Free Light Chains, High Mobility Group Box 1, and Mortality in Hemodialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Laboratory Analyses
2.3. Statistical Analyses
3. Results
3.1. Baseline Characteristics
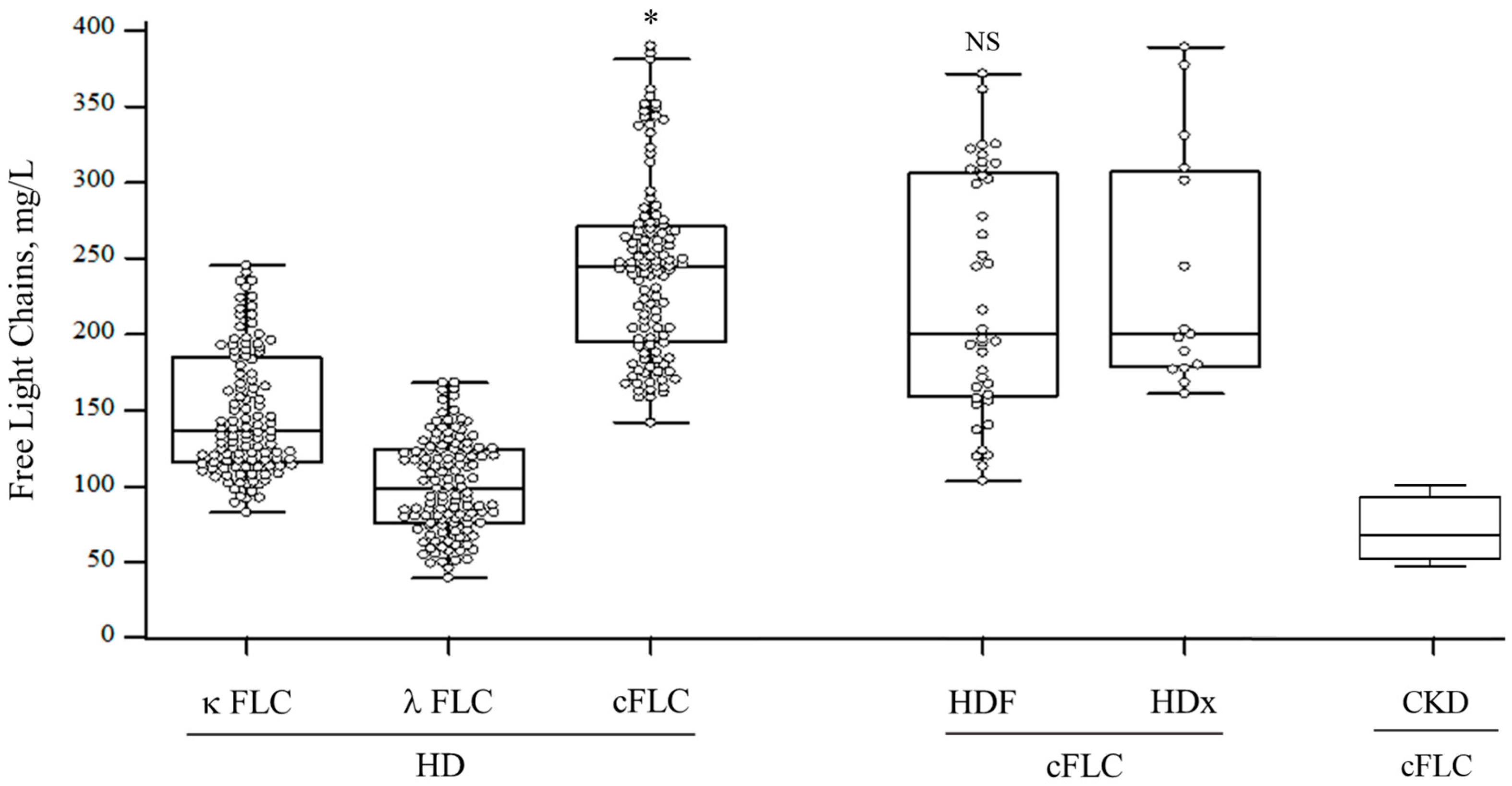
3.2. FLC Levels
3.3. Inflammatory and Immunologic Markers
3.4. Correlates of cFLCs
3.5. Mortality in HD Patients
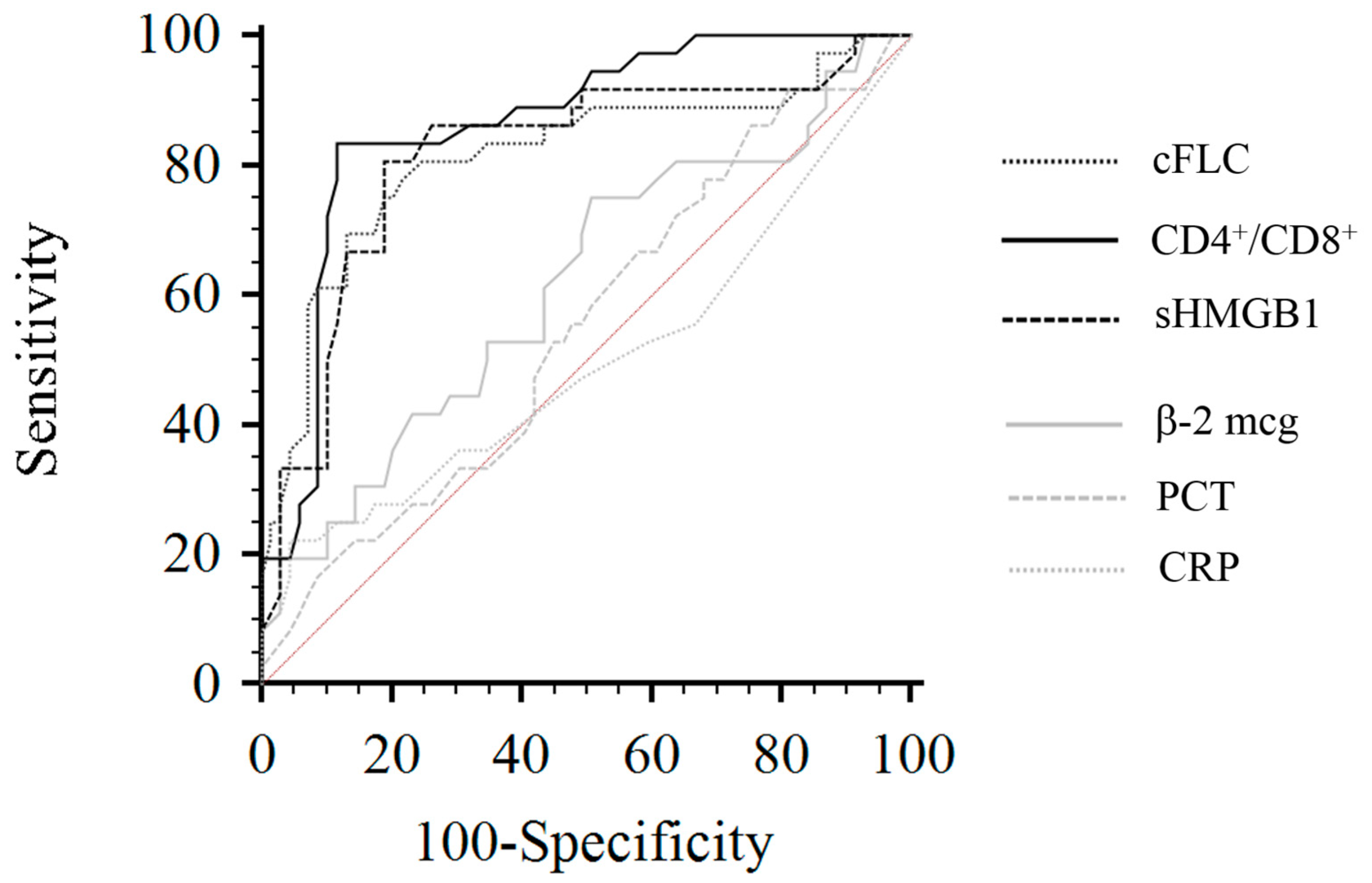
3.6. Univariate/Multiple Cox Regression Analysis and Mortality Risk in HD Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; European Uremic Toxin Work Group. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savica, V.; Calò, L.A.; Monardo, P.; Santoro, D.; Mallamace, A.; Muraca, U.; Bellinghieri, G. Salivary Phosphorus and Phosphate Content of Beverages: Implications for the Treatment of Uremic Hyperphosphatemia. J. Ren. Nutr. 2009, 19, 69–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savica, V.; Calò, L.A.; Monardo, P.; Caldarera, R.; Cavaleri, A.; Santoro, D.; Muraca, U.; Mallamace, A.; Bellinghieri, G. High phosphate content beverages in dialysis patients: Relevance for hyperphosphatemia and cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2008, 18, e39–e40. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Rudnicki, M.; Horl, W.H. Uremic toxins modulate the spontaneous apoptotic cell death and essential functions of neutrophils. Kidney Int. Suppl. 2011, 78, S48–S52. [Google Scholar]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxylsulfate and p-cresylsulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef] [Green Version]
- Monardo, P.; Lacquaniti, A.; Campo, S.; Bucca, M.; di Tocco, T.C.; Rovito, S.; Ragusa, A.; Santoro, A. Updates on hemodialysis techniques with a common denominator: The personalization of the dialytic therapy. Semin. Dial. 2021, 34, 183–195. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P. Inflammation in end-stage renal disease—What have we learned in 10 years? Semin. Dial. 2010, 23, 498–509. [Google Scholar] [CrossRef]
- Campo, S.; Lacquaniti, A.; Trombetta, D.; Smeriglio, A.; Monardo, P. Immune System Dysfunction and Inflammation in Hemodialysis Patients: Two Sides of the Same Coin. J. Clin. Med. 2022, 11, 3759. [Google Scholar] [CrossRef]
- Meier, P.; Golshayan, D.; Blanc, E.; Pascual, M.; Burnier, M. Oxidized LDL Modulates Apoptosis of Regulatory T Cells in Patients with ESRD. J. Am. Soc. Nephrol. 2009, 20, 1368–1384. [Google Scholar] [CrossRef] [Green Version]
