COVID-19 Infection in Rheumatic Patients on Chronic Antimalarial Drugs: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
- Full-length reports published in peer-reviewed journals (preprint papers were excluded);
- Prospective observational cohorts or clinical trials of adult patients (aged ≥ 18 years);
- Patients were diagnosed with a COVID-19 infection through a validated test.
2.2. Data Analysis
3. Results
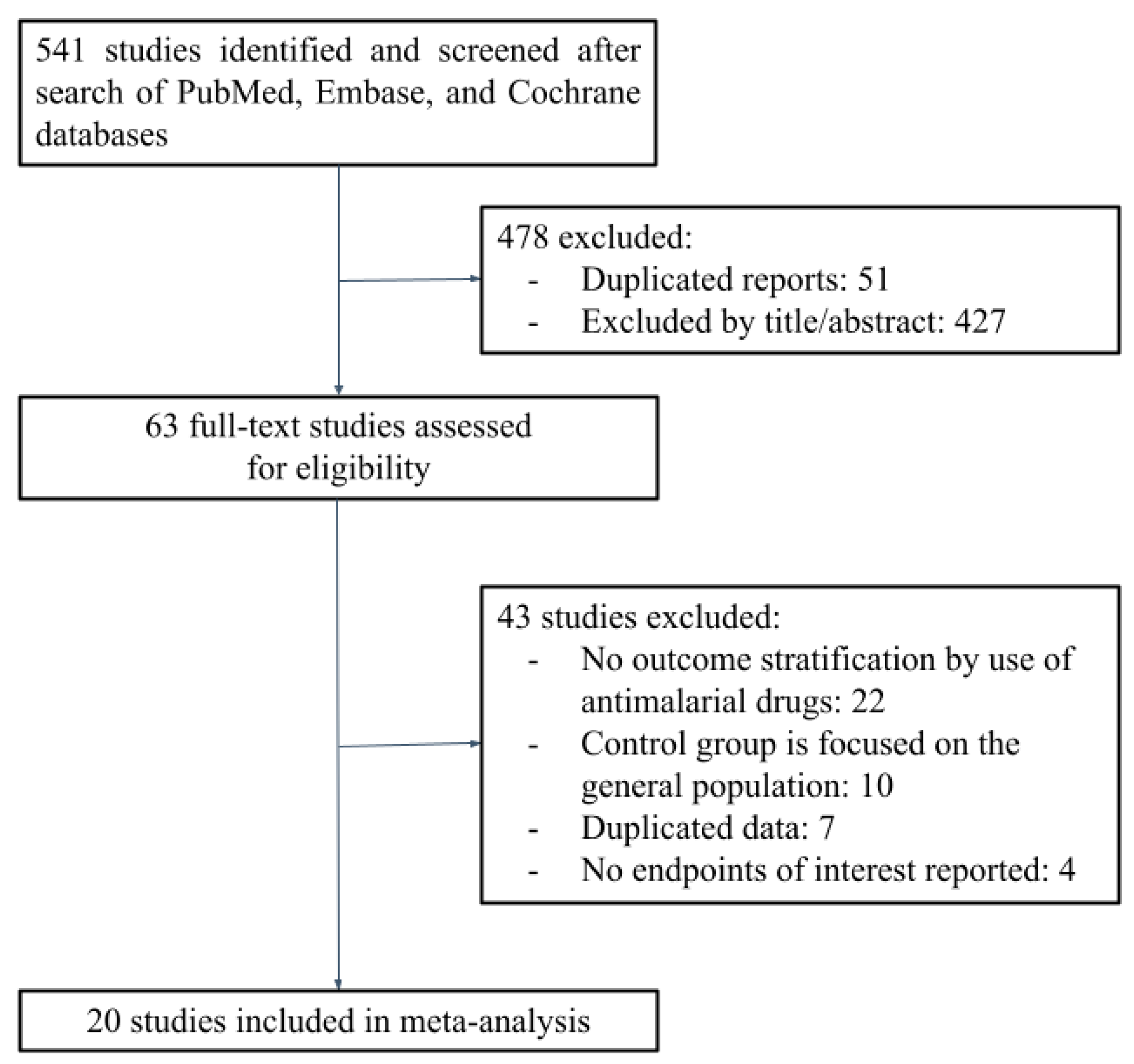
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. Risk of Bias
3.4. Outcomes
3.4.1. All-Cause Mortality
3.4.2. Hospitalization
3.4.3. COVID-19 Infection
3.4.4. ICU Admission
3.4.5. Mechanical Ventilation
3.4.6. Oxygen Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Search Strategy
- 1-PubMed
- (((((chloroquine[MeSH Terms]) OR (chloroquine OR (CQ))) OR ((hydroxychloroquine[MeSH Terms]) OR (Hydroxychloroquine OR (HCQ)))) OR ((antimalarials[MeSH Terms]) OR (Antimalarial*))) AND ((((((COVID-19[MeSH Terms]) OR ((COVID-19) OR “2019 nCoV” OR “2019 Novel Coronavirus” OR “2019-nCoV” OR “2019-New Coronavirus” OR “Coronavirus Disease 19” OR “Coronavirus Disease 2019” OR “Coronavirus Disease-19”OR “COVID 19” OR “COVID19” OR “Disease 2019, Coronavirus” OR “SARS Coronavirus 2” OR “SARS-CoV 2”)) OR ((SARS-CoV-2[MeSH Terms]) OR (“Severe Acute Respiratory Syndrome coronavirus 2” OR (SARS-CoV-2)))) OR ((Coronavirus Infections[MeSH Terms]) OR (Coronavirus))) OR ((Betacoronavirus[MeSH Terms]) OR (Betacoronavirus))) OR (coronavirus[MeSH Terms]))) AND (((((((((((((((((((arthritis[MeSH Terms]) OR (arthritis OR Arthritides)) OR ((arthritis, rheumatoid[MeSH Terms]) OR ((Arthritis, Rheumatoid) OR (RA)))) OR ((((arthritis, juvenile chronic[MeSH Terms]) OR (arthritis, juvenile idiopathic[MeSH Terms])) OR (arthritis, juvenile rheumatoid[MeSH Terms])) OR (Arthritis, Juvenile))) OR ((Spondylitis, Ankylosing[MeSH Terms]) OR (Spondylitis, Ankylosing))) OR ((rheumatoid vasculitis[MeSH Terms]) OR ((Rheumatoid Vasculitis) OR (Rheumatoid Vasculitides)))) OR ((Lupus Erythematosus, Systemic[MeSH Terms]) OR ((Lupus Erythematosus, Systemic) OR (SLE)))) OR ((osteoarthritis[MeSH Terms]) OR (osteoarthritis))) OR ((arthritis, psoriatic[MeSH Terms]) OR ((arthritis, psoriatic) OR (PsA)))) OR (((spondylarthritis[MeSH Terms]) OR (spondylarthritis)) OR ((SpA)))) OR ((rheumatic diseases[MeSH Terms]) OR (rheumatic diseases))) OR ((musculoskeletal diseases[MeSH Terms]) OR (Musculoskeletal Diseases))) OR ((Axial Spondyloarthritis[MeSH Terms]) OR (Axial Spondyloarthritis))) OR ((Connective Tissue Diseases[MeSH Terms]) OR (Connective Tissue Diseases))) OR ((Sjogren’s Syndrome[MeSH Terms]) OR ((Sjogren’s Syndrome) OR (pSS)))) OR ((scleroderma, systemic[MeSH Terms]) OR (scleroderma, systemic))) OR ((dermatomyositis[MeSH Terms]) OR (dermatomyositis))) OR ((sarcoidosis[MeSH Terms]) OR (sarcoidosis))) OR ((polymyositis[MeSH Terms]) OR (polymyositis)))
- 2-Cochrane Central
- chloroquine OR CQ OR hydroxychloroquine OR HCQ in Title Abstract Keyword
