Pre-Diagnosis Sleep Status and Survival after a Diagnosis of Ovarian Cancer: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
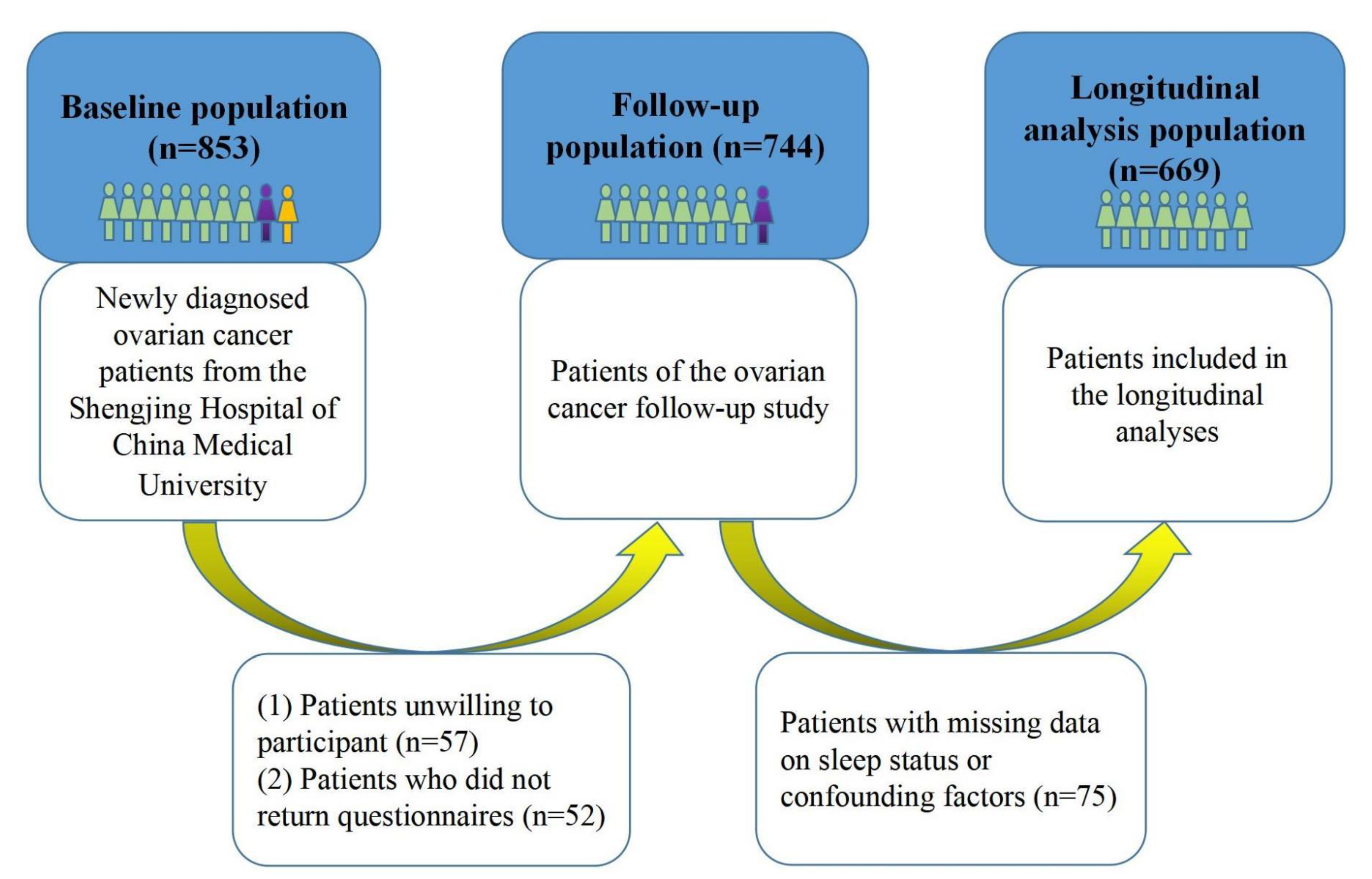
2.1. Study Population
2.2. Data Collection
2.3. Exposure Assessment
2.4. Follow-Up and Outcome
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coburn, S.B.; Bray, F.; Sherman, M.E.; Trabert, B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int. J. Cancer 2017, 140, 2451–2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Chen, W.; Zheng, R.; Zhang, S.; Ji, J.S.; Zou, X.; Xia, C.; Sun, K.; Yang, Z.; Li, H.; et al. Changing cancer survival in China during 2003-15: A pooled analysis of 17 population-based cancer registries. Lancet Glob. Health 2018, 6, e555–e567. [Google Scholar] [CrossRef] [Green Version]
- Goode, E.L.; Maurer, M.J.; Sellers, T.A.; Phelan, C.M.; Kalli, K.R.; Fridley, B.L.; Vierkant, R.A.; Armasu, S.M.; White, K.L.; Keeney, G.L.; et al. Inherited determinants of ovarian cancer survival. Clin. Cancer Res. 2010, 16, 995–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011, 61, 183–203. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, A.S. Clinical Staging of Ovarian Cancer. Methods Mol. Biol. 2022, 2424, 3–10. [Google Scholar] [CrossRef]
- Polterauer, S.; Vergote, I.; Concin, N.; Braicu, I.; Chekerov, R.; Mahner, S.; Woelber, L.; Cadron, I.; Van Gorp, T.; Zeillinger, R.; et al. Prognostic value of residual tumor size in patients with epithelial ovarian cancer FIGO stages IIA-IV: Analysis of the OVCAD data. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2012, 22, 380–385. [Google Scholar] [CrossRef]
- Zhou, F.; Ding, J. Prognosis and factors affecting colorectal cancer with ovarian metastasis. Updat. Surg. 2021, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Sassone-Corsi, P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018, 24, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.A.; Knudsen, K.E. Cancer and the Circadian Clock. Cancer Res. 2019, 79, 3806–3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulli, G.; Lam, M.T.Y.; Panda, S. Interplay between Circadian Clock and Cancer: New Frontiers for Cancer Treatment. Trends Cancer 2019, 5, 475–494. [Google Scholar] [CrossRef] [PubMed]
