuracy of ferritin, transferrin saturation and soluble transferrin receptor for the diagnosis of iron deficiency in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020, 51, 1087–1095. [Google Scholar] [CrossRef]
- Garg, M.; Chand, S.; Weenink, P.; Wu, K.Y.; Cheng, R.K.Y. Letter: Assessing iron deficiency in patients with IBD-a step in the right direction, but uncertainty remains. Aliment. Pharmacol. Ther. 2020, 52, 413–415. [Google Scholar] [CrossRef]
- Cacoub, P.; Choukroun, G.; Cohen-Solal, A.; Luporsi, E.; Peyrin-Biroulet, L.; Peoc’h, K.; Andrieu, V.; Lasocki, S.; Puy, H.; Trochu, J. Iron deficiency screening is a key issue in chronic inflammatory diseases: A call to action. J. Intern. Med. 2022, 292, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Gulhar, R.; Ashraf, M.A.; Jialal, I. Physiology, acute phase reactants. In StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Mahalhal, A.; Burkitt, M.; Duckworth, C.; Hold, G.; Campbell, B.; Pritchard, D.; Probert, C. Long-term iron deficiency and dietary iron excess exacerbate acute dextran sodium sulphate-induced colitis and are associated with significant dysbiosis. Int. J. Mol. Sci. 2021, 22, 3646. [Google Scholar] [CrossRef]
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.R.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.-F.; Gasche, C.; Geboes, K.; et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a working party of the 2005 montreal world congress of gastroenterology. Can. J. Gastroenterol. 2005, 19 (Suppl. A), 5a–36a. [Google Scholar] [CrossRef] [PubMed]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J. The montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooijkaas, H.M.K.; Souverijn, J.H.M.; Smeets, L.C. Tax GHM Handboek Medische Laboratoriumdiagnostiek; Prelum Uitgevers: Utrecht, The Netherlands, 2013. [Google Scholar]
- Nederlands Huisartsen Genootschap. Laboratoriumdiagnostiek Vitamine B12-Deficiëntie (LESA). May 2018. Available online: https://www.nhg.org/themas/publicaties/laboratoriumdiagnostiek-vitamine-b12-volledige-tekst (accessed on 18 October 2022).
- Nederlandse Vereniging voor Klinische Chemie en Laboratoriumgeneeskunde. Algemeen Overzicht Referentiewaarden. 11 October 2017. Available online: https://www.nvkc.nl/algemeen-overzicht-referentiewaarden (accessed on 18 October 2022).
- Testa, A.; Rispo, A.; Romano, M.; Riegler, G.; Selvaggi, F.; Bottiglieri, E.; Martorano, M.; Rea, M.; Gravina, A.; Nardone, O.M.; et al. The burden of anaemia in patients with inflammatory bowel diseases. Dig. Liver Dis. 2016, 48, 267–270. [Google Scholar] [CrossRef]
- Voegtlin, M.; Vavricka, S.R.; Schoepfer, A.M.; Straumann, A.; Voegtlin, J.; Rogler, G.; Ballabeni, P.; Pittet, V.; Buser, A.; Fried, M.; et al. Prevalence of anaemia in inflammatory bowel disease in Switzerland: A cross-sectional study in patients from private practices and university hospitals. J. Crohn’s Colitis 2010, 4, 642–648. [Google Scholar] [CrossRef] [Green Version]
- Bager, P.; Befrits, R.; Wikman, O.; Lindgren, S.; Moum, B.; Hjortswang, H.; Dahlerup, J.F. The prevalence of anemia and iron deficiency in IBD outpatients in Scandinavia. Scand. J. Gastroenterol. 2011, 46, 304–309. [Google Scholar] [CrossRef]
- Filmann, N.; Rey, J.; Schneeweiss, S.; Ardizzone, S.; Bager, P.; Bergamaschi, G.; Koutroubakis, I.; Lindgren, S.; Morena, F.D.L.; Moum, B.; et al. Prevalence of anemia in inflammatory bowel diseases in european countries: A systematic review and individual patient data meta-analysis. Inflamm. Bowel Dis. 2014, 20, 936–945. [Google Scholar] [CrossRef]