- Hendrikx, T.K.; van Gurp, E.A.; Mol, W.M.; Schoordijk, W.; Sewgobind, V.D.; Ijzermans, J.N.; Weimar, W.; Baan, C.C. End-stage renal failure and regulatory activities of CD4+CD25 bright +FoxP3+ T-cells. Nephrol. Dial. Transplant. 2009, 24, 1969–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirico, V.; Lacquaniti, A.; Salpietro, V.; Munafò, C.; Calabrò, M.P.; Buemi, M.; Arrigo, T.; Salpietro, C. High-mobility group box 1 (HMGB1) in childhood: From bench to bedside. Eur. J. Pediatr. 2014, 173, 1123–1136. [Google Scholar] [CrossRef]
- Chirico, V.; Lacquaniti, A.; Vinci, S.; Piraino, B.; Manti, S.; Marseglia, L.; Salpietro, A.; Gitto, E.; Arrigo, T.; Salpietro, C.; et al. High-mobilitygroup box 1 in allergic and non allergic upper airway inflammation. J. Biol. Regul. Homeost. Agents 2015, 29, 55–57. [Google Scholar]
- Chimenz, R.; Chirico, V.; Basile, P.; Carcione, A.; Conti, G.; Monardo, P.; Lacquaniti, A. HMGB-1 and TGFβ-1 highlight im-muno-inflammatory and fibrotic processes before proteinuria onset in pe-diatric patients with Alport syndrome. J. Nephrol. 2021, 34, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Bruchfeld, A.; Qureshi, A.R.; Lindholm, B.; Barany, P.; Yang, L.; Stenvinkel, P.; Tracey, K.J. High Mobility Group Box Protein-1 Correlates with Renal Function in Chronic Kidney Disease (CKD). Mol. Med. 2008, 14, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hu, Z.; Zeng, R.; Yao, Y. HMGB1 in kidney diseases. Life Sci. 2020, 259, 118203. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Son, M.; Diamond, B. HMGB1 in Systemic Lupus Erythematosus. Front. Immunol. 2020, 11, 1057. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, G.; Kong, X. Serum level of high mobility group box protein-1 and prognosis of patients with end-stage renal disease on hemodialysis and peritoneal dialysis. Medicine 2021, 100, e24275. [Google Scholar] [CrossRef] [PubMed]
- Chimenz, R.; Lacquaniti, A.; Colavita, L.; Chirico, V.; Fede, C.; Buemi, M.; Fede, C. High mobility group box 1 and tumor growth factor β: Useful biomarkers in pediatric patients receiving peritoneal dialysis. Ren. Fail. 2016, 38, 1370–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Li, S.; Li, H.; Xiong, L.; Zhou, Y.; Fan, J.; Yu, X.; Mao, H. The Potential Role of HMGB1 Release in Peritoneal Dialysis-Related Peritonitis. PLoS ONE 2013, 8, e54647. [Google Scholar] [CrossRef]