- AND
- COVID-19 OR “2019 nCoV” OR “2019 Novel Coronavirus” OR “2019-nCoV” OR “2019-New Coronavirus” OR “Coronavirus Disease 19” OR “Coronavirus Disease 2019” OR “Coronavirus Disease-19” OR “COVID 19” OR “COVID19” OR “Disease 2019, Coronavirus” OR “SARS Coronavirus 2” OR “SARS-CoV 2” OR SARS-CoV-2 OR “Severe Acute Respiratory Syndrome coronavirus 2” OR SARS-CoV-2 OR “Coronavirus Infections” OR Coronavirus OR Betacoronavirus in Title Abstract Keyword AND
- (arthritis OR Arthritides) OR “rheumatoid arthritis” (RA) OR “juvenile arthritis” OR “Ankylosing Spondylitis” OR “rheumatoid vasculitis” OR “Rheumatoid Vasculitides” OR “ Systemic Lupus Erythematosus” OR (SLE) OR osteoarthritis OR “psoriatic arthritis” OR (PsA) OR spondylarthritis OR (SpA) OR “rheumatic diseases” OR “rheumatic disease” OR “musculoskeletal diseases” OR “Musculoskeletal Disease” OR “Axial Spondyloarthritis” OR “Connective Tissue Disease” OR “Connective Tissue Diseases” OR “Sjogren’s Syndrome” OR (pSS) OR “systemic scleroderma” OR dermatomyositis OR sarcoidosis OR polymyositis in Title Abstract Keyword (Word variations have been searched)
- 3-Embase
- (chloroquine:ti,ab,kw OR hydroxychloroquine:ti,ab,kw OR ‘hydroxychloroquine sulfate’:ti,ab,kw OR ‘antimalarial agent’:ti,ab,kw) AND (‘coronavirus disease 2019′:ti,ab,kw OR ‘severe acute respiratory syndrome coronavirus 2′:ti,ab,kw OR ‘coronavirus infection’:ti,ab,kw OR betacoronavirus:ti,ab,kw) AND (arthritis:ti,ab,kw OR ‘rheumatoid arthritis’:ti,ab,kw OR ‘psoriatic arthritis’:ti,ab,kw OR osteoarthritis:ti,ab,kw OR spondylarthritis:ti,ab,kw OR ‘juvenile rheumatoid arthritis’:ti,ab,kw OR spondylitis:ti,ab,kw OR ‘ankylosing spondylitis’:ti,ab,kw OR vasculitis:ti,ab,kw OR ‘lupus erythematosus’:ti,ab,kw OR ‘systemic lupus erythematosus’:ti,ab,kw OR ‘rheumatic disease’:ti,ab,kw OR ‘musculoskeletal disease’:ti,ab,kw OR ‘axial spondyloarthritis’:ti,ab,kw OR ‘connective tissue disease’:ti,ab,kw OR ‘sjoegren syndrome’:ti,ab,kw OR ‘systemic sclerosis’:ti,ab,kw OR scleroderma:ti,ab,kw OR dermatomyositis:ti,ab,kw OR sarcoidosis:ti,ab,kw OR polymyositis:ti,ab,kw)
Appendix B
- Duration of HCQ/CQ treatment
- Duration of HCQ/CQ treatment reported in the studies:
- (1)
- Espinosa, 2021: at least 6 months;
- (2)
- Gentry, 2020: at least 3 months;
- (3)
- Guillaume, 2021: at least 6 months;
- (4)
- Kim 2022: at least 3 months;
- (5)
- Macías, 2021: approximately 4 months;
- (6)
- Pham, 2021: at least 1 month;
- (7)
- Rentsch, 2021: at least 6 months;
- (8)
- Walbi, 2022: at least 6 months;
- (9)
- Yousefghahari, 2021: at least 8 months.
Appendix C
| Selection | Comparability | Outcome | NOS Considered Score | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NOS recommendation | Representativeness of the exposed cohort | Selection of the non- exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at the start of the study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for the outcomes to occur? | Adequacy of follow-up of cohorts | |