- Eriksson, C.; Henriksson, I.; Brus, O.; Zhulina, Y.; Nyhlin, N.; Tysk, C.; Montgomery, S.; Halfvarson, J. Incidence, prevalence and clinical outcome of anaemia in inflammatory bowel disease: A population-based cohort study. Aliment. Pharmacol. Ther. 2018, 48, 638–645. [Google Scholar] [CrossRef]
- Parra, R.S.; Feitosa, M.R.; Ferreira, S.D.C.; da Rocha, J.J.R.; Troncon, L.E.D.A.; Féres, O. Anemia and iron deficiency in inflammatory bowel disease patients in a referral center in Brazil: Prevalence and risk factors. Arq. Gastroenterol. 2020, 57, 272–277. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Bouguen, G.; Laharie, D.; Pellet, G.; Savoye, G.; Gilletta, C.; Michiels, C.; Buisson, A.; Fumery, M.; Trochu, J.-N.; et al. Iron deficiency in patients with inflammatory bowel diseases: A prospective multicenter cross-sectional study. Dig. Dis. Sci. 2022, 67, 5637–5646. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, G.; Castiglione, F.; D’Incà, R.; Astegiano, M.; Fries, W.; Milla, M.; Ciacci, C.; Rizzello, F.; Saibeni, S.; Ciccocioppo, R.; et al. Prevalence, pathogenesis and management of anemia in inflammatory bowel disease: An IG-IBD multicenter, prospective, and observational study. Inflamm. Bowel Dis. 2022, izac054. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, G.; Di Sabatino, A.; Albertini, R.; Ardizzone, S.; Biancheri, P.; Bonetti, E.; Cassinotti, A.; Cazzola, P.; Markopoulos, K.; Massari, A.; et al. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor- treatment. Haematologica 2010, 95, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Høivik, M.L.; Reinisch, W.; Cvancarova, M.; Moum, B.; the IBSEN study group. Anaemia in inflammatory bowel disease: A population-based 10-year follow-up. Aliment. Pharmacol. Ther. 2014, 39, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Lucendo, A.J.; Arias, Á.; Roncero, Ó.; Hervías, D.; Verdejo, C.; Naveas-Polo, C.; Bouhmidi, A.; Lorente, R.; Alcázar, L.M.; Salueña, I.; et al. Anemia at the time of diagnosis of inflammatory bowel disease: Prevalence and associated factors in adolescent and adult patients. Dig. Liver. Dis. 2017, 49, 405–411. [Google Scholar] [CrossRef]
- Bengi, G.; Keyvan, H.; Durmaz, S.B.; Akpınar, H. Frequency, types, and treatment of anemia in Turkish patients with inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 4186–4196. [Google Scholar] [CrossRef]
- Madanchi, M.; Fagagnini, S.; Fournier, N.; Biedermann, L.; Zeitz, J.; Battegay, E.; Zimmerli, L.; Vavricka, S.R.; Rogler, G.; Scharl, M.; et al. The Relevance of Vitamin and Iron Deficiency in Patients with Inflammatory Bowel Diseases in Patients of the Swiss IBD Cohort. Inflamm. Bowel Dis. 2018, 24, 1768–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupu, A.; Diculescu, M.; Diaconescu, R.; Tantau, M.; Tantau, A.; Visovan, I.; Gheorghe, C.; Lupei, C.; Gheorghe, L.; Cerban, R.; et al. Prevalence of anemia and iron deficiency in romanian patients with inflammatory bowel disease: A prospective multicenter study. J. Gastrointest. Liver Dis. 2015, 24, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foteinogiannopoulou, K.; Karmiris, K.; Axiaris, G.; Velegraki, M.; Gklavas, A.; Kapizioni, C.; Karageorgos, C.; Kateri, C.; Katsoula, A.; Kokkotis, G.; et al. The burden and management of anemia in Greek patients with inflammatory bowel disease: A retrospective, multicenter, observational study. BMC Gastroenterol. 2021, 21, 269. [Google Scholar]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Published 2011. Available online: https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf?ua=1 (accessed on 9 November 2022).