- Leelahavanichkul, A.; Huang, Y.; Hu, X.; Zhou, H.; Tsuji, T.; Chen, R.; Kopp, J.B.; Schnermann, J.; Yuen, P.S.; Star, R.A. Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing High Mobility Group Box Pro-tein-1. Kidney Int. 2011, 80, 1198–1211. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Ren, J.; Liu, Q.; Hu, Q.; Wu, X.; Wang, G.; Hong, Z.; Ren, H.; Li, J. Effects of Changes in the Levels of Damage-Associated Molecular Patterns Following Continuous Veno–Venous Hemofiltration Therapy on Outcomes in Acute Kidney Injury Patients With Sepsis. Front. Immunol. 2019, 9, 3052. [Google Scholar] [CrossRef] [Green Version]
- Lisowska, K.A.; Radzka, M.; Witkowski, J.M.; Rutkowski, B.; Bryl, E.; Debska-Slizien, A. Recombinant Human Erythropoietin Treatment of Chronic Renal Failure Patients Normalizes Altered Phenotype and Proliferation of CD4-positive T Lymphocytes. Artif. Organs 2010, 34, E77–E84. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, K.A.; Dębska-Ślizień, M.A.; Jasiulewicz, A.; Heleniak, Z.; Bryl, E.; Witkowski, J.M. Hemodialysis Affects Phenotype and Proliferation of CD4-Positive T Lymphocytes. J. Clin. Immunol. 2011, 32, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasiulewicz, A.; Lisowska, K.A.; Pietruczuk, K.; Frąckowiak, J.; Fulop, T.; Witkowski, J.M. Homeostatic ‘bystander’ proliferation of human peripheral blood B cells in response to polyclonal T-cell stimulation in vitro. Int. Immunol. 2015, 27, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, N.D.; Pahl, M.V.; Crum, A.; Norris, K. Effect of Uremia on Structure and Function of Immune System. J. Ren. Nutr. 2012, 22, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Cohen, G. Immunoglobulin light chains in uremia. Kidney Int. 2003, 63, S15–S18. [Google Scholar] [CrossRef] [Green Version]
- Brebner, J.A.; Stockley, R.A. Polyclonal free light chains: A biomarker of inflammatory disease or treatment target? F1000 Med. Rep. 2013, 5, 4. [Google Scholar] [CrossRef]
- Hutchison, C.A.; Harding, S.; Hewins, P.; Mead, G.P.; Townsend, J.; Bradwell, A.R.; Cockwell, P. Quantitative Assessment of Serum and Urinary Polyclonal Free Light Chains in Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1684–1690. [Google Scholar] [CrossRef] [Green Version]
- Katzmann, J.A.; Clark, R.J.; Abraham, R.S.; Bryant, S.; Lymp, J.F.; Bradwell, A.R.; Kyle, R.A. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: Relative sensitivity for detection of monoclonal light chains. Clin. Chem. 2022, 48, 1437–1444. [Google Scholar] [CrossRef] [Green Version]
- Miettinen, T.A.; Kekki, M. Effect of impaired hepatic and renal function on [131] bence jones protein catabolism in human subjects. Clin. Chim. Acta 1967, 18, 395–407. [Google Scholar] [CrossRef]