| Considered to score | (a) representative of a general population ✵ (b) selected group of users (e.g., age, sex not described) (c) no description of the derivation of the cohort | (a) drawn from the same source and exposure and non-exposure groups were identified ✵ (b) drawn from a different source (c) no description of the derivation of the non-exposed cohort | (a) secure record (e.g., laboratory records) ✵ (b) structured interview ✵ (c) written self-report (d) no description | (a) yes (all patients were tested for COVID-19 and exposure and non-exposure groups were identified) ✵ (b) no | (a) sample characteristics clearly described (age, sex, and sample size) ✵ (b) study describes at least one characteristics of the sample (age, sex, or sample size) ✵ (c) study does not describe the period | (a) independent blind assessment ✵ (b) record linkage ✵ (c) self-report (d) no description | (a) yes (follow all patients at least until hospital discharge or death)✵ (b) no | (a) complete follow-up—all subjects accounted for ✵ (b) subjects lost to follow-up unlikely to introduce bias (small number lost, >90% follow-up, or description provided of those lost) ✵ (c) follow-up rate < 90% and no description of those lost (d) no statement | |
| Alzahrani, 2021 [21] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Annamalai, 2021 [23] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Batibay, 2021 [24] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Espinosa, 2021 [25] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 6 | ||
| Eviatar, 2021 [26] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Fernandez-Ruiz, 2020 [27] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 6 | ||
| Gentry, 2020 [28] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Guillaume, 2021 [29] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Jung, 2021 [30] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Kim, 2022 [31] | ✵ | ✵ | ✵ | ✵ | ✵ | 5 | |||
| Macías, 2021 [32] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Montero, 2020 [33] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Pham, 2021 [34] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Ramirez, 2020 [35] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 6 | ||
| Rentsch, 2021 [36] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Trefond, 2021 [37] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Ugarte-Gil, 2021 [38] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Walbi, 2022 [39] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Ye, 2021 [40] | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| Yousefghahari, 2021 [41] | ✵ | ✵ | ✵ | ✵ | ✵ | 5 | |||
Appendix D
- Sensitivity Analysis
Appendix E
- Funnel Plots with Fixed-Effect
- The graphics below are the funnel plots for each outcome using a fixed-effect. Each dot indicates a study included. The outer dashed lines represent the triangular region within 95% of studies are expected to locate in the absence of bias and heterogeneity.