- Hurley, S.; Goldberg, D.; Bernstein, L.; Reynolds, P. Sleep duration and cancer risk in women. Cancer Causes Control 2015, 26, 1037–1045. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Harris, H.R.; Hendryx, M.; Shadyab, A.H.; Hale, L.; Li, Y.; Crane, T.E.; Cespedes Feliciano, E.M.; Stefanick, M.L.; Luo, J. Sleep Characteristics and Risk of Ovarian Cancer Among Postmenopausal Women. Cancer Prev. Res. 2021, 14, 55–64. [Google Scholar] [CrossRef]
- Phipps, A.I.; Bhatti, P.; Neuhouser, M.L.; Chen, C.; Crane, T.E.; Kroenke, C.H.; Ochs-Balcom, H.; Rissling, M.; Snively, B.M.; Stefanick, M.L.; et al. Pre-diagnostic Sleep Duration and Sleep Quality in Relation to Subsequent Cancer Survival. J. Clin. Sleep Med. 2016, 12, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Yuan, Y.; Xue, Q.; Li, X.; Wang, M.; Ma, H.; Heianza, Y.; Qi, L. Adherence to a healthy sleep pattern is associated with lower risks of all-cause, cardiovascular and cancer-specific mortality. J Intern. Med. 2022, 291, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Gong, T.T.; Gao, S.; Li, X.Q.; Liu, F.H.; Wen, Z.Y.; Wei, Y.F.; Yan, S.; Hou, R.; Wu, Q.J. Pre-diagnosis Dairy Product Intake and Ovarian Cancer Mortality: Results from the Ovarian Cancer Follow-Up Study (OOPS). Front. Nutr. 2021, 8, 750801. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.F.; Hao, Y.Y.; Gao, S.; Li, X.Q.; Liu, F.H.; Wen, Z.Y.; Wang, H.Y.; Zhang, S.; Yan, S.; Luan, M.; et al. Pre-diagnosis Cruciferous Vegetables and Isothiocyanates Intake and Ovarian Cancer Survival: A Prospective Cohort Study. Front. Nutr. 2021, 8, 778031. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Ji, A.; Lou, H.; Lou, P.; Xu, C.; Zhang, P.; Qiao, C.; Yang, Q. Interactive effect of sleep duration and sleep quality on risk of stroke: An 8-year follow-up study in China. Sci. Rep. 2020, 10, 8690. