Impact of the Timing of Maternal Peripartum Depression on Infant Social and Emotional Development at 18 Months
Abstract
:1. Introduction
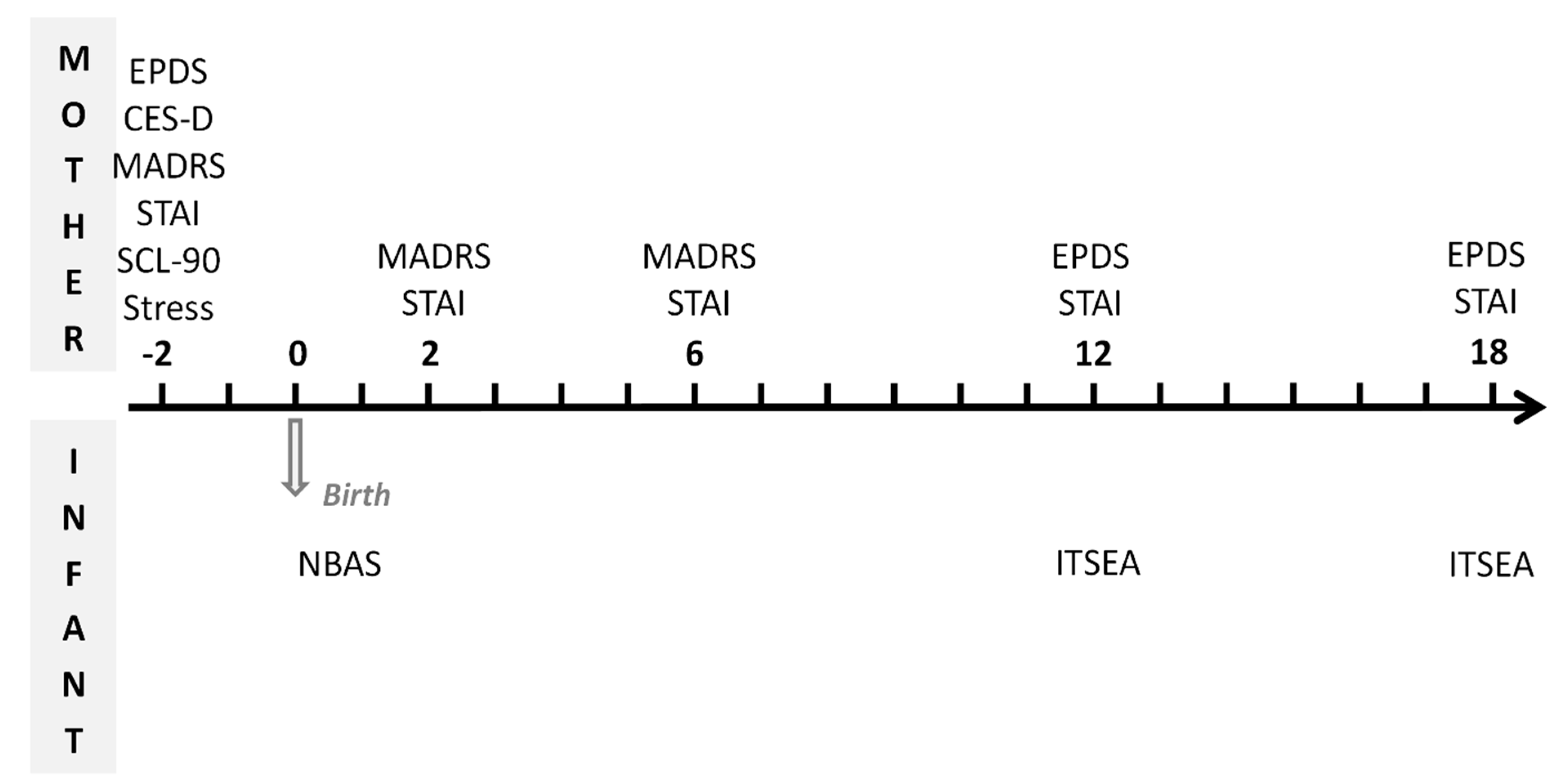
2. Materials & Methods
2.1. Design
2.2. Participants, Eligibility and Enrollment
2.3. Data Analysis
3. Results
3.1. Trajectories of Maternal Depressive Symptoms
3.2. Socio-Demographic and Newborn Features between the Groups
3.3. Pre- and Postnatal Maternal Psychopathology
3.4. Association with Infant Social-Emotional Development
3.5. Effect of the Duration of Maternal Depression on Infant Social-Emotional Development
3.6. Effect of Prenatal Anxiety on Infant Social-Emotional Development
4. Discussion
4.1. Comments on the Main Findings
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Social Determinants of Mental Health; World Health Organization and Calouste Gulbenkian Foundation: Geneva, Switzerland, 2014. [Google Scholar]
- Woody, C.A.; Ferrari, A.J.; Siskind, D.J.; Whiteford, H.A.; Harris, M.G. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect. Disord. 2017, 219, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Fairbrother, N.; Young, A.H.; Zhang, A.; Janssen, P.; Antony, M.M. The prevalence and incidence of perinatal anxiety disorders among women experiencing a medically complicated pregnancy. Arch. Womens Ment. Health 2017, 20, 311–319. [Google Scholar] [CrossRef]
- Bauer, A.; Knapp, M.; Parsonage, M. Lifetime costs of perinatal anxiety and depression. J. Affect. Disord. 2016, 192, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Babineau, V.; Green, C.G.; Jolicoeur-Martineau, A.; Bouvette-Turcot, A.A.; Minde, K.; Sassi, R.; St-André, M.; Carrey, N.; Atkinson, L.; Kennedy, J.L.; et al. Prenatal depression and 5-HTTLPR interact to predict dysregulation from 3 to 36 months—A differential susceptibility model. J. Child Psychol. Psychiatry 2015, 56, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Laurent, H.K.; Ablow, J.C.; Measelle, J. Risky shifts: How the timing and course of mothers’ depressive symptoms across the perinatal period shape their own and infant's stress response profiles. Dev. Psychopathol. 2011, 23, 521–538. [Google Scholar] [CrossRef]
- Monk, C.; Spicer, J.; Champagne, F.A. Linking prenatal maternal adversity to developmental outcomes in infants: The role of epigenetic pathways. Dev. Psychopathol. 2012, 24, 1361–1376. [Google Scholar] [CrossRef] [Green Version]
- Barker, E.D.; Kirkham, N.; Ng, J.; Jensen, S.K.G. Prenatal maternal depression symptoms and nutrition, and child cognitive function. Br. J. Psychiatry 2013, 203, 417–421. [Google Scholar] [CrossRef] [Green Version]
- Madlala, S.S.; Kassier, S.M. Antenatal and postpartum depression: Effects on infant and young child health and feeding practices. South Afr. J. Clin. Nutr. 2018, 31, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Netsi, E.; Pearson, R.M.; Murray, L.; Cooper, P.; Craske, M.G.; Stein, A. Association of Persistent and Severe Postnatal Depression with Child Outcomes. JAMA Psychiatry 2018, 75, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Postpartum Depression: Action Towards Causes and Treatment (PACT) Consortium. Heterogeneity of postpartum depression: A latent class analysis. Lancet Psychiatry 2015, 2, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Santos, H., Jr.; Tan, X.; Salomon, R. Heterogeneity in perinatal depression: How far have we come? A systematic review. Arch. Women's Ment. Health 2017, 20, 11–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.A.; Chua, T.E.; Malhotra, R.; Allen, J.C.; Teo, I.; Chern, B.S.M.; Tan, K.H.; Chen, H. Identifying trajectories of antenatal depression in women and their associations with gestational age and neonatal anthropometry: A prospective cohort study. Gen. Hosp. Psychiatry 2019, 61, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Bayrampour, H.; Tomfohr, L.; Tough, S. Trajectories of Perinatal Depressive and Anxiety Symptoms in a Community Cohort. J. Clin. Psychiatry 2016, 77, e1467–e1473. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.A.; Hammen, C.; Andersen, M.J.; Bor, W.; Najman, J.M.; Williams, G.M. Chronicity, severity, and timing of maternal depressive symptoms: Relationships with child outcomes at age 5. Dev. Psychol. 2000, 36, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Melotti, R.; Heron, J.; Ramchandani, P.; Wiles, N.; Murray, L.; Stein, A. The timing of maternal depressive symptoms and child cognitive development: A longitudinal study. J. Child. Psychol. Psychiatry 2012, 53, 632–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piteo, A.M.; Yelland, L.N.; Makrides, M. Does maternal depression predict developmental outcome in 18 month old infants? Early Hum. Dev. 2012, 88, 651–655. [Google Scholar] [CrossRef]
- Field, T.; Diego, M.; Dieter, J.; Hernandez-Reif, M.; Schanberg, S.; Kuhn, C.; Yando, R.; Bendell, D. Prenatal depression effects on the fetus and the newborn. Infant Behav. Dev. 2004, 27, 216–229. [Google Scholar] [CrossRef]
- Park, M.; Brain, U.; Grunau, R.E.; Diamond, A.; Oberlander, T.F. Maternal depression trajectories from pregnancy to 3 years postpartum are associated with children’s behavior and executive functions at 3 and 6 years. Arch. Womens Ment. Health 2018, 21, 353–363. [Google Scholar] [CrossRef]
- van der Waerden, J.; Galéra, C.; Larroque, B.; Saurel-Cubizolles, M.J.; Sutter-Dallay, A.L.; Melchior, M. Maternal Depression Trajectories and Children’s Behavior at Age 5 Years. J. Pediatr. 2015, 166, 1440–1448.e1. [Google Scholar] [CrossRef]
- Tietz, A.; Zietlow, A.L.; Reck, C. Maternal bonding in mothers with postpartum anxiety disorder: The crucial role of subclinical depressive symptoms and maternal avoidance behaviour. Arch. Womens Ment. Health 2014, 17, 433–442. [Google Scholar] [CrossRef]
- Dubber, S.; Reck, C.; Müller, M.; Gawlik, S. Postpartum bonding: The role of perinatal depression, anxiety and maternal-fetal bonding during pregnancy. Arch. Womens Ment. Health 2015, 18, 187–195. [Google Scholar] [CrossRef]
- Kingston, D.; McDonald, S.; Austin, M.-P.; Tough, S. Association between Prenatal and Postnatal Psychological Distress and Toddler Cognitive Development: A Systematic Review. PLoS ONE 2015, 10, e0126929. [Google Scholar] [CrossRef] [Green Version]