Appendix F
Appendix G
- Forest Plots with Fixed-Effect
References
- World Health Organization (WHO). COVID-19 Dashboard; World Health Organization: Geneva, Switzerland, 2022; Available online: https://covid19.who.int/ (accessed on 20 November 2022).
- Guan, W.-J.; Liang, W.-H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed]
- Strangfeld, A.; Schäfer, M.; Gianfrancesco, M.A.; Lawson-Tovey, S.; Liew, J.W.; Ljung, L.; Mateus, E.F.; Richez, C.; Santos, M.J.; Schmajuk, G.; et al. Factors associated with COVID-19-related death in people with rheumatic diseases: Results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2021, 80, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) In Vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Zhou, D.; Dai, S.-M.; Tong, Q. COVID-19: A recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 2020, 75, 1667–1670. [Google Scholar] [CrossRef]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 732–739. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Mafham, M.; Linsell, L.; Bell, J.L.; Staplin, N.; Emberson, J.R.; Wiselka, M.; Ustianowski, A.; Elmahi, E.; et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020, 383, 2030–2040. [Google Scholar] [CrossRef]
- Self, W.H.; Semler, M.W.; Leither, L.M.; Casey, J.D.; Angus, D.C.; Brower, R.G.; Chang, S.Y.; Collins, S.P.; Eppensteiner, J.C.; Filbin, M.R.; et al. Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19. JAMA 2020, 324, 2165–2176. [Google Scholar] [CrossRef]
- Tett, S.; Cutler, D.; Day, R. Antimalarials in rheumatic diseases. Baillieres Clin. Rheumatol. 1990, 4, 467–489. [Google Scholar] [CrossRef]
- Tett, S.; Cutler, D.; Day, R.; Brown, K. A dose-ranging study of the pharmacokinetics of hydroxy-chloroquine following intravenous administration to healthy volunteers. Br. J. Clin. Pharmacol. 1988, 26, 303–313. [Google Scholar] [CrossRef]
- Munster, T.; Gibbs, J.P.; Shen, D.; Baethge, B.A.; Botstein, G.R.; Caldwell, J.; Dietz, F.; Ettlinger, R.; Golden, H.E.; Lindsley, H.; et al. Hydroxychloroquine concentration-response relationships in patients with rheumatoid arthritis. Arthritis Rheum. 2002, 46, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, A.H. Pharmacologic actions of 4-aminoquinoline compounds. Am. J. Med. 1983, 75, 5–10. [Google Scholar] [CrossRef]
- Browning, D.J. Pharmacology of Chloroquine and Hydroxychloroquine. In Hydroxychloroquine and Chloroquine Retinopathy; Springer: New York, NY, USA, 2014; pp. 35–63. [Google Scholar] [CrossRef]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F. Preferred Reporting Items for a Systematic Review and Meta-analysis of Individual Participant Data: The PRISMA-IPD statement. JAMA 2015, 313, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2021; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 16 December 2019).
- Friedrich, J.O.; Adhikari, N.K.J.; Beyene, J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med. Res. Methodol. 2007, 7, 5–6. [Google Scholar] [CrossRef]
- Sweeting, M.; Sutton, A.J.; Lambert, P. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat. Med. 2004, 23, 1351–1375. [Google Scholar] [CrossRef]
- Harbord, R.M.; Egger, M.; Sterne, J. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006, 25, 3443–3457. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: London, UK, 2022; Available online: https://handbook-5-1.cochrane.org/chapter_9/9_7_sensitivity_analyses.htm (accessed on 20 November 2022).