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.W.; Han, J.W.; Lee, J.R.; Byun, S.; Kwon, S.J.; Oh, S.H.; Lee, K.H.; Han, G.; Hong, J.W.; Kwak, K.P.; et al. Sleep and cognitive decline: A prospective nondemented elderly cohort study. Ann. Neurol. 2018, 83, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Shinzawa, M.; Isaka, Y.; Yamakoshi, E.; Imai, E.; Ohashi, Y.; Hishida, A. Sleep Quality and Sleep Duration with CKD are Associated with Progression to ESKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 1825–1832. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Fan, Y.; Zhao, B.; Yang, L.; Yang, J.; Ma, X.; Yan, B. Later bedtime is associated with angina pectoris in middle-aged and older adults: Results from the Sleep Heart Health Study. Sleep Med. 2021, 79, 1–5. [Google Scholar] [CrossRef]
- Andersson, T.; Alfredsson, L.; Källberg, H.; Zdravkovic, S.; Ahlbom, A. Calculating measures of biological interaction. Eur. J. Epidemiol. 2005, 20, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, J.; Wu, Q.V.; Gooley, T.A. Cubic splines to model relationships between continuous variables and outcomes: A guide for clinicians. Bone Marrow Transplant. 2020, 55, 675–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottfried, T.; Kamer, I.; Salant, I.; Urban, D.; Lawrence, Y.R.; Onn, A.; Bar, J. Self-reported sleep quality as prognostic for survival in lung cancer patients. Cancer Manag. Res. 2020, 12, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Marinac, C.R.; Nelson, S.H.; Flatt, S.W.; Natarajan, L.; Pierce, J.P.; Patterson, R.E. Sleep duration and breast cancer prognosis: Perspectives from the Women’s Healthy Eating and Living Study. Breast Cancer Res. Treat. 2017, 162, 581–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Q.; Arem, H.; Pfeiffer, R.; Matthews, C. Prediagnosis Sleep Duration, Napping, and Mortality Among Colorectal Cancer Survivors in a Large US Cohort. Sleep 2017, 40, zsx010. [Google Scholar] [CrossRef] [Green Version]
- Aiello, I.; Fedele, M.L.M.; Román, F.; Marpegan, L.; Caldart, C.; Chiesa, J.J.; Golombek, D.A.; Finkielstein, C.V.; Paladino, N. Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Sci. Adv. 2020, 6, eaaz4530. [Google Scholar] [CrossRef] [PubMed]
