Evolving Role of Catheter Ablation for Atrial Fibrillation: Early and Effective Rhythm Control
Abstract
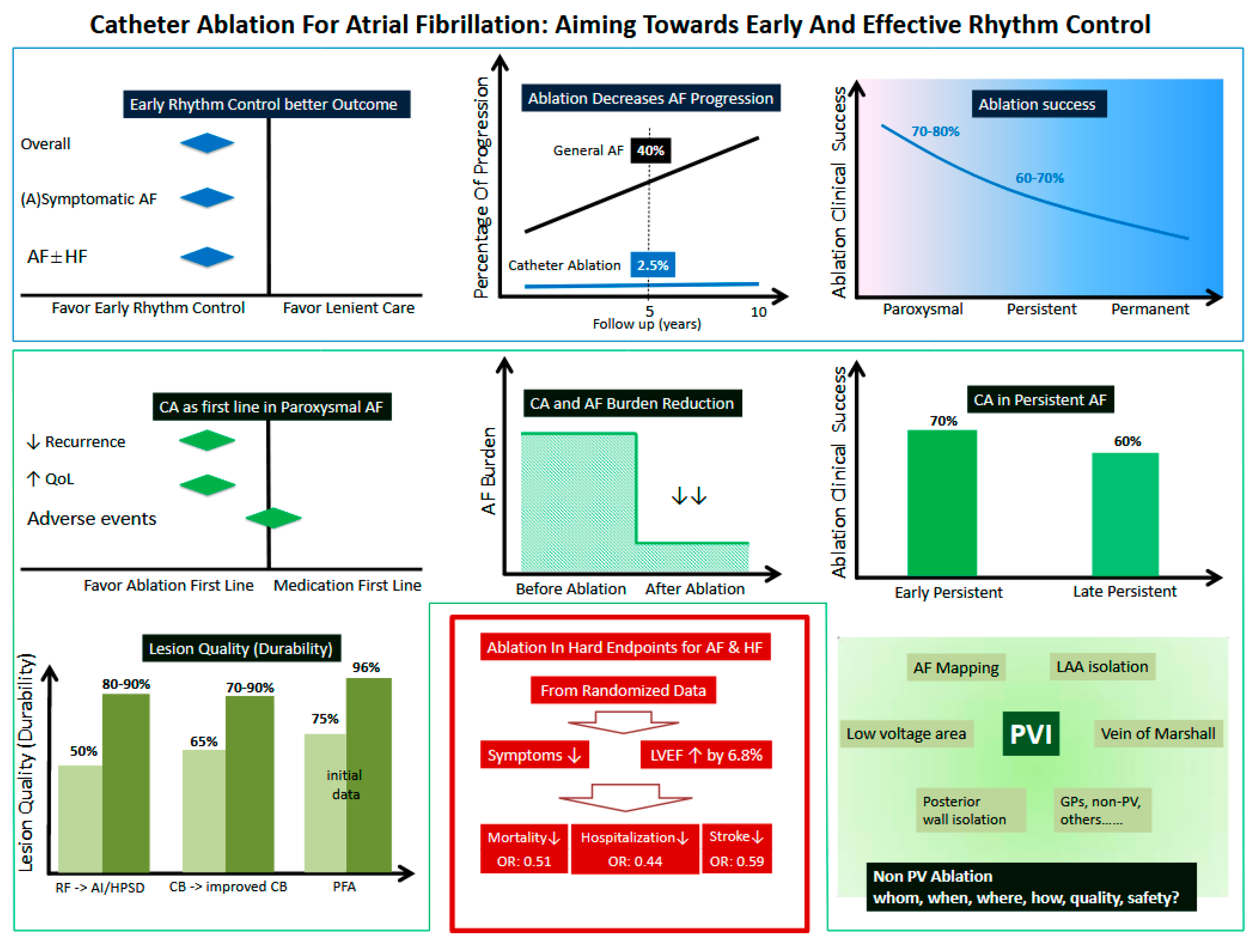
1. Introduction
2. CA as First Line Therapy in Treating Paroxysmal AF
2.1. The Concept of Diagnosis-to-Intervention Time
2.2. Early AADs Rhythm Control vs. Lenient Rate Control
2.3. Early Ablation Rhythm Control vs. AADs Rhythm Control
3. Effect of CA on Progression of AF
4. CA in Treating Persistent AF (Figure 4)

4.1. CA in Treating Persistent AF and Longstanding Persistent AF
4.2. Adjunctive Ablation Strategies beyond PVI in Treating Persistent AF
4.2.1. Ablation of Atrial Low Voltage Area
4.2.2. Left Atrial Posterior Wall Isolation
4.2.3. Ablation Vein of Marshall
4.2.4. Left Atrial Appendage Isolation/Ablation
5. Effect of CA on Hard Clinical Endpoints in AF
6. CA and AF Burden (Figure 6)

6.1. CA and AF Burden in Paroxysmal AF
6.2. CA and AF Burden in Persistent AF
7. Ablation Technologies and Concepts (Figure 7)

8. Safety Considerations
9. Other Aspect: Gender Differences
10. Summary (Figure 8 Graphic Summary)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; ESC Scientific Document Group; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Pürerfellner, H.; Meyer, C.; Sommer, P.; Kiuchi, M.G.; Martinek, M.; Futyma, P.; Zanchi, S.; Zhu, L.; Schratter, A.; et al. Anticoagulation in atrial fibrillation and liver disease: A pooled-analysis of >20000 patients. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 8, 336–345. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019, 74, 104–132. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; EAST-AFNET 4 Trial Investigators; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. New Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Kuniss, M.; Pavlovic, N.; Velagic, V.; Hermida, J.S.; Healey, S.; Arena, G.; Badenco, N.; Meyer, C.; Chen, J.; Cryo-FIRST Investigators; et al. Cryoballoon ablation vs. antiarrhythmic drugs: First-line therapy for patients with paroxysmal atrial fibrillation. Europace 2021, 23, 1033–1041. [Google Scholar] [CrossRef]
- Wazni, O.M.; Dandamudi, G.; Sood, N.; Hoyt, R.; Tyler, J.; Durrani, S.; Niebauer, M.; Makati, K.; Halperin, B.; STOP AF First Trial Investigators; et al. Cryoballoon Ablation as Initial Therapy for Atrial Fibrillation. New Engl. J. Med. 2021, 384, 316–324. [Google Scholar] [CrossRef]
- Andrade, J.G.; Wells, G.A.; Deyell, M.W.; Bennett, M.; Essebag, V.; Champagne, J.; Roux, J.-F.; Yung, D.; Skanes, A.; EARLY-AF Investigators; et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. New Engl. J. Med. 2021, 384, 305–315. [Google Scholar] [CrossRef]
- Morillo, C.A.; Verma, A.; Connolly, S.J.; Kuck, K.H.; Nair, G.M.; Champagne, J.; Sterns, L.D.; Beresh, H.; Healey, J.S.; RAAFT-2 Investigators; et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): A randomized trial. JAMA 2014, 311, 692–700. [Google Scholar] [CrossRef]
- Nielsen, J.C.; Johannessen, A.; Raatikainen, P.; Hindricks, G.; Walfridsson, H.; Kongstad, O.; Pehrson, S.; Englund, A.; Hartikainen, J.; Mortensen, L.S.; et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. New Engl. J. Med. 2012, 367, 1587–1595. [Google Scholar] [CrossRef]
- Wazni, O.M.; Marrouche, N.F.; Martin, D.O.; Verma, A.; Bhargava, M.; Saliba, W.; Bash, D.; Schweikert, R.; Brachmann, J.; Gunther, J.; et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: A randomized trial. JAMA 2005, 293, 2634–2640. [Google Scholar] [CrossRef]