- Alzahrani, Z.A.; Alghamdi, K.A.; Almaqati, A.S. Clinical characteristics and outcome of COVID-19 in patients with rheumatic diseases. Rheumatol. Int. 2021, 41, 1097–1103. [Google Scholar] [CrossRef]
- Mohanasundaram, K.; Annamalai, S.; Santhanam, S.; Nambi, T.; Sankaran, S.; Natarajan, R.; Mohandas, P. COVID-19 and Rheumatic Diseases in Tamil Nadu—A multicenter retrospective observational study. Indian J. Rheumatol. 2021, 16, 441–446. [Google Scholar] [CrossRef]
- Batıbay, S.; Ulucaköy, R.K.; Özdemir, B.; Günendi, Z.; Göğüş, F.N. Clinical outcomes of Covid-19 in patients with rheumatic diseases and the effects of the pandemic on rheumatology outpatient care: A single-centre experience from Turkey. Int. J. Clin. Pract. 2021, 75, e14442. [Google Scholar] [CrossRef]
- Espinosa, G.; Prieto-González, S.; Llevadot, M.; Marco-Hernández, J.; Martínez-Artuña, A.; Pérez-Isidro, A.; Rifé, E.; Cervera, R. The impact of SARS-CoV-2 coronavirus infection in patients with systemic lupus erythematosus from a single center in Catalonia. Clin. Rheumatol. 2021, 40, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Eviatar, T.; Elalouf, O.; Furer, V.; Goldstein-Lahat, Y.; Paran, Y.; Pel, S.; Nevo, S.; Zisapel, M.; Alcalay, Y.; Elkayam, O. Prevalence of COVID-19 and seroprevalence to SARS-CoV-2 in a rheumatologic patient population from a tertiary referral clinic in Israel. Intern. Med. J. 2021, 51, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, R.; Masson, M.; Kim, M.; Myers, B.; Haberman, R.; Scher, J.; Castillo, R.; Guttmann, A.; Carlucci, P.; Deonaraine, K.; et al. COVID-19 in Patients with Systemic Lupus Erythematosus. Arthritis Rheumatol. 2020, 72. Available online: https://acrabstracts.org/abstract/covid-19-in-patients-with-systemic-lupus-erythematosus/ (accessed on 7 July 2022). [CrossRef] [PubMed]
- Gentry, C.A.; Humphrey, M.B.; Thind, S.K.; Hendrickson, S.C.; Kurdgelashvili, G.; Williams, R.J. Long-term hydroxychloroquine use in patients with rheumatic conditions and development of SARS-CoV-2 infection: A retrospective cohort study. Lancet Rheumatol. 2020, 2, e689–e697. [Google Scholar] [CrossRef]
- Guillaume, D.; Magalie, B.; Sina, E.; Imène, S.-M.; Frédéric, V.; Mathieu, D.; Aurore, M.; Yoni, G.; Emma, E.; Charlotte, B.; et al. Antirheumatic Drug Intake Influence on Occurrence of COVID-19 Infection in Ambulatory Patients with Immune-Mediated Inflammatory Diseases: A Cohort Study. Rheumatol. Ther. 2021, 8, 1887–1895. [Google Scholar] [CrossRef]
- Jung, S.-Y.; Kim, M.-S.; Kim, M.-C.; Choi, S.-H.; Chung, J.-W.; Choi, S.T. Effect of hydroxychloroquine pre-exposure on infection with SARS-CoV-2 in rheumatic disease patients: A population-based cohort study. Clin. Microbiol. Infect. 2021, 27, 611–617. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kwak, S.G.; Lee, H.; Kim, S.-K.; Choe, J.-Y.; Park, S.-H. Baseline use of hydroxychloroquine or immunosuppressive drugs and the risk of coronavirus disease 2019. Korean J. Intern. Med. 2022, 37, 673–680. [Google Scholar] [CrossRef]
- Macías, J.; González-Moreno, P.; Sánchez-García, E.; Morillo-Verdugo, R.; Pérez-Venegas, J.J.; Pinilla, A.; Macho, M.; Martínez, M.; González-Serna, A.; Corma, A.; et al. Similar incidence of coronavirus disease 2019 (COVID-19) in patients with rheumatic diseases with and without hydroxychloroquine therapy. PLoS ONE 2021, 16, e0249036. [Google Scholar] [CrossRef]
- Montero, F.; Martínez-Barrio, J.; Serrano-Benavente, B.; González, T.; Rivera, J.; Collada, J.M.; Castrejón, I.; Álvaro-Gracia, J. Coronavirus disease 2019 (COVID-19) in autoimmune and inflammatory conditions: Clinical characteristics of poor outcomes. Rheumatol. Int. 2020, 40, 1593–1598. [Google Scholar] [CrossRef]
- Pham, K.; Torres, H.; Satlin, M.J.; Goyal, P.; Gulick, R.M. Failure of chronic hydroxychloroquine in preventing severe complications of COVID-19 in patients with rheumatic diseases. Rheumatol. Adv. Pract. 2021, 5, rkab014. [Google Scholar] [CrossRef]