- Rokkam, V.R.; Kotagiri, R. Secondary thrombocytosis. In StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Stein, J.; Bager, P.; Befrits, R.; Gasche, C.; Gudehus, M.; Lerebours, E.; Magro, F.; Mearin, F.; Mitchell, D.; Oldenburg, B.; et al. Anaemia management in patients with inflammatory bowel disease: Routine practice across nine European countries. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1456–1463. [Google Scholar] [CrossRef]
- Schaefer, B.; Tobiasch, M.; Wagner, S.; Glodny, B.; Tilg, H.; Wolf, M.; Zoller, H. Hypophosphatemia after intravenous iron therapy: Comprehensive review of clinical findings and recommendations for management. Bone 2022, 154, 116202. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, N.U.; Zeder, C.; Brittenham, G.M.; Moretti, D.; Zimmermann, M.B. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica 2020, 105, 1232–1239. [Google Scholar] [CrossRef] [Green Version]
- Stoffel, N.U.; Cercamondi, C.I.; Brittenham, G.; Zeder, C.; Geurts-Moespot, A.J.; Swinkels, D.W.; Moretti, D.; Zimmermann, M.B. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: Two open-label, randomised controlled trials. Lancet Haematol. 2017, 4, e524–e533. [Google Scholar] [CrossRef]
- Glaspy, J.A.; Wolf, M.; Strauss, W.E. Intravenous iron-induced hypophosphatemia: An emerging syndrome. Adv. Ther. 2021, 38, 3531–3549. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Gomollon, F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am. J. Gastroenterol. 2008, 103, 1299–1307. [Google Scholar] [CrossRef]


| IBD (n = 2197) | CD (n = 1271) | UC (n = 926) | p-Value | Available Data (n) | |
|---|---|---|---|---|---|
| Gender: female (n, %) | 1272 (57.9%) | 780 (61.4%) | 492 (53.1%) | p < 0.001 | 2197 |
| Age (years) | 44.0 [31.0–57.0] | 42.0 [29.0–55.0] | 48.0 [33.0–61.0] | p < 0.001 | 2196 |
| Age at diagnosis | p < 0.001 | 2142 | |||
| <17 years old | 285 (13.3%) | 212 (17.1%) | 73 (8.1%) | ||
| 17–40 years old | 1292 (60.3%) | 777 (62.7%) | 515 (57.0%) | ||
| >40 years old | 565 (26.4%) | 250 (20.2%) | 315 (34.9%) | ||
| Disease location | 1245 | ||||
| Terminal ileum | 464 (37.3%) | ||||
| Colon | 260 (20.9%) | ||||
| Ileocolon | 521 (41.8%) | ||||
| upper GI-involvement * | 112 (9.8%) | 1138 | |||
| Disease behavior | 1113 | ||||
| Inflammatory | 652 (58.6%) | ||||
| Stricturing | 318 (28.6%) | ||||
| Penetrating | 143 (12.8%) | ||||
| Perianal disease ** | 284 (24.9%) | 1142 | |||
| Disease extension | 885 | ||||
| Ulcerative proctitis | 123 (13.9%) | ||||
| Left-sided colitis | 347 (39.2%) | ||||
| Pancolitis | 415 (46.9%) | ||||
| Anemia (n, %) | 393 (18.0%) | 236 (18.7%) | 157 (17.0%) | NS | 2186 |
| Females | 183 (14.5%) | 124 (16.0%) | 59 (12.0%) | p < 0.05 | |
| Males | 210 (22.8%) | 112 (22.9%) | 98 (22.8%) | NS | |
| Iron deficiency (n, %) | 566 (43.4%) | 333 (43.4%) | 233 (43.5%) | NS | 1303 |
| Females | 396 (51.8%) | 236 (49.8%) | 160 (55.2%) | NS | |
| Males | 170 (31.5%) | 97 (33.1%) | 73 (29.7%) | NS | |
| Iron-deficiency anemia (n, %) | 159 (12.2%) | 95 (12.4%) | 64 (11.9%) | NS | 1303 |
| Females | 90 (11.8%) | 59 (12.4%) | 31 (10.7%) | NS | |
| Males | 69 (12.8%) | 36 (12.3%) | 33 (13.4%) | NS | |
| Biochemical inflammation (n, %) | 848 (39.8%) | 526 (42.3%) | 322 (36.3%) | p < 0.01 | 2132 |