- Gerardin, P.; Wendland, J.; Bodeau, N.; Galin, A.; Bialobos, S.; Tordjman, S.; Mazet, P.; Darbois, Y.; Nizard, J.; Dommergues, M.; et al. Depression during pregnancy: Is the developmental impact earlier in boys? A prospective case-control study. J. Clin. Psychiatry 2011, 72, 378–387. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radloff, L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Montgomery, S.A.; Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Zenasni, F.; Granier-Deferre, C.; Cabrol, D.; Cappelle, C.; Dallay, D. Presentation and Validation of the “Sensations during Pregnancy and Life Events Questionnaire”; International Society for Developmental Psychobiology: Aix-en-Provence, France, 2004. [Google Scholar]
- Derogatis, L. SCL-90-R Version Manual I. Clinical Psychometric Research; Clinical Psychometric Research: Baltimore, MD, USA, 1977. [Google Scholar]
- Spielberger, C.D. Manual for the State Trait Anxiety Inventory (Form y); Consulting Psychologists Press: Palo-Alto, CA, USA, 1983. [Google Scholar]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. S20), 22–33; quiz 34–57. [Google Scholar] [PubMed]
- Brazelton, T.B. Neonatal Behavioral Assessment Scale; Mac Keith Press: London, UK, 1984. [Google Scholar]
- Briggs-Gowan, M.J.; Carter, A.S. Preliminary acceptability and psychometrics of the Infant-Toddler Social and Emotional Assessment (ITSEA): A new adult-report questionnaire. Infant Ment. Health J. 1998, 19, 422–445. [Google Scholar] [CrossRef]
- Huot, R.L.; Brennan, P.A.; Stowe, Z.N.; Plotsky, P.M.; Walker, E.F. Negative affect in offspring of depressed mothers is predicted by infant cortisol levels at 6 months and maternal depression during pregnancy, but not postpartum. Ann. N. Y. Acad. Sci. 2004, 1032, 234–236. [Google Scholar] [CrossRef]
- McGrath, J.M.; Records, K.; Rice, M. Maternal depression and infant temperament characteristics. Infant Behav. Dev. 2008, 31, 71–80. [Google Scholar] [CrossRef]
- Velders, F.P.; Dieleman, G.; Henrichs, J.; Jaddoe, V.W.; Hofman, A.; Verhulst, F.C.; Hudziak, J.J.; Tiemeier, H. Prenatal and postnatal psychological symptoms of parents and family functioning: The impact on child emotional and behavioural problems. Eur. Child. Adolesc. Psychiatry 2011, 20, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Cents, R.A.; Diamantopoulou, S.; Hudziak, J.J.; Jaddoe, V.W.; Hofman, A.; Verhulst, F.C.; Lambregtse-van den Berg, M.P.; Tiemeier, H. Trajectories of maternal depressive symptoms predict child problem behaviour: The Generation R study. Psychol. Med. 2013, 43, 13–25. [Google Scholar] [CrossRef]
- Leis, J.A.; Heron, J.; Stuart, E.A.; Mendelson, T. Associations between maternal mental health and child emotional and behavioral problems: Does prenatal mental health matter? J. Abnorm. Child. Psychol. 2014, 42, 161–171. [Google Scholar] [CrossRef]