- Turagam, M.K.; Musikantow, D.; Whang, W.; Koruth, J.S.; Miller, M.A.; Langan, M.N.; Sofi, A.; Choudry, S.; Dukkipati, S.R.; Reddy, V.Y. Assessment of Catheter Ablation or Antiarrhythmic Drugs for First-line Therapy of Atrial Fibrillation: A Meta-analysis of Randomized Clinical Trials. JAMA Cardiol. 2021, 6, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Pürerfellner, H.; Ouyang, F.; Kiuchi, M.G.; Meyer, C.; Martinek, M.; Futyma, P.; Zhu, L.; Schratter, A.; Wang, J.; et al. Catheter ablation vs. antiarrhythmic drugs as ‘first-line’ initial therapy for atrial fibrillation: A pooled analysis of randomized data. Europace 2021, 23, 1950–1960. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.R.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. New Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G.; Champagne, J.; Dubuc, M.; Deyell, M.W.; Verma, A.; Macle, L.; Leong-Sit, P.; Novak, P.; Badra-Verdu, M.; Sapp, J.; et al. Cryoballoon or Radiofrequency Ablation for Atrial Fibrillation Assessed by Continuous Monitoring: A Randomized Clinical Trial. Circulation 2019, 140, 1779–1788. [Google Scholar] [CrossRef]
- Proietti, R.; Hadjis, A.; AlTurki, A.; Thanassoulis, G.; Roux, J.F.; Verma, A.; Healey, J.S.; Bernier, M.L.; Birnie, D.; Nattel, S.; et al. A Systematic Review on the Progression of Paroxysmal to Persistent Atrial Fibrillation: Shedding New Light on the Effects of Catheter Ablation. JACC Clin. Electrophysiol. 2015, 1, 105–115. [Google Scholar] [CrossRef]
- Kuck, K.H.; Lebedev, D.S.; Mikhaylov, E.N.; Romanov, A.; Gellér, L.; Kalējs, O.; Neumann, T.; Davtyan, K.; On, Y.K.; Popov, S.; et al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: The randomized controlled atrial fibrillation progression trial (ATTEST). Europace 2021, 23, 362–369. [Google Scholar] [CrossRef]
- Chew, D.S.; Black-Maier, E.; Loring, Z.; Noseworthy, P.A.; Packer, D.L.; Exner, D.V.; Mark, D.B.; Piccini, J.P. Diagnosis-to-Ablation Time and Recurrence of Atrial Fibrillation Following Catheter Ablation: A Systematic Review and Meta-Analysis of Observational Studies. Circ. Arrhythmia Electrophysiol. 2020, 13, e008128. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, X.; Zhu, M.; Chen, S.; Chen, J.; Cai, H.; Dai, J.; Xu, X.; Mao, W. Catheter ablation versus medical therapy for patients with persistent atrial fibrillation: A systematic review and meta-analysis of evidence from randomized controlled trials. J. Interv. Card. Electrophysiol. 2018, 52, 9–18. [Google Scholar] [CrossRef]
- Mansour, M.; Calkins, H.; Osorio, J.; Pollak, S.J.; Melby, D.; Marchlinski, F.E.; Athill, C.A.; Delaughter, C.; Patel, A.M.; Gentlesk, P.J.; et al. Persistent Atrial Fibrillation Ablation With Contact Force-Sensing Catheter: The Prospective Multicenter PRECEPT Trial. JACC Clin. Electrophysiol. 2020, 6, 958–969. [Google Scholar] [CrossRef]
- Schmidt, B.; Neuzil, P.; Luik, A.; Osca Asensi, J.; Schrickel, J.W.; Deneke, T.; Bordignon, S.; Petru, J.; Merkel, M.; Sediva, L.; et al. Laser Balloon or Wide-Area Circumferential Irrigated Radiofrequency Ablation for Persistent Atrial Fibrillation: A Multicenter Prospective Randomized Study. Circ. Arrhythmia Electrophysiol. 2017, 10, e005767. [Google Scholar] [CrossRef]
