Effectiveness and Minimal-Invasiveness of Zone 0 Landing Thoracic Endovascular Aortic Repair Using Branched Endograft
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Study Design
2.2. Preoperative Measurements and Treatment Strategy
2.3. Surgical Procedure
2.3.1. Branched TEVAR
2.3.2. Hybrid TEVAR
2.3.3. Follow-Up
2.4. Statistical Analyses
3. Results
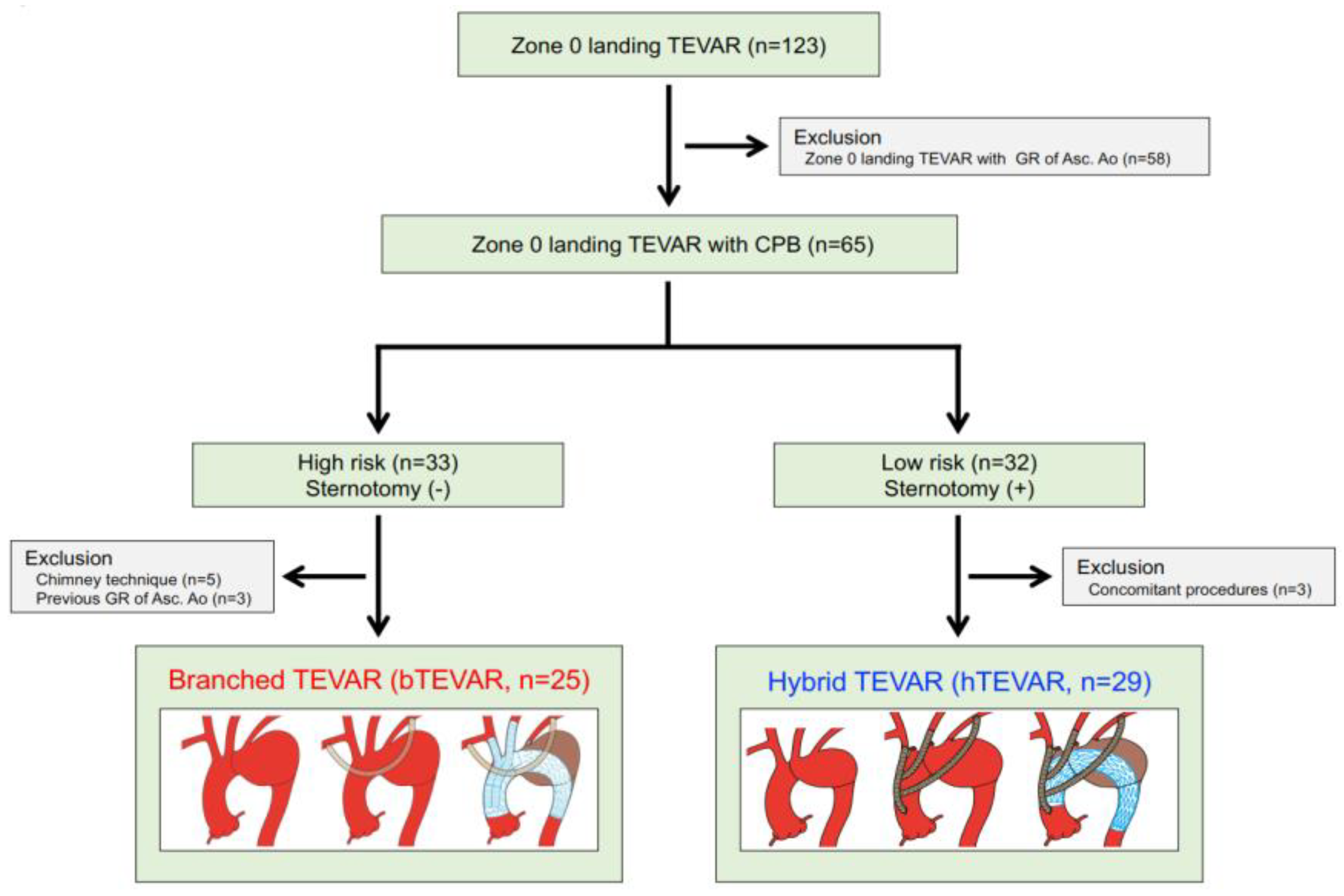
3.1. Study Population
3.2. Patients’ Characteristics
3.3. Preoperative Measurements and Stent-Grafts
3.4. Operative and in-Hospital Outcomes
3.5. Late Outcomes
3.6. Survival
3.7. Aorta-Related Death
3.8. Aortic Events
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iafrancesco, M.; Ranasinghe, A.M.; Dronavalli, V.; Adam, D.J.; Claridge, M.W.; Riley, P.; McCafferty, I.; Mascaro, J.G. Open aortic arch replacement in high-risk patients: The gold standard. Eur. J. Cardiothorac. Surg. 2016, 49, 646–651; discussion 651. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Mikamo, A.; Suzuki, R.; Kurazumi, H.; Kudo, T.; Takahashi, M.; Ikenaga, S.; Shirasawa, B.; Hamano, K. Mortality and morbidity after total aortic arch replacement. Ann. Thorac. Surg. 2014, 97, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, Y.; Kuratani, T.; Shimamura, K.; Torikai, K.; Sakamoto, T.; Shijo, T.; Sawa, Y. The efficacy and short-term results of hybrid thoracic endovascular repair into the ascending aorta for aortic arch pathologies. Eur. J. Cardiothorac. Surg. 2014, 45, 298–304; discussion 304. [Google Scholar] [CrossRef]
- Lotfi, S.; Clough, R.E.; Ali, T.; Salter, R.; Young, C.P.; Bell, R.; Modarai, B.; Taylor, P. Hybrid repair of complex thoracic aortic arch pathology: Long-term outcomes of extra-anatomic bypass grafting of the supra-aortic trunk. Cardiovasc. Intervent. Radiol. 2013, 36, 46–55. [Google Scholar] [CrossRef]