- Verlande, A.; Masri, S. Circadian Clocks and Cancer: Timekeeping Governs Cellular Metabolism. Trends Endocrinol. Metab. 2019, 30, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S. A Profound Relationship between Circadian Rhythm Dysfunction and Cancer Progression: An Approach to Exploration. Crit. Rev. Oncog. 2021, 26, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Fritschi, L.; Glass, D.C.; Heyworth, J.S.; Aronson, K.; Girschik, J.; Boyle, T.; Grundy, A.; Erren, T.C. Hypotheses for mechanisms linking shiftwork and cancer. Med. Hypotheses 2011, 77, 430–436. [Google Scholar] [CrossRef]
- Nesse, R.M.; Finch, C.E.; Nunn, C.L. Does selection for short sleep duration explain human vulnerability to Alzheimer’s disease? Evol. Med. Public Health 2017, 2017, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Ferlazzo, N.; Andolina, G.; Cannata, A.; Costanzo, M.G.; Rizzo, V.; Currò, M.; Ientile, R.; Caccamo, D. Is Melatonin the Cornucopia of the 21st Century? Antioxidants 2020, 9, 1088. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H. Melatonin and Cancer Hallmarks. Molecules 2018, 23, 518. [Google Scholar] [CrossRef] [Green Version]
- Steel, J.L.; Terhorst, L.; Collins, K.P.; Geller, D.A.; Vodovotz, Y.; Kim, J.; Krane, A.; Antoni, M.; Marsh, J.W.; Burke, L.E.; et al. Prospective Analyses of Cytokine Mediation of Sleep and Survival in the Context of Advanced Cancer. Psychosom. Med. 2018, 80, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, J.L.; Robinson, M.E.; McCrae, C.S.; Kacel, E.L.; Wong, S.S.; Patidar, S.; Sannes, T.S.; Garey, S.; Castagno, J.C.; Pereira, D.B. Associations Among Sleep Latency, Subjective Pain, and Thermal Pain Sensitivity in Gynecologic Cancer. Pain Med. 2020, 21, 5–12. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Neugebauer, V.; Koob, G.; Edwards, S.; Levine, J.D.; Ferrari, L.; Egli, M.; Regunathan, S. Neural mechanisms of pain and alcohol dependence. Pharmacol. Biochem. Behav. 2013, 112, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Grubbs, A.; Barber, E.L. Sleep and gynecological cancer outcomes: Opportunities to improve quality of life and survival. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2022, 32, 669–675. [Google Scholar] [CrossRef]