- Chun, J.K.R.; Bordignon, S.; Last, J.; Mayer, L.; Tohoku, S.; Zanchi, S.; Bianchini, L.; Bologna, F.; Nagase, T.; Urbanek, L.; et al. Cryoballoon Versus Laserballoon: Insights From the First Prospective Randomized Balloon Trial in Catheter Ablation of Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2021, 14, e009294. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Jiang, C.Y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to catheter ablation for persistent atrial fibrillation. New Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.; Das, M.; Riva, S.; Morgan, M.; Ronayne, C.; Sahni, A.; Shaw, M.; Todd, D.; Hall, M.; Modi, S.; et al. Use of Ablation Index-Guided Ablation Results in High Rates of Durable Pulmonary Vein Isolation and Freedom From Arrhythmia in Persistent Atrial Fibrillation Patients: The PRAISE Study Results. Circ. Arrhythmia Electrophysiol. 2018, 11, e006576. [Google Scholar] [CrossRef] [PubMed]
- Ciconte, G.; Baltogiannis, G.; de Asmundis, C.; Sieira, J.; Conte, G.; Di Giovanni, G.; Saitoh, Y.; Irfan, G.; Mugnai, G.; Hunuk, B.; et al. Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: A comparison between radiofrequency catheter ablation and second-generation cryoballoon ablation. Europace 2015, 17, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Omran, H.; Gutleben, K.J.; Molatta, S.; Fischbach, T.; Wellmann, B.; Horstkotte, D.; Körber, B.; Nölker, G. Second generation cryoballoon ablation for persistent atrial fibrillation: An updated meta-analysis. Clin. Res. Cardiol. 2018, 107, 182–192. [Google Scholar] [CrossRef]
- Liu, X.H.; Gao, X.F.; Jin, C.L.; Chen, C.F.; Chen, B.; Xu, Y.Z. Cryoballoon versus radiofrequency ablation for persistent atrial fibrillation: A systematic review and meta-analysis. Kardiol. Pol. 2020, 78, 20–29. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: The DECAAF study. JAMA 2014, 311, 498–506. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Greene, T.; Dean, J.M.; Kholmovski, E.G.; Boer, L.M.; Mansour, M.; Calkins, H.; Marchlinski, F.; Wilber, D.; Hindricks, G.; et al. Efficacy of LGE-MRI-guided fibrosis ablation versus conventional catheter ablation of atrial fibrillation: The DECAAF II trial: Study design. J. Cardiovasc. Electrophysiol. 2021, 32, 916–924. [Google Scholar] [CrossRef]
- Lee, J.M.; Shim, J.; Park, J.; Yu, H.T.; Kim, T.H.; Park, J.K.; Uhm, J.S.; Kim, J.B.; Joung, B.; Lee, M.H.; et al. The Electrical Isolation of the Left Atrial Posterior Wall in Catheter Ablation of Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2019, 5, 1253–1261. [Google Scholar] [CrossRef]
- Kuck, K.H.; Albenque, J.P.; Chun, K.J.; Fürnkranz, A.; Busch, M.; Elvan, A.; Schlüter, M.; Braegelmann, K.M.; Kueffer, F.J.; Hemingway, L.; et al. Repeat Ablation for Atrial Fibrillation Recurrence Post Cryoballoon or Radiofrequency Ablation in the FIRE AND ICE Trial. Circ. Arrhythmia Electrophysiol. 2019, 12, e007247. [Google Scholar] [CrossRef]
- Aryana, A.; Allen, S.L.; Pujara, D.K.; Bowers, M.R.; O’Neill, P.G.; Yamauchi, Y.; Shigeta, T.; Vierra, E.C.; Okishige, K.; Natale, A. Concomitant Pulmonary Vein and Posterior Wall Isolation Using Cryoballoon With Adjunct Radiofrequency in Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2021, 7, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liao, J.; Ling, Z.; Meyer, C.; Sommer, P.; Futyma, P.; Martinek, M.; Schratter, A.; Acou, W.J.; Wang, J.; et al. Adjunctive Left Atrial Posterior Wall Isolation in Treating Atrial Fibrillation: Insight From a Large Secondary Analysis. JACC Clin. Electrophysiol. 2022, 8, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, T.; Derval, N.; Duchateau, J.; Denis, A.; Nakashima, T.; Takagi, T.; Ramirez, F.D.; André, C.; Krisai, P.; Nakatani, Y.; et al. Vein of Marshall Ethanol Infusion: Feasibility, Pitfalls, and Complications in Over 700 Patients. Circ. Arrhythmia Electrophysiol. 2021, 14, e010001. [Google Scholar] [CrossRef]
