Changes in Serum Creatinine May Cause Hypoglycemia among Non-Critically Ill Patients Admitted to Internal Medicine Units
Abstract
:1. Introduction
2. Methods
3. Results
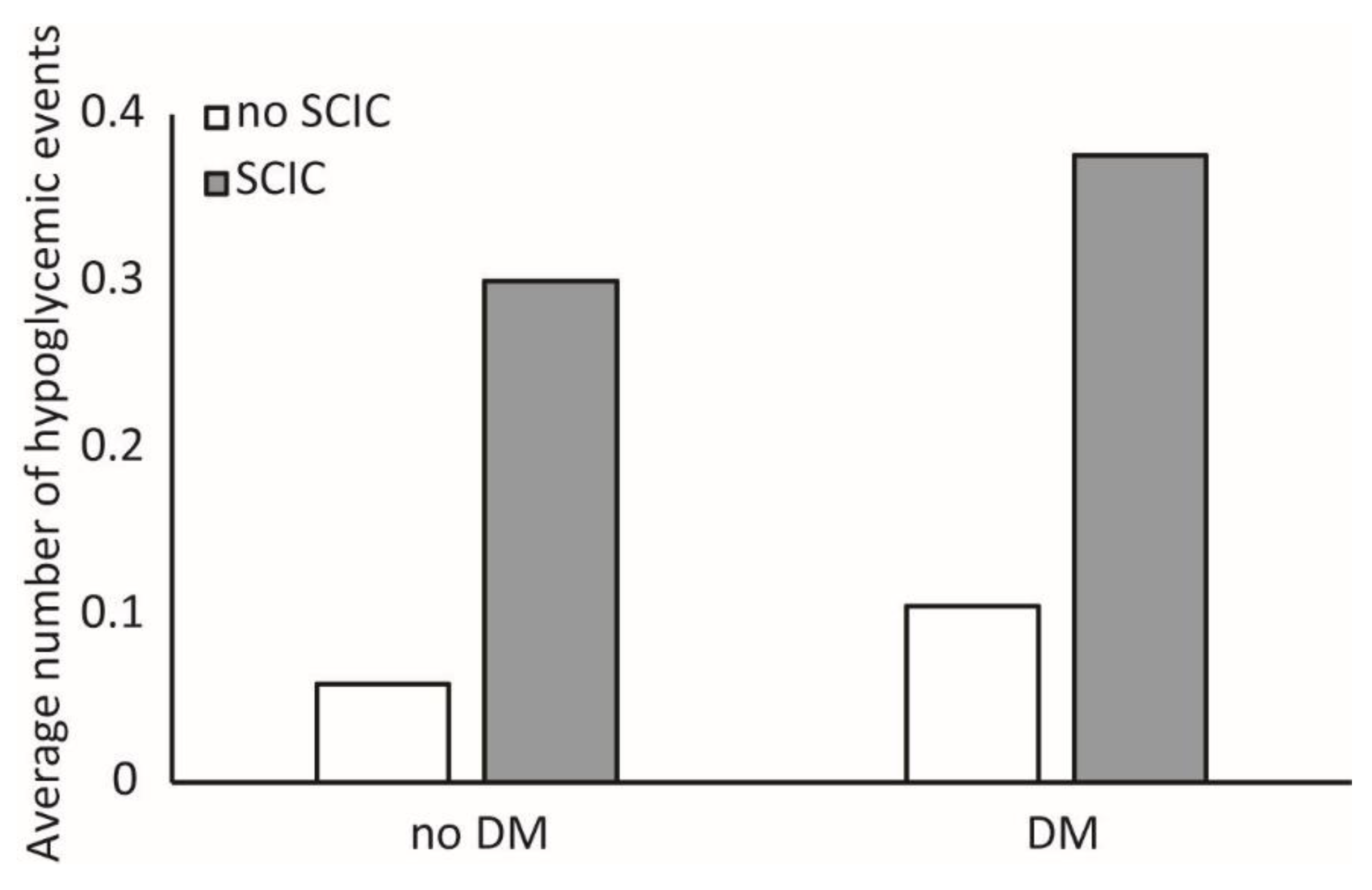
3.1. Association between SCIC Status and Hypoglycemia
3.2. Association between Hypoglycemia and SCIC Magnitude
3.3. Ascertaining Timing of SCIC and Hypoglycemia
3.4. Association between Age, Incident Hypoglycemia and SCIC
3.5. “Deteriorating” SCIC vs. “Improving” SCIC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegelaar, S.E.; Hermanides, J.; Oudemans-van Straaten, H.M.; van der Voort, P.H.; Bosman, R.J.; Zandstra, D.F.; DeVries, J.H. Mean glucose during ICU admission is related to mortality by a U-shaped curve in surgical and medical patients: A retrospective cohort study. Crit. Care 2010, 14, R224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendez, C.E.; Mok, K.-T.; Ata, A.; Tanenberg, R.J.; Calles-Escandon, J.; Umpierrez, G.E. Increased Glycemic Variability Is Independently Associated With Length of Stay and Mortality in Noncritically Ill Hospitalized Patients. Diabetes Care 2013, 36, 4091–4097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodovicz, K.G.; Mehta, V.; Zhang, Q.; Zhao, C.; Davies, M.J.; Chen, J.; Radican, L.; Engel, S.S. Association between hypoglycemia and inpatient mortality and length of hospital stay in hospitalized, insulin-treated patients. Curr. Med. Res. Opin. 2013, 29, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Humphrey, L.L.; Chou, R.; Snow, V.; Shekelle, P. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: A clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2011, 154, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Legouis, D.; Faivre, A.; Cippà, P.E.; de Seigneux, S. Renal gluconeogenesis: An underestimated role of the kidney in systemic glucose metabolism. Nephrol. Dial. Transplant. 2020, 37, 1417–1425. [Google Scholar] [CrossRef]
- Leibovitz, E.; Wainstein, J.; Boaz, M. Association of albumin and cholesterol levels with incidence of hypoglycaemia in people admitted to general internal medicine units. Diabet. Med. 2018, 35, 1735–1741. [Google Scholar] [CrossRef]
- Khanimov, I.; Wainstein, J.; Boaz, M.; Shimonov, M.; Leibovitz, E. Reduction of serum albumin in non-critically ill patients during hospitalization is associated with incident hypoglycaemia. Diabetes Metab. 2019, 46, 27–32. [Google Scholar] [CrossRef]
- Khanimov, I.; Boaz, M.; Shimonov, M.; Wainstein, J.; Leibovitz, E. Systemic treatment with glucocorticoids is associated with incident hypoglycemia and mortality: A historical prospective analysis. Am. J. Med. 2020, 133, 831–838. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef]