- O’Donnell, K.J.; Meaney, M.J. Fetal Origins of Mental Health: The Developmental Origins of Health and Disease Hypothesis. Am. J. Psychiatry 2017, 174, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Hiroi, R.; Carbone, D.L.; Zuloaga, D.G.; Bimonte-Nelson, H.A.; Handa, R.J. Sex dependent programming effects of prenatal glucocorticoid treatment on the developing serotonin system and stress-related behaviors in adulthood. Neurosci. Lett. 2016, 320, 43–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardon, M.-C.; Gerardin, P.; Joubert, C.; Perez-Diaz, F.; Cohen-Salmon, C. Influence of prepartum chronic ultramild stress on maternal pup care behavior in mice. Biol. Psychiatry 2000, 47, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, R.A.; Di Maio, R.; Volonte, D.; Galbiati, F.; Lewis, M.; Romero, G. Nongenomic glucocorticoid receptor action regulates gap junction intercellular communication and neural progenitor cell proliferation. Proc. Natl. Acad. Sci. USA 2011, 108, 16657–16662. [Google Scholar] [CrossRef] [Green Version]
- Cruceanu, C.; Dony, L.; Krontira, A.C.; Fischer, D.S.; Roeh, S.; Di Giaimo, R.; Kyrousi, C.; Kaspar, L.; Arloth, J.; Czamara, D.; et al. Cell-Type-Specific Impact of Glucocorticoid Receptor Activation on the Developing Brain: A Cerebral Organoid Study. Am. J. Psychiatry 2022, 179, 375–387. [Google Scholar] [CrossRef]
- Fitzgerald, E.; Parent, C.; Kee, M.Z.L.; Meaney, M.J. Maternal Distress and Offspring Neurodevelopment: Challenges and Opportunities for Pre-clinical Research Models. Front. Hum. Neurosci. 2021, 15, 635304. [Google Scholar] [CrossRef] [PubMed]
- Qiu, A.; Anh, T.T.; Li, Y.; Chen, H.; Rifkin-Graboi, A.; Broekman, B.F.; Kwek, K.; Saw, S.M.; Chong, Y.S.; Gluckman, P.D.; et al. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl. Psychiatry 2015, 5, e508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rifkin-Graboi, A.; Meaney, M.J.; Chen, H.; Bai, J.; Hameed, W.R.; Tint, M.; Broekman, B.F.P.; Chong, Y.-S.; Gluckman, P.D.; Fortier, M.V.; et al. Antenatal maternal anxiety predicts variations in neural structures implicated in anxiety disorders in newborns. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 313–321.e2. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Pearson, R.M.; Goodman, S.H.; Rapa, E.; Rahman, A.; McCallum, M.; Howard, L.M.; Pariante, C.M. Effects of perinatal mental disorders on the fetus and child. Lancet 2014, 384, 1800–1819. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.M.; Evans, J.; Kounali, D.; Lewis, G.; Heron, J.; Ramchandani, P.G.; O’Connor, T.G.; Stein, A. Maternal depression during pregnancy and the postnatal period: Risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry 2013, 70, 1312–1319. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.A.; Goodman, M. Development of emotion regulation: More than meets the eye. In Emotion Regulation and Psychopathology: A Transdiagnostic Approach to Etiology and Treatment; Kring, A.M., Sloan, D.M., Eds.; The Guilford Press: New York, NY, USA, 2010; pp. 38–58. [Google Scholar]