- Nakashima, T.; Pambrun, T.; Vlachos, K.; Goujeau, C.; André, C.; Krisai, P.; Ramirez, F.D.; Kamakura, T.; Takagi, T.; Nakatani, Y.; et al. Impact of Vein of Marshall Ethanol Infusion on Mitral Isthmus Block: Efficacy and Durability. Circ. Arrhythmia Electrophysiol. 2020, 13, e008884. [Google Scholar] [CrossRef] [PubMed]
- Valderrábano, M.; Peterson, L.E.; Swarup, V.; Schurmann, P.A.; Makkar, A.; Doshi, R.N.; DeLurgio, D.; Athill, C.A.; Ellenbogen, K.A.; Natale, A.; et al. Effect of Catheter Ablation With Vein of Marshall Ethanol Infusion vs Catheter Ablation Alone on Persistent Atrial Fibrillation: The VENUS Randomized Clinical Trial. JAMA 2020, 324, 1620–1628. [Google Scholar] [CrossRef]
- Lador, A.; Peterson, L.E.; Swarup, V.; Schurmann, P.A.; Makkar, A.; Doshi, R.N.; DeLurgio, D.; Athill, C.A.; Ellenbogen, K.A.; Natale, A.; et al. Determinants of outcome impact of vein of Marshall ethanol infusion when added to catheter ablation of persistent atrial fibrillation: A secondary analysis of the VENUS randomized clinical trial. Heart Rhythm 2021, 18, 1045–1054. [Google Scholar] [CrossRef]
- Di Biase, L.; Burkhardt, J.D.; Mohanty, P.; Mohanty, S.; Sanchez, J.E.; Trivedi, C.; Güneş, M.; Gökoğlan, Y.; Gianni, C.; Horton, R.P.; et al. Left Atrial Appendage Isolation in Patients With Longstanding Persistent AF Undergoing Catheter Ablation: BELIEF Trial. J. Am. Coll. Cardiol. 2016, 68, 1929–1940. [Google Scholar] [CrossRef]
- Romero, J.; Gabr, M.; Patel, K.; Briceno, D.; Diaz, J.C.; Alviz, I.; Trivedi, C.; Mohanty, S.; Polanco, D.; Della Rocca, D.G.; et al. Efficacy and safety of left atrial appendage electrical isolation during catheter ablation of atrial fibrillation: An updated meta-analysis. Europace 2021, 23, 226–237. [Google Scholar] [CrossRef]
- Tohoku, S.; Chen, S.; Bordignon, S.; Chun, J.K.; Schmidt, B. Hot or cold? Feasibility, safety, and outcome after radiofrequency-guided versus cryoballoon-guided left atrial appendage isolation. J. Arrhythmia 2022, 38, 316–326. [Google Scholar] [CrossRef]
- Chen, S.; Schmidt, B.; Bordignon, S.; Bologna, F.; Lindhoff-Last, E.; Chun, K.R.J. Thrombus Formation in Isolated Left Atrial Appendage After Multiple Atrial Fibrillation Ablations Despite Oral Anticoagulation Followed by Percutaneous Appendage Closure. JACC Clin. Electrophysiol. 2019, 5, 398–400. [Google Scholar] [CrossRef]
- Chen, S.; Schmidt, B.; Tohoku, S.; Trolese, L.; Bordignon, S.; Chun, K.R.J. Transesophageal echocardiography-guided closure of electrically isolated left atrial appendage to constrain a rapidly growing thrombus despite anticoagulation and sinus rhythm. J. Cardiovasc. Electrophysiol. 2020, 31, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Zender, N.; Weise, F.K.; Bordignon, S.; Herrmann, E.; Konstantinou, A.; Bologna, F.; Nagase, T.; Chen, S.; Chun, K.R.J.; Schmidt, B. Thromboembolism after electrical isolation of the left atrial appendage: A new indication for interventional closure? Europace 2019, 21, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- DeLurgio, D.B.; Crossen, K.J.; Gill, J.; Blauth, C.; Oza, S.R.; Magnano, A.R.; Mostovych, M.A.; Halkos, M.E.; Tschopp, D.R.; Kerendi, F.; et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation: Results of CONVERGE Clinical Trial. Circ. Arrhythmia Electrophysiol. 2020, 13, e009288. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.H.; Fan, J.; Ji, C.C.; Cheng, Y.J.; Chen, X.M.; Jiang, J.Z.; Wu, S.H. Long-Term Outcomes and Improvements in Quality of Life in Patients with Atrial Fibrillation Treated with Catheter Ablation vs. Antiarrhythmic Drugs. Am. J. Cardiovasc. Drugs 2021, 21, 299–320. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. New Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019, 321, 1261–1274. [Google Scholar] [CrossRef]