- Lewington, A.J.P.; Cerdá, J.; Mehta, R.L. Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int. 2013, 84, 457–467. [Google Scholar] [CrossRef]
- Bihorac, A.; Yavas, S.; Subbiah, S.; Hobson, C.E.; Schold, J.D.; Gabrielli, A.; Layon, A.J.; Segal, M.S. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann. Surg. 2009, 249, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Lameire, N.H.; Bagga, A.; Cruz, D.; De Maeseneer, J.; Endre, Z.; Kellum, J.A.; Liu, K.D.; Mehta, R.L.; Pannu, N.; Van Biesen, W.; et al. Acute kidney injury: An increasing global concern. Lancet 2013, 382, 170–179. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. 1), S1–S2. [CrossRef] [PubMed]
- Khanimov, I.; Shimonov, M.; Wainstein, J.; Leibovitz, E. Hypoglycemia, Malnutrition and Body Composition. Adv. Exp. Med. Biol. 2020, 4, 71–84. [Google Scholar]
- Khanimov, I.; Ditch, M.; Adler, H.; Giryes, S.; Felner Burg, N.; Boaz, M.; Leibovitz, E. Prediction of Hypoglycemia during Admission of Non-Critically Ill Patients: Results from the MENU Study. Horm. Metab. Res. 2020, 52, 660–668. [Google Scholar] [CrossRef]
- Khanimov, I.; Zingerman, B.; Korzetz, A.; Boaz, M.; Shimonov, M.; Wainstein, J. Leibovitz E Association Between estimated GFR and Incident Hypoglycemia During Hospitalization. Nephrology 2021, 27, 162–170. [Google Scholar]
- Gerich, J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: Therapeutic implications. Diabet. Med. 2010, 27, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Gerich, J.E.; Woerle, H.J.; Meyer, C.; Stumvoll, M. Renal gluconeogenesis: Its importance in human glucose homeostasis. Diabetes Care 2001, 24, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Schoolwerth, A.C.; Smith, B.C.; Culpepper, R.M. Renal gluconeogenesis. Miner. Electrolyte Metab. 1988, 14, 347–361. [Google Scholar]
- Legouis, D.; Ricksten, S.E.; Faivre, A.; Verissimo, T.; Gariani, K.; Verney, C.; Galichon, P.; Berchtold, L.; Feraille, E.; Fernandez, M.; et al. Altered proximal tubular cell glucose metabolism during acute kidney injury is associated with mortality. Nat. Metab. 2020, 2, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lee, T.W.; DeFronzo, R.A. Why do SGLT2 inhibitors inhibit only 30-50% of renal glucose reabsorption in humans? Diabetes 2012, 61, 2199–2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horii, T.; Oikawa, Y.; Kunisada, N.; Shimada, A.; Atsuda, K. Real-world risk of hypoglycemia-related hospitalization in Japanese patients with type 2 diabetes using SGLT2 inhibitors: A nationwide cohort study. BMJ Open Diabetes Res. Care 2020, 8, e001856. [Google Scholar] [CrossRef] [PubMed]
- Disease-Related Malnutrition: An Evidence-Based Approach to Treatment; CABI: Wallingford, UK, 2003; ISBN 9780851996486.