- Haynes, R.; Hutchison, C.A.; Emberson, J.; Dasgupta, T.; Wheeler, D.C.; Townend, J.N.; Landray, M.J.; Cockwell, P. Serum Free Light Chains and the Risk of ESRD and Death in CKD. Clin. J. Am. Soc. Nephrol. 2011, 6, 2829–2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desjardins, L.; Liabeuf, S.; Lenglet, A.; Lemke, H.D.; Vanholder, R.; Choukroun, G.; Massy, Z.A. European Uremic Toxin (EUTox)Work Group: Association between free light chain levels, and disease progression and mortality in chronic kidney disease. Toxins 2013, 5, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, C.A.; Burmeister, A.; Harding, S.J.; Basnayake, K.; Church, H.; Jesky, M.D.; White, K.; Green, C.E.; Stringer, S.J.; Bassett, P.; et al. Serum Polyclonal Immunoglobulin Free Light Chain Levels Predict Mortality in People With Chronic Kidney Disease. Mayo Clin. Proc. 2014, 89, 615–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamy, T.; Henri, P.; Lobbedez, T.; Comby, E.; Ryckelynck, J.-P.; Ficheux, M. Comparison between On-Line High-Efficiency Hemodiafiltration and Conventional High-Flux Hemodialysis for Polyclonal Free Light Chain Removal. Blood Purif. 2014, 37, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Dellepiane, S.; Marengo, M.; D’Arezzo, M.; Donati, G.; Fabbrini, P.; Lacquaniti, A.; Ronco, C.; Cantaluppi, V. The Next Evolution of HemoDialysis eXpanded: From a Delphi Questionnaire-Based Approach to the Real Life of Italian Dialysis Units. Blood Purif. 2022, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, A.H.; Lyko, R.; Nilsson, L.-G.; Beck, W.; Amdahl, M.; Lechner, P.; Schneider, A.; Wanner, C.; Rosenkranz, A.R.; Krieter, D.H. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol. Dial. Transplant. 2016, 32, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Dispenzieri, A.; Katzmann, J.A.; Kyle, R.A.; Larson, D.R.; Therneau, T.M.; Colby, C.L.; Clark, R.J.; Mead, G.P.; Kumar, S.; Melton, L.J.; et al. Use of Nonclonal Serum Immunoglobulin Free Light Chains to Predict Overall Survival in the General Population. Mayo Clin. Proc. 2012, 87, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, J.; Assi, L.K.; Burmeister, A.; Hoefield, R.; Cockwell, P.; Kalra, P.A. Association of Serum Ig Free Light Chains with Mortality and ESRD among Patients with Non dialysis-Dependent CKD. Clin. J. Am. Soc. Nephrol. 