- Packer, D.L.; Piccini, J.P.; Monahan, K.H.; Al-Khalidi, H.R.; Silverstein, A.P.; Noseworthy, P.A.; Poole, J.E.; Bahnson, T.D.; Lee, K.L.; Mark, D.B.; et al. Ablation Versus Drug Therapy for Atrial Fibrillation in Heart Failure: Results From the CABANA Trial. Circulation 2021, 143, 1377–1390. [Google Scholar] [CrossRef]
- Wu, G.; Huang, H.; Cai, L.; Yang, Y.; Liu, X.; Yu, B.; Tang, Y.; Jiang, H.; Huang, C.; CAPA Study Investigators. Long-term observation of catheter ablation vs. pharmacotherapy in the management of persistent and long-standing persistent atrial fibrillation (CAPA study). Europace 2021, 23, 731–739. [Google Scholar] [CrossRef]
- Chen, S.; Pürerfellner, H.; Meyer, C.; Acou, W.J.; Schratter, A.; Ling, Z.; Liu, S.; Yin, Y.; Martinek, M.; Kiuchi, M.G.; et al. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: A stratified pooled analysis of randomized data. Eur. Heart J. 2020, 41, 2863–2873. [Google Scholar] [CrossRef]
- Steinberg, J.S.; O’Connell, H.; Li, S.; Ziegler, P.D. Thirty-Second Gold Standard Definition of Atrial Fibrillation and Its Relationship With Subsequent Arrhythmia Patterns: Analysis of a Large Prospective Device Database. Circ. Arrhythmia Electrophysiol. 2018, 11, e006274. [Google Scholar] [CrossRef]
- Chen, L.Y.; Chung, M.K.; Allen, L.A.; Ezekowitz, M.; Furie, K.L.; McCabe, P.; Noseworthy, P.A.; Perez, M.V.; Turakhia, M.P.; American Heart Association Council on Clinical Cardiology; et al. Atrial Fibrillation Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e623–e644. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Reynolds, K.; Yang, J.; Gupta, N.; Lenane, J.; Sung, S.H.; Harrison, T.N.; Liu, T.I.; Solomon, M.D. Association of Burden of Atrial Fibrillation With Risk of Ischemic Stroke in Adults With Paroxysmal Atrial Fibrillation: The KP-RHYTHM Study. JAMA Cardiol. 2018, 3, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Duytschaever, M.; De Pooter, J.; Demolder, A.; El Haddad, M.; Phlips, T.; Strisciuglio, T.; Debonnaire, P.; Wolf, M.; Vandekerckhove, Y.; Knecht, S.; et al. Long-term impact of catheter ablation on arrhythmia burden in low-risk patients with paroxysmal atrial fibrillation: The CLOSE to CURE study. Heart Rhythm 2020, 17, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Brachmann, J.; Sohns, C.; Andresen, D.; Siebels, J.; Sehner, S.; Boersma, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Schunkert, H.; et al. Atrial Fibrillation Burden and Clinical Outcomes in Heart Failure: The CASTLE-AF Trial. JACC Clin. Electrophysiol. 2021, 7, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.H.; Merkely, B.; Zahn, R.; Arentz, T.; Seidl, K.; Schlüter, M.; Tilz, R.R.; Piorkowski, C.; Gellér, L.; Kleemann, T.; et al. Catheter Ablation Versus Best Medical Therapy in Patients With Persistent Atrial Fibrillation and Congestive Heart Failure: The Randomized AMICA Trial. Circ. Arrhythmia Electrophysiol. 2019, 12, e007731. [Google Scholar] [CrossRef]
- Chen, S.; Schmidt, B.; Bordignon, S.; Perrotta, L.; Bologna, F.; Chun, K.R.J. Impact of Cryoballoon Freeze Duration on Long-Term Durability of Pulmonary Vein Isolation: ICE Re-Map Study. JACC Clin. Electrophysiol. 2019, 5, 551–559. [Google Scholar] [CrossRef]