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. Nature Publishing Group 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, D.; Mohan, A.; Subev, E.; Sarav, M.; Sturgill, D. Acute Kidney Injury Incidence in Hospitalized Patients and Implications for Nutrition Support. Nutr. Clin. Pract. 2020, 35, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Leibovitz, E.; Adler, H.; Giryes, S.; Ditch, M.; Burg, N.F.; Boaz, M. Malnutrition risk is associated with hypoglycemia among general population admitted to internal medicine units. Results from the MENU study. Eur. J. Clin. Nutr. 2018, 72, 888–893. [Google Scholar] [CrossRef]
- Poston, J.T.; Koyner, J.L. Sepsis associated acute kidney injury. BMJ 2019, 364, k4891. [Google Scholar] [CrossRef]
- Miller, S.I.; Wallace, R.J.; Musher, D.M.; Septimus, E.J.; Kohl, S.; Baughn, R.E. Hypoglycemia as a manifestation of sepsis. Am. J. Med. 1980, 68, 649–654. [Google Scholar] [CrossRef]
- Cryer, P.E.; Axelrod, L.; Grossman, A.B.; Heller, S.R.; Montori, V.M.; Seaquist, E.R.; Service, F.J. Evaluation and management of adult hypoglycemic disorders: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2009, 94, 709–728. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.S.; Ko, S.H. Avoiding or coping with severe hypoglycemia in patients with type 2 diabetes. Korean J. Intern. Med. 2015, 30, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Cryer, P.E. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N. Engl. J. Med. 2013, 369, 362–372. [Google Scholar] [CrossRef] [PubMed]



| Parameter | SCIC n = 5639 | no SCIC n = 19,761 | p Value |
|---|---|---|---|
| Male sex (%) | 52.4 | 48.4 | p < 0.001 |
| Age (years) | 76.1 ± 15.7 | 68.2 ± 18.3 | p < 0.001 |
| Acute conditions in discharge letter | |||
| Exacerbation of CHF (%) | 9.7 | 4.9 | p < 0.001 |
| Exacerbation of COPD (%) | 4.6 | 3.2 | p < 0.001 |
| Acute coronary syndrome (%) | 3.4 | 1.9 | p < 0.001 |
| Acute infection (any site) (%) | 40.1 | 19.8 | p < 0.001 |
| Co-morbidities | |||
| CHF (%) | 16.0 | 8.5 | p < 0.001 |
| COPD (%) | 13.3 | 10.5 | p < 0.001 |
| CVA (%) | 9.5 | 7.1 | p < 0.001 |
| Dementia (%) | 8.2 | 3.9 | p < 0.001 |
| Hypertension (%) | 57.8 | 50.4 | p < 0.001 |
| IHD (%) | 22.0 | 18.8 | p < 0.001 |
| Hyperlipidemia (%) | 36.0 | 35.4 | 0.384 |
| Liver disease (%) | 4.2 | 3.6 | 0.029 |
| Malignancy-all types all stages | 8.5 | 6.0 | p < 0.001 |
| PVD (%) | 4.0 | 2.5 | p < 0.001 |
| Hypothyroidism (%) | 8.4 | 7.5 | p = 0.029 |
| Diabetes mellitus (%) | 39.9 | 30.0 | p < 0.001 |
| Admission measurements and laboratory data | |||
| Temperature (°C) | 36.8 ± 0.6 | 36.8 ± 0.4 | p < 0.001 |
| Heart rate (BPM) | 84.7 ± 18.8 | 79.8 ± 17.4 | p < 0.001 |
| Systolic blood pressure (mmHg) | 135 ± 28 | 141 ± 26 | p < 0.001 |
| Diastolic blood pressure (mmHg) | 73 ± 14 | 78 ± 13 | p < 0.001 |
| White blood cell count (cells per mm3 × 103) | 12.7 ± 2.0 | 10.0 ± 6.4 | p < 0.001 |