- Guyon-Harris, K.; Huth-Bocks, A.; Lauterbach, D.; Janisse, H. Trajectories of maternal depressive symptoms across the birth of a child: Associations with toddler emotional development. Arch. Womens Ment. Health 2016, 19, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Giallo, R.; Woolhouse, H.; Gartland, D.; Hiscock, H.; Brown, S. The emotional-behavioural functioning of children exposed to maternal depressive symptoms across pregnancy and early childhood: A prospective Australian pregnancy cohort study. Eur. Child Adolesc. Psychiatry 2015, 24, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Kingston, D.; Kehler, H.; Austin, M.P.; Mughal, M.K.; Wajid, A.; Vermeyden, L.; Benzies, K.; Brown, S.; Stuart, S.; Giallo, R. Trajectories of maternal depressive symptoms during pregnancy and the first 12 months postpartum and child externalizing and internalizing behavior at three years. PLoS ONE 2018, 13, e0195365. [Google Scholar] [CrossRef] [Green Version]
- Hannigan, L.J.; Eilertsen, E.M.; Gjerde, L.C.; Reichborn-Kjennerud, T.; Eley, T.C.; Rijsdijk, F.V.; Ystrom, E.; McAdams, T.A. Maternal prenatal depressive symptoms and risk for early-life psychopathology in offspring: Genetic analyses in the Norwegian Mother and Child Birth Cohort Study. Lancet Psychiatry 2018, 5, 808–815. [Google Scholar] [CrossRef] [Green Version]
- Betts, K.S.; Williams, G.M.; Najman, J.M.; Alati, R. Maternal depressive, anxious and stress symptoms during pregnancy predict internalizing problems in adolescence. Depress. Anxiety 2014, 31, 9–18. [Google Scholar] [CrossRef]
- Meaney, M.J. Perinatal Maternal Depressive Symptoms as an Issue for Population Health. Am. J. Psychiatry 2018, 175, 1084–1093. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo-Sotorrío, E.; Holgado-Tello, F.P.; Carrasco, M.Á. Incremental Validity and Informant Effect from a Multi-Method Perspective: Assessing Relations between Parental Acceptance and Children’s Behavioral Problems. Front. Psychol. 2016, 7, 664. [Google Scholar] [CrossRef]
- Chilcoat, H.D.; Breslau, N. Does psychiatric history bias mothers’ reports? An application of a new analytic approach. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlinson, M.; Cooper, P.; Murray, L. The mother-infant relationship and infant attachment in a South African peri-urban settlement. Child. Dev. 2005, 76, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Hayes, L.J.; Goodman, S.H.; Carlson, E. Maternal antenatal depression and infant disorganized attachment at 12 months. Attach. Hum. Dev. 2013, 15, 133–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhusen, J.L. A literature update on maternal-fetal attachment. J. Obstet. Gynecol. Neonatal Nurs. 2008, 37, 315–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Halfon, N. Racial and Ethnic Disparities in Birth Outcomes: A Life Course Perspective. Matern. Child Health J. 2003, 7, 13–30. [Google Scholar] [CrossRef] [PubMed]
| Trajectories of Maternal Depressive Symptoms | Kruskal–Wallis Chi [2] or Fisher’s Exact | p Value | Adjusted p-Value (Holm) | |||
|---|---|---|---|---|---|---|
| DEP−/− (No Perinatal Depression) n = 38 | DEP−/+ (Postnatal Depression Only) n = 19 | DEP+/+ (Prenatal and Postnatal Depression) n = 14 | ||||
| Socio-demographic characteristics | ||||||
| Mother’s age (years), mean ± SD | 31.4 ± 4.0 | 30.8 ± 4.5 | 32.8 ± 4.0 | 1.69 | 0.430 | 1 |