2015, 10, 740–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, G.; Rudnicki, M.; Schmaldienst, S.; Hörl, W.H. Effect of dialysis on serum/plasma levels of free immunoglobulin light chains in end-stage renal disease patients. Nephrol. Dial. Transplant. 2022, 17, 879–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, M.; Masakane, I.; Wada, A.; Nakai, S.; Nitta, K.; Nakamoto, H. Super high-flux membrane dialyzers improve mortality in patients on hemodialysis: A 3-year nationwide cohort study. Clin. Kidney J. 2021, 15, 473–483. [Google Scholar] [CrossRef]
- Fenton, A.; Jesky, M.D.; Webster, R.; Stringer, S.J.; Yadav, P.; Chapple, I.; Dasgupta, I.; Harding, S.J.; Ferro, C.; Cockwell, P. Association between urinary free light chains and progression to end stage renal disease in chronic kidney disease. PLoS ONE 2018, 13, e0197043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, W.Z.; Wang, P.X.; Aaron, K.J.; Basnayake, K.; Sanders, P.W. Immunoglobulin light chains activate nuclear factor-kappaB in renal epithelial cells through a Src-dependent mechanism. Blood 2011, 117, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hering-Smith, K.S.; Simon, E.E.; Batuman, V. Myeloma light chains induce epithelial-mesenchymal transition in human renal proximal tubule epithelial cells. Nephrol. Dial. Transplant. 2008, 23, 860–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burmeister, A.; Assi, L.K.; Ferro, C.; Hughes, R.G.; Barnett, A.H.; Bellary, S.; Cockwell, P.; Pratt, G.; Hutchison, C.A. The relationship between high-sensitivity CRP and polyclonal Free Light Chains as markers of inflammation in chronic disease. Int. J. Lab. Hematol. 2013, 36, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.; Rocco, M.V.; Yan, G.; Leypoldt, J.K.; Levin, N.W.; Greene, T.; Agodoa, L.; Bailey, J.; Beck, G.J.; Clark, W.; et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: Results of the HEMO study. J. Am. Soc. Nephrol. 2006, 17, 546–555. [Google Scholar] [CrossRef] [Green Version]
- Okuno, S.; Ishimura, E.; Kohno, K.; Fujino-Katoh, Y.; Maeno, Y.; Yamakawa, T.; Inaba, M.; Nishizawa, Y. Serum beta2-microglobulin level is a significant predictor of mortality in maintenance hemodialysis patients. Nephrol. Dial. Transplant. 2009, 24, 571–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolignano, D.; Coppolino, G.; Romeo, A.; Lacquaniti, A.; Buemi, M. Neutrophil gelatinase-associated lipocalin levels in chronic hemodialysis patients: Brief Communication. Nephrology 2010, 15, 23–26. [Google Scholar] [CrossRef]