- Ramirez, G.A.; Gerosa, M.; Beretta, L.; Bellocchi, C.; Argolini, L.M.; Moroni, L.; Della Torre, E.; Artusi, C.; Nicolosi, S.; Caporali, R.; et al. COVID-19 in systemic lupus erythematosus: Data from a survey on 417 patients. Semin. Arthritis Rheum. 2020, 50, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, C.T.; DeVito, N.J.; MacKenna, B.; Morton, C.E.; Bhaskaran, K.; Brown, J.P.; Schultze, A.; Hulme, W.J.; Croker, R.; Walker, A.J.; et al. Effect of pre-exposure use of hydroxychloroquine on COVID-19 mortality: A population-based cohort study in patients with rheumatoid arthritis or systemic lupus erythematosus using the OpenSAFELY platform. Lancet Rheumatol. 2021, 3, e19–e27. [Google Scholar] [CrossRef]
- Trefond, L.; Drumez, E.; Andre, M.; Costedoat-Chalumeau, N.; Seror, R.; Devaux, M.; Dernis, E.; Dieudonne, Y.; El Mahou, S.; Lanteri, A.; et al. Effet d’un traitement par hydroxychloroquine prescrit comme traitement de fond de rhumatismes inflammatoires chroniques ou maladies auto-immunes systémiques sur les tests diagnostiques et l’évolution de l’infection à SARS-CoV-2: Étude de 871 patients. Rev. Rhum. Ed. Fr. 2022, 89, 192–195. [Google Scholar] [CrossRef]
- Ugarte-Gil, M.F.; Alarcón, G.S.; Izadi, Z.; Duarte-García, A.; Reátegui-Sokolova, C.; Clarke, A.E.; Wise, L.; Pons-Estel, G.J.; Santos, M.J.; Bernatsky, S.; et al. Characteristics associated with poor COVID-19 outcomes in individuals with systemic lupus erythematosus: Data from the COVID-19 Global Rheumatology Alliance. Ann. Rheum. Dis. 2022, 81, 970–978. [Google Scholar] [CrossRef]
- Walbi, I.A.; Albarqi, H.A.; Alghanim, N.S.; Albadi, M.A.; Al Maimouni, H.M.; Alkahtani, S.A.; Alshabi, A.M.; Alali, A.S.; Alqahtani, F.; Al-Najjar, A.H.; et al. Effect of chronic hydroxychloroquine use on COVID-19 risk in patients with rheumatoid arthritis and systemic lupus erythematosus: A multicenter retrospective cohort. J. Int. Med. Res. 2022, 50, 3000605221090363. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Zhong, J.; Cai, S.; Dong, L.; Li, C.; Hou, X.; Chen, X.; Zhang, A.; Chen, W.; He, D.; et al. COVID-19 infection in patients with connective tissue disease: A multicity study in Hubei province, China. MedComm 2021, 2, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Yousefghahari, B.; Navari, S.; Sadeghi, M.; Soleimaniamiri, S.; Soleimaniamiri, M.; Heidari, B.; Babaei, M.; Ghodrati, K.; Guran, A.; Gholinia, H. Risk of COVID-19 infection in patients with rheumatic disease taking disease-modifying anti-rheumatic drugs. Clin. Rheumatol. 2021, 40, 4309–4315. [Google Scholar] [CrossRef] [PubMed]
- Derendorf, H. Excessive lysosomal ion-trapping of hydroxychloroquine and azithromycin. Int. J. Antimicrob. Agents 2020, 55, 106007. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, J.; Zhang, Z.; Jiang, S.; Han, S.; Yan, D.; Zhuang, R.; Hu, B.; Zhang, Z. Efficacy of Hydroxychloroquine in Patients with COVID-19: Results of a Randomized Clinical Trial. MedRxiv 2020. [Google Scholar] [CrossRef]
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 2020, 14, 72–73. [Google Scholar] [CrossRef]
- Geleris, J.; Sun, Y.; Platt, J.; Zucker, J.; Baldwin, M.; Hripcsak, G.; Labella, A.; Manson, D.K.; Kubin, C.; Barr, R.G.; et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020, 382, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.S.; Dufort, E.M.; Udo, T.; Wilberschied, L.A.; Kumar, J.; Tesoriero, J.; Weinberg, P.; Kirkwood, J.; Muse, A.; DeHovitz, J.; et al. Association of Treatment with Hydroxychloroquine or Azithromycin with In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA 2020, 323, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Randomised Evaluation of COVID-19 Therapy. No Clinical Benefit from Use of Hydroxychloroquine in Hospitalised Patients with COVID-19; RECOVERY: Oxford, UK, 2020; Available online: https://www.recoverytrial.net/files/hcq-recovery-statement-050620-final-002.pdf (accessed on 15 August 2022).
- Food and Drug Administration. Hydroxychloroquine and Chloroquine Letter; FDA: Maryland, MD, USA, 2020. Available online: https://www.fda.gov/media/138945/download (accessed on 15 August 2022).
- World Health Organization. WHO Discontinues Hydroxychloroquine and Lopinavir/Ritonavir Treatment Arms for COVID-19; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19 (accessed on 15 August 2022).