| Father’s age (years), mean ± SD | 32.3 ± 5.3 | 32.8 ± 4.7 | 34.0 ± 7.10 | 0.10 | 0.949 | 1 |
| Singles | 14% | - | 1 (8%) | - | - | 1 |
| Middle and low SES | 49% | 37% | 54% | - | - | 1 |
| Pregnancy and newborn characteristics | ||||||
| Weight gain (kg) | 14.8 ± 3.9 | 14.1 ± 3.0 | 13.8 ± 5.3 | 1.04 | 0.596 | 1 |
| Head circumference (cm) | 34.7 ± 1.4 | 35.4 ± 4.2 | 35.4 ± 1.9 | 2.78 | 0.250 | 1 |
| Weight (kg) | 3.34 ± 0.49 | 3.18 ± 0. 35 | 3.61 ± 0.50 | 6.65 | 0.036 * | 0.447 |
| Length (cm) | 49.3 ± 2.5 | 48.7 ± 1.9 | 50.5 ± 2.2 | 4.91 | 0.086 | 0.860 |
| APGAR score 5′ | 10.0 (−) | 9.9 ± 0.2 | 10 (−) | 2.42 | 0.298 | 1 |
| APGAR score 10′ | 9.8 ± 0.5 | 9.6 ± 1.0 | 9.9 ± 0.3 | 1.83 | 0.400 | 1 |
| Prenatal maternal psychopathology | ||||||
| MADRS score, prenatal | 6.2 ± 4.4 c | 8.1 ± 4.0 b | 18.2 ± 3.6 a | 34.99 | <0.001 ** | <0.001 ** |
| EPDS score, prenatal | 3.4 ± 3.7 c | 7.3 ± 5.4 b | 10.6 ± 7.0 a | 15.31 | <0.001 ** | 0.009 * |
| CES-D score, prenatal | 8.7 ± 5.5 c | 13.3 ± 6.9 b | 24.2 ± 8.9 a | 21.45 | <0.001 ** | <0.001 ** |
| STAI Y-A score, prenatal | 28.8 ± 8.0 | 35.3 ± 12.9 | 38.7 ± 14.0 | 6.74 | 0.034 * | 0.447 |
| STAI Y-B score, prenatal | 31.1 ± 8.8 c | 38.7 ± 9.9 b | 43.0 ± 9.3 a | 12.70 | 0.002 * | 0.031 * |
| Number of SLE | 7.8 ± 6.4 b | 9.4 ± 7.4 b | 19.0 ± 9.1 a | 8.04 | 0.018 * | 0.269 |
| Reactivity to SLE | 21.3 ± 20.4 b | 29.2 ± 23.1 b | 61.6 ± 27.2 a | 12.33 | 0.002 ** | 0.036 * |
| Number of SPS | 16.9 ± 10.0 | 15.0 ± 12.0 | 27.2 ± 17.5 | 2.72 | 0.256 | 1 |
| Reactivity of SPS | 40.7 ± 31.9 | 48.1 ± 44.1 | 94.0 ± 73.0 | 3.11 | 0.211 | 1 |
| Postnatal maternal psychopathology | ||||||
| MADRS score, 2 months | 3.5 ± 3.3 b | 14.5 ± 9.3 a | 17.4 ± 8.8 a | 31.88 | <0.001 ** | <0.001 ** |
| STAI Y-A, 2 months | 27.1 ± 10.4 | 35.8 ± 15.5 | 32.4 ± 10.2 | 2.90 | 0.234 | 1 |
| MADRS score, 6 months | 3.0 ± 3.0 b | 13.0 ± 8.8 a | 12.8 ± 8.2 a | 28.02 | <0.001 ** | <0.001 ** |
| STAI Y-A score, 6 months | 28.3 ± 8.2 | 29.4 ± 8.2 | 37.2 ± 6.6 | 7.40 | 0.025 * | 0.347 |
| EPDS score, 12 months | 3.4 ± 3.7 | 7.3 ± 5.4 | 10.8 ± 6.8 | 15.86 | <0.001 ** | 0.008 |
| STAI Y-A score, 12 months | 48.9 ± 2.5 | 46.4 ± 4.1 | 48.1 ± 8.9 | 10.36 | 0.006 * | 0.090 |
| EPDS score, 18 months | 2.9 ± 3.2 b | 7.6 ± 4.8 a | 6.9 ± 5.1 a | 14.34 | 0.001 | 0.015 * |
| STAI Y-A score, 18 months | 49.4 ± 2.5 | 47.1 ± 3.8 | 48.9 ± 3.6 | 5.34 | 0.069 | 0.763 |
| ITSEA Scores in Infants | Trajectories of Maternal Depressive Symptoms | Kruskal– Wallis | p Value | ||
|---|---|---|---|---|---|
| DEP−/− (No Perinatal Depression) n = 38 | DEP−/+ (Postnatal Depression Only) n = 19 | DEP+/+ (Prenatal and Postnatal Depression) n = 14 | |||
| (1) Externalizing Symptoms | |||||
| Total score | 0.36 ± 0.21 | 0.45 ± 0.28 | 0.54 ± 0.30 | 3.32 | 0.190 |
| Activity/impulsivity | 0.49 ± 0.38 | 0.70 ± 0.54 | 0.78 ± 0.39 | 5.12 | 0.077 |
| Aggression/defiance | 0.86 ± 0.46 | 0.93 ± 0.45 | 1.07 ± 0.54 | 1.16 | 0.559 |
| Peer aggression | 0.20 ± 0.23 | 0.15 ± 0.16 | 0.22 ± 0.23 | 0.66 | 0.719 |
| (2) Internalizing Symptoms | |||||