- Timmermanns, K.; Kox, M.; Scheffer, G.J.; Pickkers, P. Danger in the intensive care unit: Damps in critically ill patients. Shock 2016, 45, 108–116. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Antoniadi, G.; Liakopoulos, V.; Kartsios, C.; Stefanidis, I. Basic Science and Dialysis: Disturbances of Acquired Immunity in Hemodialysis Patients. Semin. Dial. 2007, 20, 440–451. [Google Scholar] [CrossRef]
- Betjes, M.G.H. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013, 9, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Litjens, N.H.; Van Druningen, C.J.; Betjes, M. Progressive loss of renal function is associated with activation and depletion of naive T lymphocytes. Clin. Immunol. 2006, 118, 83–91. [Google Scholar] [CrossRef]
- Ducloux, D.; Legendre, M.; Bamoulid, J.; Rebibou, J.-M.; Saas, P.; Courivaud, C.; Crepin, T. ESRD-associated immune phenotype depends on dialysis modality and iron status: Clinical implications. Immun. Ageing 2018, 15, 16. [Google Scholar] [CrossRef]
- Betjes, M.G.; Meijers, R.W.; de Wit, E.A.; Weimar, W.; Litjens, N.H. Terminally Differentiated CD8+ Temra Cells Are Associated With the Risk for Acute Kidney Allograft Rejection. Transplantation 2012, 94, 63–69. [Google Scholar] [CrossRef]


| Variable | All Patients (n = 119) | Progressors (n = 36) | Non Progressors (n = 83) | p |
|---|---|---|---|---|
| Age, years | 71 (55.2–76.7) | 75.7 (68.5–78.9) | 60.2 (53.2–65.8) | <0.01 |
| M/F | 82/37 | 21/15 | 61/22 | 0.13 |
| Dialysis vintage, months | 57.6 ± 16.8 | 63.2± 11.3 | 58.1± 13.4 | 0.09 |
| spKt/V, weekly mean | 1.4 ± 0.3 | 1.4 ± 0.2 | 1.3 ± 0.1 | 0.37 |
| Dialysis session length, min | 240 ±0.11 | 240 ± 0.14 | 240 ± 0.10 | 0.47 |
| Diabetes, n (%) | 67 (56) | 31(86) | 36 (43) | 0.01 |
| Hypertension, n (%) | 61 (51) | 21 | 40 | 0.32 |
| Laboratory parameters | ||||
| Creatinine, mg/dL | 9.73 ± 2.8 | 9.12 ± 1.8 | 10.1 ± 1.2 | 0.67 |
| Urea, mg/dL | 167.2 ± 41.1 | 212.7 ± 23.6 | 196.3 ± 30.1 | 0.57 |
| Potassium, mmol/L | 5 ± 0.8 | 5.6 ± 0.4 | 5.9 ± 0.2 | 0.89 |
| Albumin, g/dL | 3 ± 1.3 | 3.3 ± 0.9 | 3.9 ± 0.2 | 0.77 |
| Phosphate, mg/dL | 5.1 ± 1.8 | 6.2 ± 0.7 | 5.8 ± 1.1 | 0.61 |
| Serum Calcium, mg/dL | 8.4 ± 0.7 | 8.9 ± 0.3 | 9.1 ± 0.6 | 0.45 |
| PTH, pg/mL, median (IQR) | 321 (186–428) | 387 (202–387) | 341 (232–431) | 0.09 |
| Total cholesterol, mg/dL | 140.5 ± 32.3 | 162.5 ± 22.1 | 151.1 ± 12.9 | 0.32 |
| White blood cells, mm3 | 7.07 ± 2.4 | 8.12 ± 1.9 | 8.9 ± 1.1 | 0.23 |
| Hemoglobin, g/dL | 11.5 ± 1.2 | 10.7 ± 0.9 | 11.7 ± 0.7 | 0.09 |
| TSAT, %, median (IQR) | 27.8 (22.1–40.1) | 23.1 (19.4–33.2) | 28.1 (21.7–36.1) | 0.11 |