- Ostovar-Kermani, T.; Arnaud, D.; Almaguer, A.; Garcia, I.; Gonzalez, S.; Mendez Martinez, Y.H.; Surani, S. Painful Sleep: Insomnia in Patients with Chronic Pain Syndrome and its Consequences. Folia Med. 2020, 62, 645–654. [Google Scholar] [CrossRef] [PubMed]

| Variables | Sleep Quality | p Value | |
|---|---|---|---|
| Good (PSQI Score ≤ 5) | Poor (PSQI Score > 5) | ||
| No. of patients | 317 | 352 | |
| Mean (SD) age at diagnosis (years) | 53.61 ± 9.60 | 53.78 ± 9.72 | 0.82 |
| Mean (SD) body mass index (kg/m2) | 23.04 ± 3.54 | 23.41 ± 3.64 | 0.19 |
| Mean (SD) physical activity (MET/hours/week) | 112.09 ± 79.16 | 108.33 ± 79.43 | 0.54 |
| Mean (SD) electronic product use a (hours/week) | 20.99 ± 12.31 | 23.05 ± 14.62 | <0.05 |
| Educational level | 0.57 | ||
| Junior secondary or below | 168 (53.00) | 186 (52.84) | |
| Senior high school/technical secondary school | 63 (19.87) | 80 (22.73) | |
| Junior college/university or above | 86 (27.13) | 86 (24.43) | |
| Family income per month (Yuan) | 0.81 | ||
| <5000 | 194 (61.20) | 208 (59.09) | |
| 5000 to <10,000 | 87 (27.44) | 99 (28.13) | |
| ≥10,000 | 36 (11.36) | 45 (12.78) | |
| Parity | 0.22 | ||
| ≤1 | 236 (74.45) | 247 (70.17) | |
| ≥2 | 81 (25.55) | 105 (29.83) | |
| Menopausal status (yes) | 220 (69.40) | 259 (73.58) | 0.23 |
| Cigarette smoking (yes) | 27 (8.52) | 38 (10.80) | 0.32 |
| Alcohol drinking (yes) | 52 (16.40) | 87 (24.72) | <0.05 |
| Tea drinking (yes) | 108 (34.07) | 107 (30.40) | 0.31 |
| Rotating night shift work (yes) | 21 (6.62) | 33 (9.38) | 0.19 |
| Taking a nap during the day (yes) | 184 (58.04) | 227 (64.49) | 0.09 |
| Mean (SD) night bedtime (hour in 24 h format) | 21.49 ± 1.43 | 21.62 ± 2.12 | 0.37 |
| Mean (SD) wake-up time (hour in 24 h format) | 5.97 ± 0.92 | 5.88 ± 1.29 | 0.26 |
| Mean (SD) night sleep duration (hours/day) | 7.67 ± 0.86 | 6.11 ± 1.14 | <0.05 |
| Mean (SD) daytime napping duration b (minutes/day) | 44.48 ± 23.66 | 45.24 ± 24.27 | 0.75 |