- Murashita, T.; Matsuda, H.; Domae, K.; Iba, Y.; Tanaka, H.; Sasaki, H.; Ogino, H. Less invasive surgical treatment for aortic arch aneurysms in high-risk patients: A comparative study of hybrid thoracic endovascular aortic repair and conventional total arch replacement. J. Thorac. Cardiovasc. Surg. 2012, 143, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Kuratani, T.; Shimamura, K.; Sakamoto, T.; Kin, K.; Masada, K.; Shijo, T.; Torikai, K.; Maeda, K.; Sawa, Y. Type 1a endoleak following Zone 1 and Zone 2 thoracic endovascular aortic repair: Effect of bird-beak configuration. Eur. J. Cardiothorac. Surg. 2017, 52, 718–724. [Google Scholar] [CrossRef]
- Kudo, T.; Kuratani, T.; Shimamura, K.; Sakaniwa, R.; Sawa, Y. Long-term results of hybrid aortic arch repair using landing zone 0: A single-centre study. Eur. J. Cardiothorac. Surg. 2021, 59, 1227–1235. [Google Scholar] [CrossRef]
- Kudo, T.; Kuratani, T.; Shimamura, K.; Sawa, Y. Determining the Optimal Proximal Landing Zone for TEVAR in the Aortic Arch: Comparing the Occurrence of the Bird-Beak Phenomenon in Zone 0 vs. Zones 1 and 2. J. Endovasc. Ther. 2020, 27, 368–376. [Google Scholar] [CrossRef]
- Preventza, O.; Garcia, A.; Cooley, D.A.; Haywood-Watson, R.J.; Simpson, K.; Bakaeen, F.G.; Cornwell, L.D.; Omer, S.; de la Cruz, K.I.; Price, M.D.; et al. Total aortic arch replacement: A comparative study of zone 0 hybrid arch exclusion versus traditional open repair. J. Thorac. Cardiovasc. Surg. 2015, 150, 1591–1598; discussion 1598–1600. [Google Scholar] [CrossRef]
- Preventza, O.; Tan, C.W.; Orozco-Sevilla, V.; Euhus, C.J.; Coselli, J.S. Zone zero hybrid arch exclusion versus open total arch replacement. Ann. Cardiothorac. Surg. 2018, 7, 372–379. [Google Scholar] [CrossRef]
- Shimizu, H.; Hirahara, N.; Motomura, N.; Miyata, H.; Takamoto, S. Current status of cardiovascular surgery in Japan, 2015 and 2016: Analysis of data from Japan Cardiovascular Surgery Database. 4-Thoracic aortic surgery. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Kuratani, T.; Shimamura, K.; Sawa, Y. Early and midterm results of thoracic endovascular aortic repair using a branched endograft for aortic arch pathologies: A retrospective single-center study. JTCVS Tech. 2020, 4, 17–25. [Google Scholar] [CrossRef]