| Total score | 0.49 ± 0.21 b | 0.46 ± 0.24 b | 0.65 ± 0.18 a | 6.03 | 0.049 * |
| Depression/withdrawal | 0.04 ± 0.06 | 0.06 ± 0.10 | 0.13 ± 0.19 | 1.96 | 0.376 |
| Anxiety/worries | 0.15 ± 0.20 c | 0.23 ± 0.17 b | 0.33 ± 0.21 a | 9.70 | 0.008 ** |
| General anxiety | 0.13 ± 0.17 c | 0.17 ± 0.11 b | 0.26 ± 0.16 a | 7.73 | 0.021 * |
| Separation distress | 1.02 ± 0.42 | 0.93 ± 0.51 | 1.19 ± 0.43 | 2.62 | 0.270 |
| Inhibition to novelty | 0.76 ± 0.48 | 0.67 ± 0.44 | 1.02 ± 0.42 | 2.99 | 0.225 |
| (3) Dysregulation | |||||
| Total score | 0.37 ± 0.19 | 0.38 ± 0.22 | 0.52 ± 0.22 | 4.04 | 0.132 |
| Sleep | 0.43 ± 0.54 | 0.44 ± 0.50 | 0.56 ± 0.49 | 1.68 | 0.432 |
| Negative emotionality | 0.53 ± 0.31 | 0.54 ± 0.32 | 0.84 ± 0.44 | 4.60 | 0.100 |
| Eating | 0.17 ± 0.31 | 0.16 ± 0.31 | 0.22 ± 0.26 | 1.95 | 0.377 |
| Sensory sensitivity | 0.31 ± 0.22 | 0.34 ± 0.34 | 0.43 ± 0.26 | 1.62 | 0.446 |
| (4) Competence | |||||
| Total score | 1.11 ± 0.34 | 1.05 ± 0.23 | 0.97 ± 0.18 | 1.86 | 0.395 |
| Compliance | 1.06 ± 0.34 | 1.05 ± 0.34 | 0.87 ± 0.27 | 2.72 | 0.257 |
| Attention | 1.27 ± 0.46 | 1.26 ± 0.44 | 1.03 ± 0.52 | 1.99 | 0.370 |
| Imitation/play | 1.15 ± 0.50 | 1.07 ± 0.34 | 1.03 ± 0.53 | 0.21 | 0.900 |
| Mastery motivation | 1.41 ± 0.39 | 1.49 ± 0.36 | 1.39 ± 0.18 | 0.90 | 0.638 |
| Empathy | 0.82 ± 0.62 | 0.63 ± 0.48 | 0.66 ± 0.46 | 0.82 | 0.663 |
| Prosocial peer relation | 0.88 ± 0.46 | 0.66 ± 0.43 | 0.74 ± 0.46 | 2.25 | 0.325 |
| (5) Additional Indices | |||||
| Maladaptive | 0.15 ± 0.17 | 0.16 ± 0.16 | 0.16 ± 0.13 | 0.54 | 0.764 |
| Social relatedness | 1.70 ± 0.24 a | 1.59 ± 0.22 b | 1.52 ± 0.20 b | 6.65 | 0.036 * |
| Atypical | 0.31 ± 0.27 | 0.34 ± 0.32 | 0.36 ± 0.31 | 0.24 | 0.887 |
| ITSEA Scores in Infants | n | Kendall τ | Z | p Value |
|---|---|---|---|---|
| 1. Externalizing Symptoms | ||||
| Total score | 64 | 0.25 | 2.47 | 0.013 * |
| Activity/impulsivity | 64 | 0.26 | 2.47 | 0.013 * |
| Aggression/defiance | 64 | 0.26 | 2.28 | 0.023 * |
| Peer aggression | 52 | 0.03 | 0.22 | 0.827 |
| 2. Internalizing Symptoms | ||||
| Total score | 64 | 0.13 | 1.31 | 0.189 |
| Depression/withdrawal | 64 | 0.14 | 1.22 | 0.224 |
| Anxiety/worries | 64 | 0.34 | 3.19 | 0.001 ** |
| General anxiety | 64 | 0.29 | 2.76 | 0.006 ** |
| Separation distress | 64 | 0.10 | 0.94 | 0.348 |
| Inhibition to novelty | 64 | 0.03 | 0.29 | 0.772 |
| 3. Dysregulation | ||||
| Total score | 64 | 0.15 | 1.49 | 0.136 |
| Sleep | 64 | 0.16 | 1.50 | 0.135 |
| Negative emotionality | 64 | 0.15 | 1.44 | 0.151 |
| Eating | 63 | 0.05 | 0.48 | 0.631 |
| Sensory sensitivity | 64 | 0.05 | 0.46 | 0.647 |
| 4. Competence | ||||
| Total score | 64 | −0.03 | −0.35 | 0.725 |
| Compliance | 64 | −0.07 | −0.69 | 0.488 |
| Attention | 63 | −0.11 | −1.08 | 0.281 |
| Imitation/play | 64 | 0.05 | 0.49 | 0.622 |
| Mastery motivation | 64 | 0.10 | 0.94 | 0.347 |
| Empathy | 62 | 0.03 | 0.31 | 0.759 |
| Prosocial peer relation | 52 | −0.08 | −0.67 | 0.505 |
| 5. Additional Indices | ||||
| Maladaptive | 64 | 0.11 | 1.05 | 0.294 |
| Social relatedness | 64 | −0.19 | −1.89 | 0.059 |
| Atypical | 63 | 0.03 | 0.32 | 0.752 |