| Mean (SD) total sleep duration (hours/day) | 8.10 ± 0.99 | 6.59 ± 1.29 | <0.05 |
| Characteristics | No. of Deaths/Total (%) | Adjusted HR a (95% CI) |
|---|---|---|
| Age at diagnosis | ||
| ≤50 | 43/246 (17.48) | 1.00 (ref) |
| >50 | 80/423 (18.91) | 1.19 (0.82, 1.74) |
| Histological type | ||
| Serous | 88/458 (19.21) | 1.00 (ref) |
| Non-serous | 35/211 (16.59) | 1.69 (1.07, 2.66) |
| Histopathologic grade | ||
| Poorly differentiated | 112/569 (19.68) | 1.00 (ref) |
| Moderately differentiated | 7/48 (14.58) | 0.66 (0.30, 1.48) |
| Well differentiated | 4/52 (7.69) | 0.49 (0.18, 1.36) |
| FIGO stage | ||
| Ⅰ–Ⅱ | 39/335 (11.64) | 1.00 (ref) |
| Ⅲ–Ⅳ | 84/312 (26.92) | 2.73 (1.75, 4.26) |
| Residual lesions | ||
| No | 77/523 (14.72) | 1.00 (ref) |
| ≤1 cm | 30/104 (28.85) | 1.66 (1.06, 2.60) |
| >1 cm | 16/42 (38.10) | 2.33 (1.32, 4.09) |
| Comorbidities | ||
| No | 69/367 (18.80) | 1.00 (ref) |
| Yes | 54/302 (17.88) | 0.97 (0.67, 1.39) |
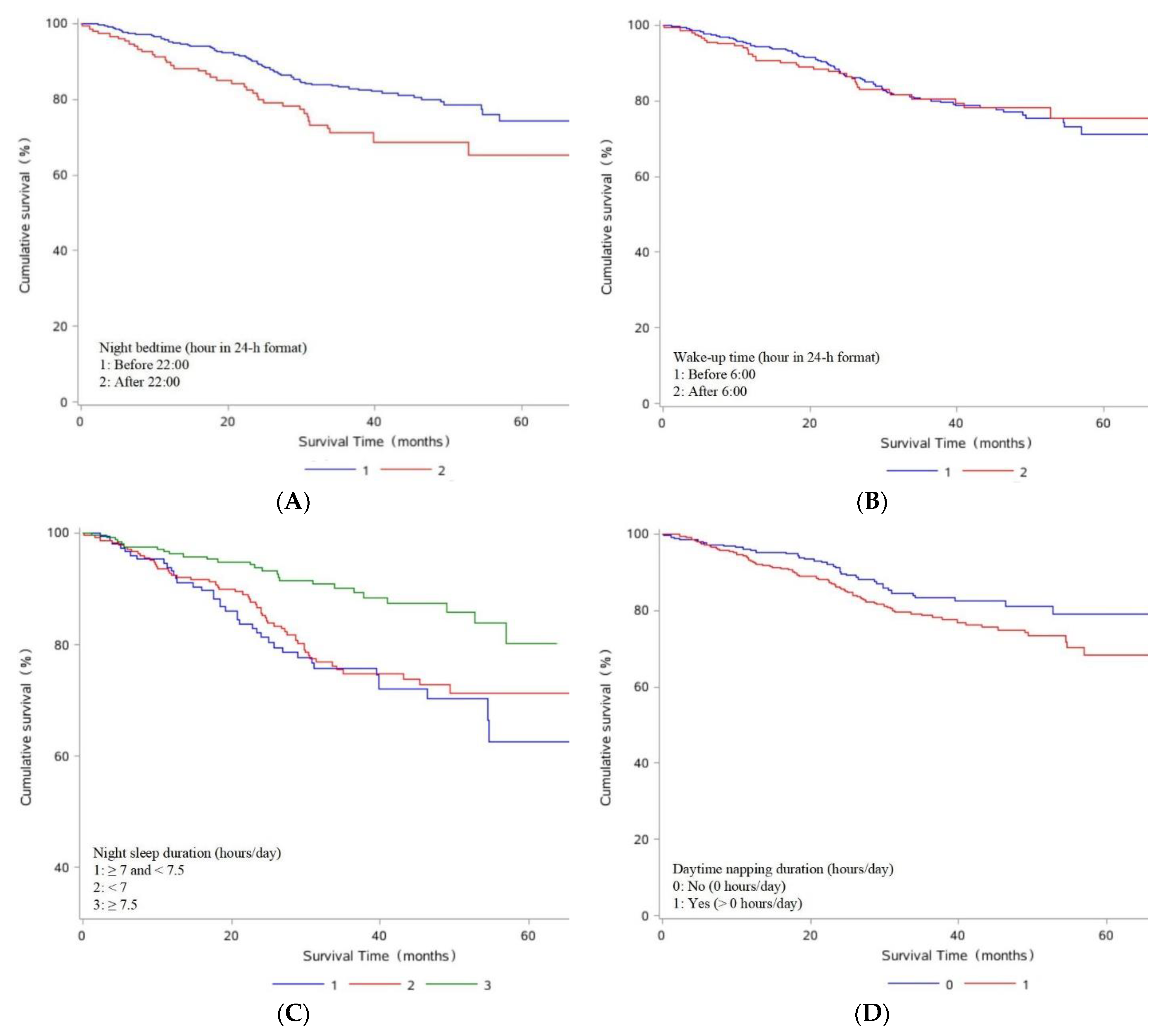
| Sleep Parameters | Deaths, N (% of Total Deaths) | Model 1 a HR (95% CI) | Model 2 b HR (95% CI) |
|---|---|---|---|
| Night bedtime (hour in 24 h format) | |||
| Before 22:00 | 83/518 (16.02) | 1.00 (Ref) | 1.00 (Ref) |
| After 22:00 | 40/151 (26.49) | 1.73 (1.19, 2.53) | 2.13 (1.42, 3.18) |
| the change of every 30 min | 1.39 (0.99, 1.95) | 1.49 (1.03, 2.16) | |
| Wake-up time (hour in 24 h format) | |||
| Before 6:00 | 86/465 (18.49) | 1.00 (Ref) | 1.00 (Ref) |
| After 6:00 | 37/204 (18.14) | 1.03 (0.70, 1.52) | 1.07 (0.71, 1.61) |
| the change of every 30 min | 0.95 (0.69, 1.32) | 1.00 (0.71, 1.41) | |
| Night sleep duration (hours/day) | |||
| <7 | 58/276 (21.01) | 0.92 (0.61, 1.39) | 0.90 (0.59, 1.37) |
| ≥7 and <7.5 | 39/148 (26.35) | 1.00 (Ref) | 1.00 (Ref) |
| ≥7.5 | 26/245 (10.61) | 0.43 (0.26, 0.71) | 0.40 (0.24, 0.67) |
| Daytime napping duration (hours/day) | |||
| No (0 h/day) | 38/258 (14.73) | 1.00 (Ref) | 1.00 (Ref) |
| Yes (> 0 h/day) | 85/411 (20.68) | 1.49 (1.01, 2.18) | 1.37 (0.93, 2.03) |
| the change of every 30 min | 1.20 (1.02, 1.41) | 1.15 (0.97, 1.37) | |