| Females | 521 (42.1%) | 340 (44.7%) | 181 (38.0%) | p < 0.05 | |
| Males | 327 (36.5%) | 186 (38.5%) | 141 (34.2%) | NS | |
| Iron deficiency | 384 (70.8%) | 223 (68.0%) | 161 (75.2%) | NS | 1303 |
| Anemia | 201 (23.7%) | 126 (24.0%) | 75 (23.3%) | NS | 2186 |
| Iron-Deficient (n = 566) | Iron-Sufficient (n = 737) | p-Value | Missing Data (n, %) | |
|---|---|---|---|---|
| Hemoglobin (mmol/L) | 8.20 [7.70–8.80] | 8.80 [8.20–9.40] | p < 0.001 | 1 (0.1%) |
| Females | 8.10 [7.50–8.50] | 8.30 [7.90–8.80] | p < 0.001 | |
| Males | 8.70 [8.00–9.20] | 9.30 [8.60–9.70] | p < 0.001 | |
| Hematocrit (%) | 0.41 [0.38–0.43] | 0.43 [0.40–0.46] | p < 0.001 | 129 (9.9%) |
| MCV (fL) | 90.00 [86.00–93.00] | 92.00 [89.00–96.00] | p < 0.001 | 6 (0.5%) |
| WBC (×109/L) | 7.50 [6.30–9.40] | 6.80 [5.60–8.30] | p < 0.001 | 18 (1.4%) |
| Platelets (×109/L) | 310.00 [262.00–362.00] | 267.00 [226.00–312.00] | p < 0.001 | 19 (1.5%) |
| LDH (U/L) | 187.00 [156.00–205.50] | 177.00 [149.50–199.00] | NS | 1089 (83.6%) |
| CRP (mg/L) | 3.80 [1.00–8.00] | 2.00 [1.00–4.00] | p < 0.001 | 20 (1.5%) |
| FCP (mg/kg) | 291.00 [84.00–924.25] | 55.00 [22.00–156.25] | p < 0.001 | 573 (44.0%) |
| Ferritin (µg/L) | 27.00 [16.00–52.50] | 112.00 [66.00–178.00] | p < 0.001 | 29 (2.2%) |
| Tsat (%) | 17.00 [11.00–23.75] | 27.00 [20.00–35.00] | p < 0.001 | 891 (68.4%) |
| Iron (µmol/L) | 11.25 [7.00–15.98] | 16.70 [13.00–21.35] | p < 0.001 | 830 (63.7%) |
| Transferrin (g/L) | 2.80 [2.50–3.10] | 2.40 [2.20–2.70] | p < 0.001 | 930 (71.4%) |
| TIBC (µmol/L) | 72.20 (± 11.27) | 62.00 [57.00–68.00] | p < 0.001 | 1088 (83.5%) |
| Folic acid (nmol/L) | 13.00 [8.90–21.55] | 15.20 [11.15–23.13] | NS | 1011 (77.6%) |
| Vitamin B12 (pmol/L) | 336.00 [240.00–483.00] | 344.00 [252.00–468.00] | NS | 753 (57.8%) |
| Anemia | ||||
|---|---|---|---|---|
| Univariable OR (95% CI) | p-Value | Multivariable OR (95% CI) | p-Value | |
| Gender (male reference) | 0.57 [0.46–0.71] | p < 0.001 | 0.43 [0.28–0.66] | p < 0.001 |
| Age (years) | 1.01 [1.00–1.02] | p < 0.01 | 1.02 [1.01–1.04] | p < 0.001 |
| MCV (fL) | 0.94 [0.92–0.96] | p < 0.001 | 0.94 [0.91–0.97] | p < 0.001 |
| Log2 WBC (×109/L) | 1.01 [0.80–1.28] | NS | ||
| Log2 Platelets (×109/L) | 2.12 [1.61–2.78] | p < 0.001 | 2.17 [1.25–3.79] | p < 0.01 |
| Log2 Ferritin (µg/L) | 0.74 [0.68–0.81] | p < 0.001 | 0.75 [0.65–0.87] | p < 0.001 |
| Tsat (%) | 0.92 [0.90–0.94] | p < 0.001 | ||
| Log2 CRP (mg/L) | 1.23 [1.15–1.32] | p < 0.001 | ||
| Log2 FCP (mg/kg) | 1.23 [1.15–1.32] | p < 0.001 | 1.18 [1.08–1.28] | p < 0.001 |
| Iron Deficiency | ||||
| Univariable OR (95% CI) | p-Value | Multivariable OR (95% CI) | p-Value | |
| Gender (male reference) | 2.34 [1.86–2.94] | p < 0.001 | 2.63 [1.84–3.75] | p < 0.001 |
| Age (years) | 0.97 [0.97–0.98] | p < 0.001 | 0.98 [0.97–0.99] | p < 0.001 |
| MCV (fL) | 0.92 [0.90–0.93] | p < 0.001 | 0.94 [0.91–0.97] | p < 0.001 |
| Log2 WBC (×109/L) | 2.13 [1.67–2.72] | p < 0.001 | ||
| Log2 Platelets (×109/L) | 3.79 [2.80–5.14] | p < 0.001 | 1.85 [1.16–2.95] | p < 0.01 |
| Log2 CRP (mg/L) | 1.31 [1.22–1.41] | p < 0.001 | ||
| Log2 FCP (mg/kg) | 1.39 [1.30–1.49] | p < 0.001 | 1.39 [1.29–1.50] | p < 0.001 |
| Iron-Deficiency Anemia | ||||