| BNP, pg/mL, median (IQR) | 5440 (1650–9960) | 10,457 (6594–12,660) | 4679 (1369–7460) | <0.01 |
| Inflammatory markers | ||||
| Ferritin, ng/mL median (IQR) | 605.5 (448–914) | 816 (723–1021) | 493 (401–613) | <0.01 |
| CRP, mg/dL median (IQR) | 0.5 (0.5–1.4) | 1.7 (1.4–2.1) | 0.4 (0.2–0.9) | 0.02 |
| PCT, ng/mL median (IQR) | 0.2 (0.1–0.3) | 0.4 (0.2–0.5) | 0.3 (0.2–0.4) | 0.21 |
| Homocistein, μmol/L | 30.19 ± 13.9 | 33.5 ± 8.7 | 31.2 ± 11.4 | 0.30 |
| α1 protein, mg/dL | 4.9 ± 1.1 | 5.2 ± 0.7 | 4.7 ± 1.3 | 0.49 |
| α2 protein, mg/dL | 10.2 ± 2 | 9.7 ± 1.3 | 10.1 ± 1.2 | 0.87 |
| β-2 MG, mg/L, median (IQR) | 28.7 (22.1–32.4) | 32.1 (28.5–36) | 21.2 (18.5–28) | 0.02 |
| cFLC, mg/L, median (IQR) | 244.4 (197.9–273.5) | 251 (205–341) | 177.5 (161–207) | <0.01 |
| Immunity markers | ||||
| sHMGB1, pg/mL, median (IQR) | 94.5 (55–302) | 67 (54–111) | 152 (98–297) | <0.01 |
| White blood cells, mm3 | 7.07 ± 2.4 | 8.1 ± 1.9 | 8.9 ± 1.1 | 0.25 |
| CD4+/CD8+ ratio, median (IQR) | 1.1 (0.7–1.5) | 0.7 (0.6–0.9) | 1.2 (0.9–1.6) | 0.01 |
| γglobulin, UA/mL | 15.1 ± 4.1 | 13.2 ± 1.8 | 14.9 ± 2.8 | 0.11 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | 1.03 | 0.91–1.06 | 0.11 | 1.03 | 0.97–1.04 | 0.16 |
| Diabetes mellitus | 1.10 | 0.95–1.21 | 0.32 | |||
| BNP | 1.03 | 1.01–1.05 | <0.01 | 1.08 | 1.04–1.10 | 0.01 |
| Ferritin | 1.06 | 0.97–1.18 | 0.37 | |||
| CRP | 1.10 | 0.98–1.09 | 0.08 | |||
| β2-MG | 1.09 | 1.01–1.13 | <0.01 | 1.09 | 1.05–1.12 | 0.02 |
| CD4+/CD8+ ratio | 1.02 | 0.97–1.04 | 0.10 | |||
| sHMGB1 | 0.83 | 0.74–0.96 | 0.02 | 0.95 | 0.89–0.98 | 0.03 |
| cFLCs | 1.02 | 1.01–1.03 | 0.01 | 1.11 | 1.06–1.13 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacquaniti, A.; Campo, S.; Falliti, G.; Caruso, D.; Gargano, R.; Giunta, E.; Monardo, P. Free Light Chains, High Mobility Group Box 1, and Mortality in Hemodialysis Patients. J. Clin. Med. 2022, 11, 6904. https://doi.org/10.3390/jcm11236904
Lacquaniti A, Campo S, Falliti G, Caruso D, Gargano R, Giunta E, Monardo P. Free Light Chains, High Mobility Group Box 1, and Mortality in Hemodialysis Patients. Journal of Clinical Medicine. 2022; 11(23):6904. https://doi.org/10.3390/jcm11236904
Chicago/Turabian StyleLacquaniti, Antonio, Susanna Campo, Giuseppe Falliti, Daniele Caruso, Romana Gargano, Elena Giunta, and Paolo Monardo. 2022. "Free Light Chains, High Mobility Group Box 1, and Mortality in Hemodialysis Patients" Journal of Clinical Medicine 11, no. 23: 6904. https://doi.org/10.3390/jcm11236904