- Chen, S.; Schmidt, B.; Bordignon, S.; Tohoku, S.; Urbanek, L.; Plank, K.; Willems, F.; Throm, C.; Konstantinou, A.; Hilbert, M.; et al. Cryoballoon pulmonary vein isolation in treating atrial fibrillation using different freeze protocols: The “ICE-T 4 minutes vs 3 minutes” propensity-matched study (Frankfurt ICE-T 4 vs. 3). J. Cardiovasc. Electrophysiol. 2020, 31, 1923–1931. [Google Scholar] [CrossRef]
- Bourier, F.; Takigawa, M.; Lam, A.; Vlachos, K.; Ramirez, F.D.; Martin, C.A.; Frontera, A.; Kitamura, T.; Duchateau, J.; Pambrun, T.; et al. Ultralow temperature cryoablation: Safety and efficacy of preclinical atrial and ventricular lesions. J. Cardiovasc. Electrophysiol. 2021, 32, 570–577. [Google Scholar] [CrossRef]
- Wielandts, J.Y.; Kyriakopoulou, M.; Almorad, A.; Hilfiker, G.; Strisciuglio, T.; Phlips, T.; El Haddad, M.; Lycke, M.; Unger, P.; Le Polain de Waroux, J.B.; et al. Prospective Randomized Evaluation of High Power During CLOSE-Guided Pulmonary Vein Isolation: The POWER-AF Study. Circ. Arrhythmia Electrophysiol. 2021, 14, e009112. [Google Scholar] [CrossRef]
- O’Brien, J.; Obeidat, M.; Kozhuharov, N.; Ding, W.Y.; Tovmassian, L.; Bierme, C.; Chin, S.H.; Chu, G.S.; Luther, V.; Snowdon, R.L.; et al. Procedural efficiencies, lesion metrics, and 12-month clinical outcomes for Ablation Index-guided 50 W ablation for atrial fibrillation. Europace 2021, 23, 878–886. [Google Scholar] [CrossRef]
- Ravi, V.; Poudyal, A.; Abid, Q.U.; Larsen, T.; Krishnan, K.; Sharma, P.S.; Trohman, R.G.; Huang, H.D. High-power short duration vs. conventional radiofrequency ablation of atrial fibrillation: A systematic review and meta-analysis. Europace 2021, 23, 710–721. [Google Scholar] [CrossRef]
- Chen, S.; Schmidt, B.; Bordignon, S.; Urbanek, L.; Tohoku, S.; Bologna, F.; Angelkov, L.; Garvanski, I.; Tsianakas, N.; Konstantinou, A.; et al. Ablation index-guided 50 W ablation for pulmonary vein isolation in patients with atrial fibrillation: Procedural data, lesion analysis, and initial results from the FAFA AI High Power Study. J. Cardiovasc. Electrophysiol. 2019, 30, 2724–2731. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chun, K.R.J.; Tohoku, S.; Bordignon, S.; Urbanek, L.; Willems, F.; Plank, K.; Hilbert, M.; Konstantinou, A.; Tsianakas, N.; et al. Esophageal Endoscopy After Catheter Ablation of Atrial Fibrillation Using Ablation-Index Guided High-Power: Frankfurt AI-HP ESO-I. JACC Clin. Electrophysiol. 2020, 6, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Schmidt, B.; Seeger, A.; Bordignon, S.; Tohoku, S.; Willems, F.; Urbanek, L.; Throm, C.; Konstantinou, A.; Plank, K.; et al. Catheter ablation of atrial fibrillation using ablation index-guided high power (50 W) for pulmonary vein isolation with or without esophageal temperature probe (the AI-HP ESO II). Heart Rhythm 2020, 17, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Schmidt, B.; Bordignon, S.; Tohoku, S.; Urban, V.C.; Schulte-Hahn, B.; Chun, K.R.J. Catheter ablation of atrial fibrillation using ablation index-guided high-power technique: Frankfurt AI high-power 15-month follow-up. J. Cardiovasc. Electrophysiol. 2021, 32, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, S.; Chen, S.; Bordignon, S.; Bianchini, L.; Tohoku, S.; Bologna, F.; Tondo, C.; Chun, K.R.J.; Schmidt, B. Ablation Index-guided high-power (50 W) short-duration for left atrial anterior and roofline ablation: Feasibility, procedural data, and lesion analysis (AI High-Power Linear Ablation). J. Cardiovasc. Electrophysiol. 2021, 32, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Yavin, H.D.; Leshem, E.; Shapira-Daniels, A.; Sroubek, J.; Barkagan, M.; Haffajee, C.I.; Cooper, J.M.; Anter, E. Impact of High-Power Short-Duration Radiofrequency Ablation on Long-Term Lesion Durability for Atrial Fibrillation Ablation. JACC Clin. Electrophysiol. 