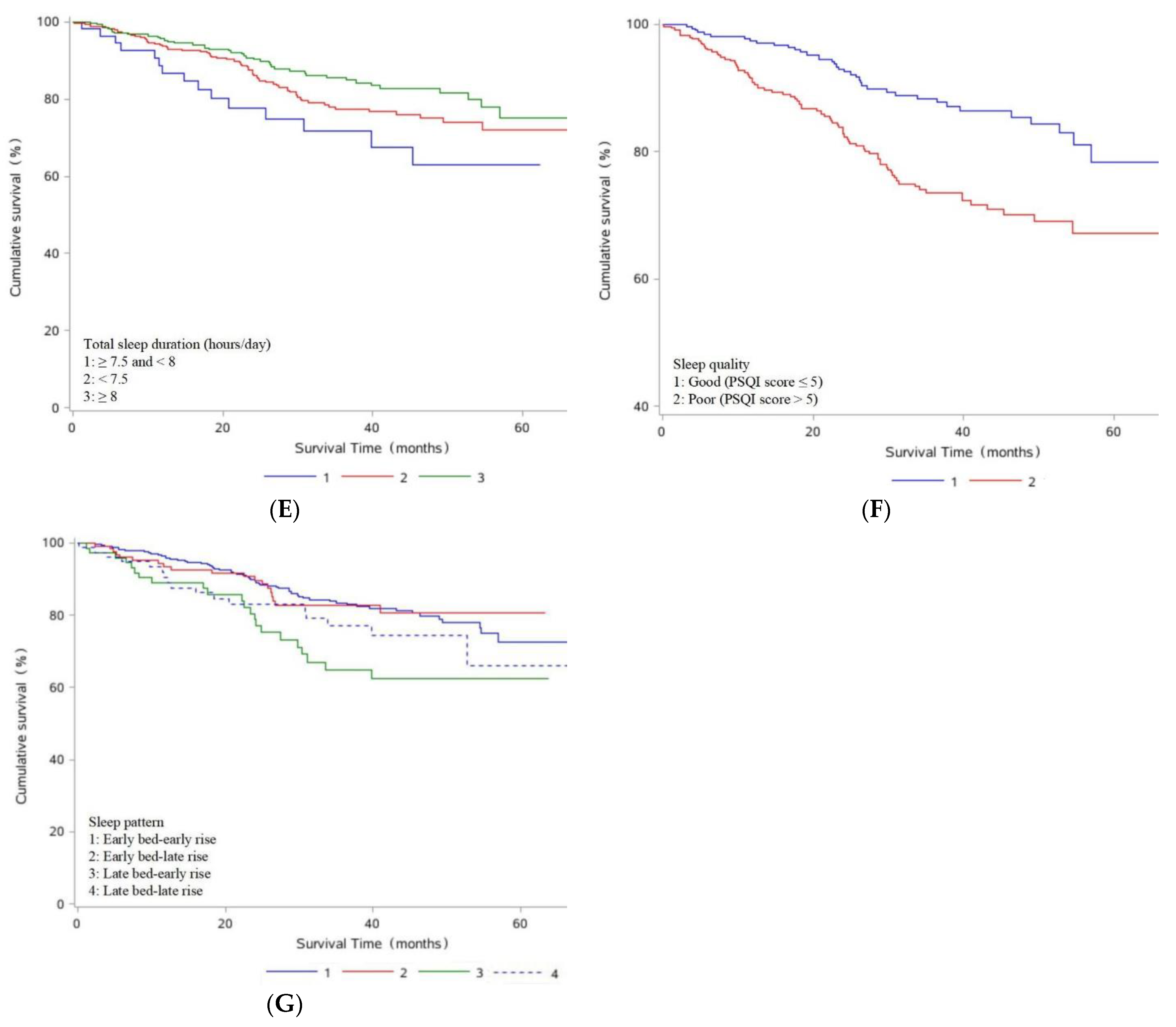
| Total sleep duration (hours/day) | |||
| <7.5 | 65/330 (19.70) | 0.61 (0.35, 1.08) | 0.80 (0.44, 1.47) |
| ≥7.5 and <8 | 15/55 (27.27) | 1.00 (Ref) | 1.00 (Ref) |
| ≥8 | 43/284 (15.14) | 0.43 (0.24, 0.78) | 0.53 (0.29, 0.98) |
| Sleep quality | |||
| Good (PSQI score ≤ 5) | 39/317 (12.30) | 1.00 (Ref) | 1.00 (Ref) |
| Poor (PSQI score > 5) | 84/352 (23.86) | 2.31 (1.57, 3.39) | 2.43 (1.64, 3.62) |
| the change of every 1 score | 1.08 (1.04, 1.13) | 1.08 (1.04, 1.13) | |
| Sleep pattern | |||
| Early bed-early rise | 64/392 (16.33) | 1.00 (Ref) | 1.00 (Ref) |
| Early bed-late rise | 19/126 (15.08) | 1.00 (0.60, 1.68) | 0.97 (0.57, 1.64) |
| Late bed-early rise | 22/73 (30.14) | 2.05 (1.26, 3.32) | 2.26 (1.37, 3.72) |
| Late bed-late rise | 18/78 (23.08) | 1.46 (0.86, 2.47) | 1.93 (1.09, 3.42) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Gao, C.; Wei, Y.; Wen, Z.; Li, X.; Liu, F.; Gong, T.; Yan, S.; Qin, X.; Gao, S.; et al. Pre-Diagnosis Sleep Status and Survival after a Diagnosis of Ovarian Cancer: A Prospective Cohort Study. J. Clin. Med. 2022, 11, 6914. https://doi.org/10.3390/jcm11236914
Li X, Gao C, Wei Y, Wen Z, Li X, Liu F, Gong T, Yan S, Qin X, Gao S, et al. Pre-Diagnosis Sleep Status and Survival after a Diagnosis of Ovarian Cancer: A Prospective Cohort Study. Journal of Clinical Medicine. 2022; 11(23):6914. https://doi.org/10.3390/jcm11236914
Chicago/Turabian StyleLi, Xiaoying, Chang Gao, Yifan Wei, Zhaoyan Wen, Xinyu Li, Fanghua Liu, Tingting Gong, Shi Yan, Xue Qin, Song Gao, and et al. 2022. "Pre-Diagnosis Sleep Status and Survival after a Diagnosis of Ovarian Cancer: A Prospective Cohort Study" Journal of Clinical Medicine 11, no. 23: 6914. https://doi.org/10.3390/jcm11236914
APA StyleLi, X., Gao, C., Wei, Y., Wen, Z., Li, X., Liu, F., Gong, T., Yan, S., Qin, X., Gao, S., Zhao, Y., & Wu, Q. (2022). Pre-Diagnosis Sleep Status and Survival after a Diagnosis of Ovarian Cancer: A Prospective Cohort Study. Journal of Clinical Medicine, 11(23), 6914. https://doi.org/10.3390/jcm11236914