| STAI Y-B Score (Maternal Trait-Anxiety) | STAI Y-A Score (Maternal State-Anxiety) | ||||||
|---|---|---|---|---|---|---|---|
| ITSEA Scores in Infants | n | Kendall τ | Z | p Value | Kendall τ | Z | p Value |
| 1. Externalizing Symptoms | |||||||
| Total score | 91 | 0.11 | 1.57 | 0.117 | 0.16 | 2.18 | 0.029 * |
| Activity/impulsivity | 91 | 0.11 | 1.42 | 0.156 | 0.17 | 2.28 | 0.023 * |
| Aggression/defiance | 91 | 0.19 | 2.39 | 0.017 * | 0.12 | 1.56 | 0.118 |
| Peer aggression | 72 | −0.13 | −1.42 | 0.155 | −0.02 | −0.19 | 0.852 |
| 2. Internalizing Symptoms | |||||||
| Total score | 91 | 0.16 | 2.15 | 0.032 * | 0.04 | 0.54 | 0.592 |
| Depression/withdrawal | 91 | 0.09 | 1.06 | 0.288 | 0.08 | 0.95 | 0.343 |
| Anxiety/worries | 91 | 0.16 | 2.02 | 0.043 * | 0.05 | 0.59 | 0.555 |
| General anxiety | 91 | 0.10 | 1.35 | 0.178 | 0.09 | 1.15 | 0.252 |
| Separation distress | 91 | 0.19 | 2.50 | 0.012 * | 0.05 | 0.61 | 0.544 |
| Inhibition to novelty | 91 | 0.05 | 0.70 | 0.487 | −0.03 | −0.35 | 0.728 |
| 3. Dysregulation | |||||||
| Total score | 91 | 0.13 | 1.80 | 0.073 | 0.07 | 0.97 | 0.333 |
| Sleep | 91 | 0.03 | 0.41 | 0.679 | −0.03 | −0.41 | 0.680 |
| Negative emotionality | 91 | 0.11 | 1.47 | 0.142 | 0.15 | 2.04 | 0.041 * |
| Eating | 91 | 0.15 | 1.85 | 0.064 | 0.16 | 2.00 | 0.045 * |
| Sensory sensitivity | 91 | 0.13 | 1.73 | 0.084 | 0.02 | 0.21 | 0.831 |
| 4. Competence | |||||||
| Total score | 91 | −0.03 | −0.42 | 0.673 | −0.14 | −1.87 | 0.061 |
| Compliance | 91 | −0.20 | −2.62 | 0.009 * | −0.11 | −1.53 | 0.126 |
| Attention | 90 | −0.04 | −0.51 | 0.609 | −0.14 | −1.88 | 0.060 |
| Imitation/play | 91 | 0.04 | 0.47 | 0.638 | −0.08 | −1.13 | 0.260 |
| Mastery motivation | 91 | 0.01 | 0.10 | 0.923 | −0.07 | −0.88 | 0.378 |
| Empathy | 90 | 0.04 | 0.52 | 0.601 | −0.04 | −0.55 | 0.579 |
| Prosocial peer relation | 73 | −0.06 | −0.71 | 0.477 | −0.09 | −1.09 | 0.275 |
| 5. Additional Indices | |||||||
| Maladaptive | 91 | 0.18 | 2.32 | 0.021 * | 0.01 | 0.13 | 0.894 |
| Social relatedness | 91 | 0.01 | 0.17 | 0.866 | −0.08 | −1.01 | 0.311 |
| Atypical | 90 | 0.09 | 1.16 | 0.245 | 0.06 | 0.77 | 0.442 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wendland, J.; Benarous, X.; Young, H.; Brahim, T.; Apter, G.; Bodeau, N.; Cohen, D.; Gérardin, P. Impact of the Timing of Maternal Peripartum Depression on Infant Social and Emotional Development at 18 Months. J. Clin. Med. 2022, 11, 6919. https://doi.org/10.3390/jcm11236919
Wendland J, Benarous X, Young H, Brahim T, Apter G, Bodeau N, Cohen D, Gérardin P. Impact of the Timing of Maternal Peripartum Depression on Infant Social and Emotional Development at 18 Months. Journal of Clinical Medicine. 2022; 11(23):6919. https://doi.org/10.3390/jcm11236919
Chicago/Turabian StyleWendland, Jaqueline, Xavier Benarous, Héloïse Young, Takoua Brahim, Gisèle Apter, Nicolas Bodeau, David Cohen, and Priscille Gérardin. 2022. "Impact of the Timing of Maternal Peripartum Depression on Infant Social and Emotional Development at 18 Months" Journal of Clinical Medicine 11, no. 23: 6919. https://doi.org/10.3390/jcm11236919