2020, 6, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Koruth, J.; Kuroki, K.; Iwasawa, J.; Enomoto, Y.; Viswanathan, R.; Brose, R.; Buck, E.D.; Speltz, M.; Dukkipati, S.R.; Reddy, V.Y. Preclinical Evaluation of Pulsed Field Ablation: Electrophysiological and Histological Assessment of Thoracic Vein Isolation. Circ. Arrhythmia Electrophysiol. 2019, 12, e007781. [Google Scholar] [CrossRef]
- Koruth, J.S.; Kuroki, K.; Kawamura, I.; Brose, R.; Viswanathan, R.; Buck, E.D.; Donskoy, E.; Neuzil, P.; Dukkipati, S.R.; Reddy, V.Y. Pulsed Field Ablation Versus Radiofrequency Ablation: Esophageal Injury in a Novel Porcine Model. Circ. Arrhythmia Electrophysiol. 2020, 13, e008303. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Neuzil, P.; Koruth, J.S.; Petru, J.; Funosako, M.; Cochet, H.; Sediva, L.; Chovanec, M.; Dukkipati, S.R.; Jais, P. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 315–326. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Anic, A.; Koruth, J.; Petru, J.; Funasako, M.; Minami, K.; Breskovic, T.; Sikiric, I.; Dukkipati, S.R.; Kawamura, I.; et al. Pulsed Field Ablation in Patients With Persistent Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 76, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, K.; Whang, W.; Eggert, C.; Lam, J.; Leavitt, J.; Kawamura, I.; Reddy, A.; Morrow, B.; Schneider, C.; Petru, J.; et al. Ostial dimensional changes after pulmonary vein isolation: Pulsed field ablation vs radiofrequency ablation. Heart Rhythm 2020, 17, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Cochet, H.; Nakatani, Y.; Sridi-Cheniti, S.; Cheniti, G.; Ramirez, F.D.; Nakashima, T.; Eggert, C.; Schneider, C.; Viswanathan, R.; Derval, N.; et al. Pulsed field ablation selectively spares the oesophagus during pulmonary vein isolation for atrial fibrillation. Europace 2021, 23, 1391–1399. [Google Scholar] [CrossRef]
- Chun, K.R.J.; Perrotta, L.; Bordignon, S.; Khalil, J.; Dugo, D.; Konstantinou, A.; Fürnkranz, A.; Schmidt, B. Complications in Catheter Ablation of Atrial Fibrillation in 3,000 Consecutive Procedures: Balloon Versus Radiofrequency Current Ablation. JACC Clin. Electrophysiol. 2017, 3, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, A.; Ueberham, L.; Schuler, E.; Wiedemann, M.; Reithmann, C.; Sause, A.; Tebbenjohanns, J.; Schade, A.; Shin, D.I.; Staudt, A.; et al. Cardiac tamponade in catheter ablation of atrial fibrillation: German-wide analysis of 21 141 procedures in the Helios atrial fibrillation ablation registry (SAFER). Europace 2018, 20, 1944–1951. [Google Scholar] [CrossRef]
- Heeger, C.H.; Sohns, C.; Pott, A.; Metzner, A.; Inaba, O.; Straube, F.; Kuniss, M.; Aryana, A.; Miyazaki, S.; Cay, S.; et al. Phrenic Nerve Injury During Cryoballoon-Based Pulmonary Vein Isolation: Results of the Worldwide YETI Registry. Circ. Arrhythmia Electrophysiol. 2022, 15, e010516. [Google Scholar] [CrossRef]
- Tohoku, S.; Chen, S.; Last, J.; Bordignon, S.; Bologna, F.; Trolese, L.; Zanchi, S.; Bianchini, L.; Schmidt, B.; Chun, K.R.J. Phrenic nerve injury in atrial fibrillation ablation using balloon catheters: Incidence, characteristics, and clinical recovery course. J. Cardiovasc. Electrophysiol. 2020, 31, 1932–1941. [Google Scholar] [CrossRef]
- Chen, S.; Schmidt, B.; Bordignon, S.; Bologna, F.; Perrotta, L.; Nagase, T.; Chun, K.R.J. Atrial fibrillation ablation using cryoballoon technology: Recent advances and practical techniques. J. Cardiovasc. Electrophysiol. 2018, 29, 932–943. [Google Scholar] [CrossRef]