- Ali, A.S.; Abdel-Rahman, M.S.; Almalikil, R.S.; Mohamed, A.S.; Alfaifi, K.A.; Fadil, A.E.; El-Shitany, N.A.; Alkreathy, H.M. Optimizing the Use of Hydroxychloroquine in the Management of COVID-19 Given Its Pharmacological Profile. J. Pharm. Res. Int. 2020, 32, 29–43. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/sea-215754 (accessed on 15 August 2022). [CrossRef]
- Arshad, U.; Pertinez, H.; Box, H.; Tatham, L.; Rajoli, R.K.R.; Curley, P.; Neary, M.; Sharp, J.; Liptrott, N.J.; Valentijn, A.; et al. Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics. Clin. Pharmacol. Ther. 2020, 108, 775–790. [Google Scholar] [CrossRef]
- Liu, H.; Maruyama, H.; Masuda, T.; Honda, A.; Arai, F. The Influence of Virus Infection on the Extracellular pH of the Host Cell Detected on Cell Membrane. Front. Microbiol. 2016, 7, 1127. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4987339/ (accessed on 15 August 2022). [CrossRef] [PubMed]
- Abarientos, C.; Sperber, K.; Shapiro, D.L.; Aronow, W.S.; Chao, C.P.; Ash, J.Y. Hydroxychloroquine in systemic lupus erythematosus and rheumatoid arthritis and its safety in pregnancy. Expert Opin. Drug Saf. 2011, 10, 705–714. [Google Scholar] [CrossRef]
- Scherbel, A.L.; Harrison, J.W.; Atdjian, M. Further observations on the use of 4-Aminoquinoline compounds in patients with rheumatoid arthritis or related diseases. Clevel. Clin. Q. 1958, 25, 95–111. [Google Scholar] [CrossRef]
- Bagnall, A.W. The value of Chloroquine in rheumatoid disease: A four-year study of continuous therapy. Can. Med. Assoc. J. 1957, 77, 182–194. [Google Scholar]
- Napoli, P.E.; Nioi, M. Global Spread of Coronavirus Disease 2019 and Malaria: An Epidemiological Paradox in the Early Stage of a Pandemic. J. Clin. Med. 2020, 9, 1138. [Google Scholar] [CrossRef]
- Osei, S.A.; Biney, R.P.; Anning, A.S.; Nortey, L.N.; Ghartey-Kwansah, G. Low incidence of COVID-19 case severity and mortality in Africa; Could malaria co-infection provide the missing link? BMC Infect. Dis. 2022, 22, 78. [Google Scholar] [CrossRef]
- Teboh-Ewungkem, M.I.; Ngwa, G.A. COVID-19 in malaria-endemic regions: Potential consequences for malaria intervention coverage, morbidity, and mortality. Lancet Infect. Dis. 2021, 21, 5–6. [Google Scholar] [CrossRef]
| Study | HCQ/CQ Users/HCQ/CQ Nonusers | HCQ/CQ/Total (%) | Male, n (%) | Mean Age, (years) | RA, n (%) | SLE, n (%) | Others, n (%) | DM, n (%) | HTN, n (%) | Lung Disease, n (%) | CVD, n (%) | Renal Disease, n (%) | CD Use, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alzahrani, 2021 [22] | 14/33 | 29.8 | 6 (12.7) | 50.8 | 25 (53.0) | 10 (21.3) | 12 (25.5) | 7 (14.8) | NA | NA | NA | NA | 21 (44.7) |
| Annamalai, 2021 [23] | 28/57 | 32.9 | 23 (27.0) | 47.7 * | 44 (51.7) | 13 (15.3) | 28 (32.9) | 21 (24.7) | 22 (25.9) | NA | NA | 3 (3.5) | 37 (43.5) |
| Batibay, 2021 [24] | 103/217 | 32.2 | 91 (28.4) | 47.9 | 109 (34.0) | 17 (5.3) | 194 (60.0) | 35 (10.9) | 70 (21.9) | 36 (11.2) | 28 (8.7) | 13 (4.0) | 113 (35.3) |
| Espinosa, 2021 [25] | 296/104 | 74.0 | 28 (7.0) | 50.7 | 0 (0.0) | 400 (100.0) | 0 (0.0) | NA | NA | NA | NA | NA | NA |
| Eviatar, 2021 [26] | 225/976 | 18.7 | 308 (25.6) | 56.0 | 183 (15.2) | 140 (11.6) | 878 (73.1) | 147 (12.2) | 296 (24.6) | 137 (11.4) | 103 (8.6) | 7 (0.58) | 157 (13.1) |
| Fernandez-Ruiz, 2020 [27] | 32/9 | 78.0 | 3 (7.3) | 47.0 * | 0 (0.0) | 41 (100.0) | 0 (0.0) | NA | NA | NA | NA | NA | 18 (43.9) |