| Univariable OR (95% CI) | p-Value | Multivariable OR (95% CI) | p-Value | |
| Gender (male reference) | 0.91 [0.65–1.27] | NS | ||
| Age (years) | 1.00 [0.99–1.01] | NS | ||
| MCV (fL) | 0.85 [0.83–0.88] | p < 0.001 | 0.87 [0.84–0.91] | p < 0.001 |
| Log2 WBC (×109/L) | 1.49 [1.04–2.12] | p < 0.05 | ||
| Log2 Platelets (×109/L) | 3.52 [2.30–5.40] | p < 0.001 | ||
| Log2 CRP (mg/L) | 1.26 [1.15–1.39] | p < 0.001 | ||
| Log2 FCP (mg/kg) | 1.37 [1.25–1.51] | p < 0.001 | 1.35 [1.22–1.49] | p < 0.001 |
| Received Iron Therapy (n = 204) | No Iron Therapy (n = 1778) | p-Value | Available Data (n, %) | |
|---|---|---|---|---|
| Gender: female (n, %) | 125 (61.3%) | 1025 (57.6%) | NS | 1982 (100.0%) |
| Biochemical inflammation (n, %) | 114 (59.1%) | 657 (38.3%) | p < 0.001 | 1908 (96.3%) |
| Anemia (n, %) | 108 (54.3%) | 254 (14.5%) | p < 0.001 | 1949 (98.3%) |
| Iron-deficiency anemia (n, %) | 71 (46.7%) | 53 (6.9%) | p < 0.001 | 915 (46.2%) |
| Iron deficiency (n, %) | 120 (78.4%) | 280 (36.7%) | p < 0.001 | 916 (46.2%) |
| Functional iron deficiency (n, %) | 8 (22.2%) | 14 (14.3%) | NS | 134 (6.8%) |
| Folic acid deficiency (n, %) | 1 (2.0%) | 2 (0.9%) | NS | 263 (13.3%) |
| Vitamin B12 deficiency (n, %) | 5 (7.6%) | 22 (6.0%) | NS | 433 (21.8%) |
| Oral iron (n, %) | 90 (44.1%) | NA | NA | 204 (100.0%) |
| Intravenous iron (n, %) | 104 (51.0%) | NA | NA | 204 (100.0%) |
| Combined therapy (n, %) | 10 (4.9%) | NA | NA | 204 (100.0%) |
| Phosphate measurement * (n, %) | 6 (5.8%) | NA | NA | 114 (100.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loveikyte, R.; Boer, M.; van der Meulen, C.N.; ter Steege, R.W.F.; Tack, G.; Kuyvenhoven, J.; Jharap, B.; Vu, M.K.; Vogelaar, L.; West, R.L.; et al. Anemia and Iron Deficiency in Outpatients with Inflammatory Bowel Disease: Ubiquitous Yet Suboptimally Managed. J. Clin. Med. 2022, 11, 6843. https://doi.org/10.3390/jcm11226843
Loveikyte R, Boer M, van der Meulen CN, ter Steege RWF, Tack G, Kuyvenhoven J, Jharap B, Vu MK, Vogelaar L, West RL, et al. Anemia and Iron Deficiency in Outpatients with Inflammatory Bowel Disease: Ubiquitous Yet Suboptimally Managed. Journal of Clinical Medicine. 2022; 11(22):6843. https://doi.org/10.3390/jcm11226843
Chicago/Turabian StyleLoveikyte, Roberta, Menno Boer, Catharina N. van der Meulen, Rinze W. F. ter Steege, Greetje Tack, Johan Kuyvenhoven, Bindia Jharap, My K. Vu, Lauran Vogelaar, Rachel L. West, and et al. 2022. "Anemia and Iron Deficiency in Outpatients with Inflammatory Bowel Disease: Ubiquitous Yet Suboptimally Managed" Journal of Clinical Medicine 11, no. 22: 6843. https://doi.org/10.3390/jcm11226843
APA StyleLoveikyte, R., Boer, M., van der Meulen, C. N., ter Steege, R. W. F., Tack, G., Kuyvenhoven, J., Jharap, B., Vu, M. K., Vogelaar, L., West, R. L., van der Marel, S., Römkens, T. E. H., Mujagic, Z., Hoentjen, F., van Bodegraven, A. A., van Schaik, F. D. M., de Vries, A. C., Dijkstra, G., & van der Meulen-de Jong, A. E., on behalf of the Dutch Initiative on Crohn and Colitis (ICC). (2022). Anemia and Iron Deficiency in Outpatients with Inflammatory Bowel Disease: Ubiquitous Yet Suboptimally Managed. Journal of Clinical Medicine, 11(22), 6843. https://doi.org/10.3390/jcm11226843