- Gandjbakhch, E.; Mandel, F.; Dagher, Y.; Hidden-Lucet, F.; Rollin, A.; Maury, P. Incidence, epidemiology, diagnosis and prognosis of atrio-oesophageal fistula following percutaneous catheter ablation: A French nationwide survey. Europace 2021, 23, 557–564. [Google Scholar] [CrossRef]
- John, R.M.; Kapur, S.; Ellenbogen, K.A.; Koneru, J.N. Atrioesophageal fistula formation with cryoballoon ablation is most commonly related to the left inferior pulmonary vein. Heart Rhythm 2017, 14, 184–189. [Google Scholar] [CrossRef]
- Takigawa, M.; Kuwahara, T.; Takahashi, A.; Watari, Y.; Okubo, K.; Takahashi, Y.; Takagi, K.; Kuroda, S.; Osaka, Y.; Kawaguchi, N.; et al. Differences in catheter ablation of paroxysmal atrial fibrillation between males and females. Int. J. Cardiol. 2013, 168, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Deshmukh, A.; Thakkar, B.; Coffey, J.O.; Agnihotri, K.; Patel, A.; Ainani, N.; Nalluri, N.; Patel, N.; Patel, N.; et al. Gender, Race, and Health Insurance Status in Patients Undergoing Catheter Ablation for Atrial Fibrillation. Am. J. Cardiol. 2016, 117, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Vallakati, A.; Reddy, M.; Sharma, A.; Kanmanthareddy, A.; Sridhar, A.; Pillarisetti, J.; Atkins, D.; Konda, B.; Bommana, S.; Di Biase, L.; et al. Impact of gender on outcomes after atrial fibrillation ablation. Int. J. Cardiol. 2015, 187, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Ngo, L.; Ali, A.; Ganesan, A.; Woodman, R.; Adams, R.; Ranasinghe, I. Gender differences in complications following catheter ablation of atrial fibrillation. Eur. Heart J.-Qual. Care Clin. Outcomes 2021, 7, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Sugumar, H.; Nanayakkara, S.; Chieng, D.; Wong, G.R.; Parameswaran, R.; Anderson, R.D.; Al-Kaisey, A.; Nalliah, C.J.; Azzopardi, S.; Prabhu, S.; et al. Arrhythmia recurrence is more common in females undergoing multiple catheter ablation procedures for persistent atrial fibrillation: Time to close the gender gap. Heart Rhythm 2020, 17 Pt A, 692–698. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Yin, Y.; Ling, Z.; Meyer, C.; Pürerfellner, H.; Martinek, M.; Kiuchi, M.G.; Futyma, P.; Zhu, L.; Schratter, A.; et al. Evolving Role of Catheter Ablation for Atrial Fibrillation: Early and Effective Rhythm Control. J. Clin. Med. 2022, 11, 6871. https://doi.org/10.3390/jcm11226871
Chen S, Yin Y, Ling Z, Meyer C, Pürerfellner H, Martinek M, Kiuchi MG, Futyma P, Zhu L, Schratter A, et al. Evolving Role of Catheter Ablation for Atrial Fibrillation: Early and Effective Rhythm Control. Journal of Clinical Medicine. 2022; 11(22):6871. https://doi.org/10.3390/jcm11226871
Chicago/Turabian StyleChen, Shaojie, Yuehui Yin, Zhiyu Ling, Christian Meyer, Helmut Pürerfellner, Martin Martinek, Márcio Galindo Kiuchi, Piotr Futyma, Lin Zhu, Alexandra Schratter, and et al. 2022. "Evolving Role of Catheter Ablation for Atrial Fibrillation: Early and Effective Rhythm Control" Journal of Clinical Medicine 11, no. 22: 6871. https://doi.org/10.3390/jcm11226871
APA StyleChen, S., Yin, Y., Ling, Z., Meyer, C., Pürerfellner, H., Martinek, M., Kiuchi, M. G., Futyma, P., Zhu, L., Schratter, A., Wang, J., Acou, W.-J., Sommer, P., Ouyang, F., Liu, S., Chun, J. K. R., & Schmidt, B. (2022). Evolving Role of Catheter Ablation for Atrial Fibrillation: Early and Effective Rhythm Control. Journal of Clinical Medicine, 11(22), 6871. https://doi.org/10.3390/jcm11226871