| Gentry, 2020 [28] | 10,703/ 21,406 | 33.3 | 24,531 (76.4) | 65.2 * | 22,242 (69.3) | 7117 (22.2) | 2750 (8.6) | NA | NA | 6771 (21.1) | 13,297 (41.4) | 7632 (23.8) | 2708 (8.4) |
| Guillaume, 2021 [29] | 181/278 | 39.4 | 43 (9.3) | 59.4 * | 149 (32.4) | 193 (42.0) | 117 (25.4) | 33 (7.1) | NA | 33 (7.2) | 167 (36.4) | NA | 268 (58.4) |
| Jung, 2021 [30] | 649/1417 | 31.4 | 574 (27.7) | 62.0 | 1877 (90.8) | 299 (14.5) | 0 (0.0) | 634 (30.7) | 1089 (52.7) | 1051 (50.9) | NA | 233 (11.3) | 1891 (91.5) |
| Kim, 2022 [31] | 318/476 | 40.0 | 48 (6.0) | NA | 511 (64.3) | 283 (35.6) | 0 (0.0) | 28 (3.5) | 23 (2.9) | 11 (1.4) | 27 (3.4) | 5 (0.6) | 528 (66.5) |
| Macías, 2021 [32] | 290/432 | 40.2 | 124 (17.2) | 56.5 | 467 (64.7) | 94 (13.0) | 158 (21.9) | NA | NA | NA | NA | NA | 261 (36.1) |
| Montero, 2020 [33] | 9/53 | 14.5 | 26 (41.9) | 60.9 | 20 (32.2) | 9 (14.5) | 13 (21) | 12 (19.3) | 27 (43.5) | 14 (22.6) | 31 (50.0) | NA | 30 (48.4) |
| Pham, 2021 [34] | 14/28 | 33.3 | 11 (26.2) | 61.0 * | 18 (42.8) | 7 (16.7) | 19 (45.2) | 12 (28.5) | 29 (67.4) | 16 (38.1) | NA | 5 (11.9) | 14 (33.3) |
| Ramirez, 2020 [35] | 289/128 | 69.3 | 33 (7.7) | NA | 0 (0.0) | 417 (100.0) | 0 (0.0) | 12 (2.9) | 123 (29.5) | 40 (9.6) | NA | NA | NA |
| Rentsch, 2021 [36] | 30,569/164,068 | 15.7 | 56,197 (28.9) | 67.0 * | 167,874 (86.2) | 26,763 (13.7) | 0 (0.0) | 34,807 (17.9) | 83,404 (42.8) | 26,680 (13.7) | NA | 26,472 (13.6) | 33,677 (17.3) |
| Trefond, 2021 [37] | 71/191 | 27.1 | 16 (6.1) | 54.4 * | 131 (50.0) | 42 (16.0) | 89 (34.0) | 24 (9.2) | 75 (28.6) | 42 (16.3) | NA | 19 (7.2) | 92 (35.1) |
| Ugarte-Gil, 2021 [38] | 665/ 1257 | 34.6 | 188 (9.8) | 44.4 | 0 (0.0) | 1922 (100.0) | 0 (0.0) | NA | NA | NA | NA | 223 (11.6) | 825 (43.0) |
| Walbi, 2022 [39] | 207/304 | 40.5 | 92 (18.0) | 44.5 | 325 (63.6) | 151 (29.5) | 35 (6.8) | 78 (15.3) | 105 (20.5) | 44 (8.6) | 24 (4.7) | NA | 138 (27.0) |
| Ye, 2021 [40] | 18/82 | 18.0 | 60 (60.0) | 59.2 * | 0 (0.0) | 0 (0.0) | 100 (100.0) | 6 (6.0) | 23 (23.0) | 15 (15.0) | NA | NA | 22 (22.0) |
| Yousefghahari, 2021 [41] | 430/370 | 53.7 | NA | NA | 473 (59.1) | 110 (13.8) | 217 (27.1) | 94 (11.8) | 56 (7.0) | 58 (7.3) | 30 (3.8) | NA | 716 (89.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landsteiner de Sampaio Amêndola, I.; Aires Pinheiro, J.; Póvoa, P.; Cés de Souza Dantas, V.; Serafim, R.B. COVID-19 Infection in Rheumatic Patients on Chronic Antimalarial Drugs: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 6865. https://doi.org/10.3390/jcm11226865
Landsteiner de Sampaio Amêndola I, Aires Pinheiro J, Póvoa P, Cés de Souza Dantas V, Serafim RB. COVID-19 Infection in Rheumatic Patients on Chronic Antimalarial Drugs: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(22):6865. https://doi.org/10.3390/jcm11226865
Chicago/Turabian StyleLandsteiner de Sampaio Amêndola, Isabela, Jonathan Aires Pinheiro, Pedro Póvoa, Vicente Cés de Souza Dantas, and Rodrigo Bernardo Serafim. 2022. "COVID-19 Infection in Rheumatic Patients on Chronic Antimalarial Drugs: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 22: 6865. https://doi.org/10.3390/jcm11226865
APA StyleLandsteiner de Sampaio Amêndola, I., Aires Pinheiro, J., Póvoa, P., Cés de Souza Dantas, V., & Serafim, R. B. (2022). COVID-19 Infection in Rheumatic Patients on Chronic Antimalarial Drugs: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(22), 6865. https://doi.org/10.3390/jcm11226865


