| Hemoglobin (g/dL) | 12.2 ± 2.3 | 12.8 ± 2.0 | p < 0.001 |
| Sodium (meq/L) | 137 ± 6 | 137 ± 5 | p < 0.001 |
| Potassium (meq/L) | 4.3 ± 0.7 | 4.1 ± 0.5 | p < 0.001 |
| Cholesterol (mg/dL) | 147 ± 47 | 165 ± 45 | p < 0.001 |
| Serum albumin (g/dL) | 3.4 ± 0.6 | 3.9 ± 0.5 | p < 0.001 |
| C-Reactive Protein (mg/dL) | 9.5 ± 10.6 | 4.1 ± 6.6 | p < 0.001 |
| Mean glucose during hospitalization (mg/dL) | 150 ± 54 | 133 ± 46 | p < 0.001 |
| Incident hypoglycemia (%) | 13.1 | 4.1 | p < 0.001 |
| Deteriorating SCIC n = 2129 | Improving SCIC n = 3510 | p Value | |
|---|---|---|---|
| Age (years) | 78.7 ± 0.3 | 74.5 ± 16.6 | <0.001 |
| Male sex (%) | 49.4 | 54.2 | <0.001 |
| Length of stay (days) | 14.5 ± 0.8 | 8.8 ± 9.3 | <0.001 |
| Admission measurements and laboratory data | |||
| Temperature (°C) | 36.8 ± 0 | 36.9 ± 0.5 | <0.001 |
| Heart rate (BPM) | 86.2 ± 0.4 | 83.8 ± 18.3 | <0.001 |
| Systolic blood pressure (mmHg) | 137.3 ± 0.7 | 133 ± 26.4 | <0.001 |
| Diastolic blood pressure (mmHg) | 73.6 ± 0.4 | 72.4 ± 13.4 | 0.005 |
| White blood cell count (cells per cmm × 103) | 12.4 ± 0.2 | 12.9 ± 10.1 | 0.041 |
| Hemoglobin (g/dL) | 11.8 ± 0 | 12.4 ± 2.3 | <0.001 |
| Cholesterol (mg/dL) | 148.2 ± 1.1 | 146.4 ± 46.3 | 0.170 |
| Serum albumin (g/dL) | 3.4 ± 0 | 3.5 ± 0.6 | <0.001 |
| C-Reactive Protein (mg/dL) | 9.2 ± 0.2 | 9.8 ± 10.8 | 0.048 |
| Admission creatinine (mg/dL) | 1.39 ± 0.65 | 1.55 ± 0.59 | <0.001 |
| Co-morbidities | |||
| Acute infection (any site) (%) | 41.2 | 39.4 | 0.183 |
| CHF (%) | 25.0 | 10.6 | <0.001 |
| Hypertension (%) | 62.4 | 55.0 | <0.001 |
| Diabetes mellitus (%) | 41.7 | 38.9 | 0.037 |
| Incident Hypoglycemia (%) | 16.9 | 10.7 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zingerman, B.; Khanimov, I.; Shimonov, M.; Boaz, M.; Rozen-Zvi, B.; Leibovitz, E. Changes in Serum Creatinine May Cause Hypoglycemia among Non-Critically Ill Patients Admitted to Internal Medicine Units. J. Clin. Med. 2022, 11, 6852. https://doi.org/10.3390/jcm11226852
Zingerman B, Khanimov I, Shimonov M, Boaz M, Rozen-Zvi B, Leibovitz E. Changes in Serum Creatinine May Cause Hypoglycemia among Non-Critically Ill Patients Admitted to Internal Medicine Units. Journal of Clinical Medicine. 2022; 11(22):6852. https://doi.org/10.3390/jcm11226852
Chicago/Turabian StyleZingerman, Boris, Israel Khanimov, Mordechai Shimonov, Mona Boaz, Benaya Rozen-Zvi, and Eyal Leibovitz. 2022. "Changes in Serum Creatinine May Cause Hypoglycemia among Non-Critically Ill Patients Admitted to Internal Medicine Units" Journal of Clinical Medicine 11, no. 22: 6852. https://doi.org/10.3390/jcm11226852
APA StyleZingerman, B., Khanimov, I., Shimonov, M., Boaz, M., Rozen-Zvi, B., & Leibovitz, E. (2022). Changes in Serum Creatinine May Cause Hypoglycemia among Non-Critically Ill Patients Admitted to Internal Medicine Units. Journal of Clinical Medicine, 11(22), 6852. https://doi.org/10.3390/jcm11226852