- Okita, Y.; Miyata, H.; Motomura, N.; Takamoto, S.; Japan Cardiovascular Surgery Database Organization. A study of brain protection during total arch replacement comparing antegrade cerebral perfusion versus hypothermic circulatory arrest, with or without retrograde cerebral perfusion: Analysis based on the Japan Adult Cardiovascular Surgery Database. J. Thorac. Cardiovasc. Surg. 2015, 149, S65–S73. [Google Scholar] [CrossRef] [PubMed]
- Minatoya, K.; Inoue, Y.; Sasaki, H.; Tanaka, H.; Seike, Y.; Oda, T.; Omura, A.; Iba, Y.; Ogino, H.; Kobayashi, J. Total arch replacement using a 4-branched graft with antegrade cerebral perfusion. J. Thorac. Cardiovasc. Surg. 2019, 157, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Kurazumi, H.; Mikamo, A.; Kudo, T.; Suzuki, R.; Takahashi, M.; Shirasawa, B.; Zempo, N.; Hamano, K. Aortic arch surgery in octogenarians: Is it justified? Eur. J. Cardiothorac. Surg. 2014, 46, 672–677. [Google Scholar] [CrossRef][Green Version]
- Leshnower, B.G.; Kilgo, P.D.; Chen, E.P. Total arch replacement using moderate hypothermic circulatory arrest and unilateral selective antegrade cerebral perfusion. J. Thorac. Cardiovasc. Surg. 2014, 147, 1488–1492. [Google Scholar] [CrossRef]
- Misfeld, M.; Leontyev, S.; Borger, M.A.; Gindensperger, O.; Lehmann, S.; Legare, J.F.; Mohr, F.W. What is the best strategy for brain protection in patients undergoing aortic arch surgery? A single center experience of 636 patients. Ann. Thorac. Surg. 2012, 93, 1502–1508. [Google Scholar] [CrossRef]
- De Rango, P.; Ferrer, C.; Coscarella, C.; Musumeci, F.; Verzini, F.; Pogany, G.; Montalto, A.; Cao, P. Contemporary comparison of aortic arch repair by endovascular and open surgical reconstructions. J. Vasc. Surg. 2015, 61, 339–346. [Google Scholar] [CrossRef]
- Iba, Y.; Minatoya, K.; Matsuda, H.; Sasaki, H.; Tanaka, H.; Oda, T.; Kobayashi, J. How should aortic arch aneurysms be treated in the endovascular aortic repair era? A risk-adjusted comparison between open and hybrid arch repair using propensity score-matching analysis. Eur. J. Cardiothorac. Surg. 2014, 46, 32–39. [Google Scholar] [CrossRef]
- Bavaria, J.; Vallabhajosyula, P.; Moeller, P.; Szeto, W.; Desai, N.; Pochettino, A. Hybrid approaches in the treatment of aortic arch aneurysms: Postoperative and midterm outcomes. J. Thorac. Cardiovasc. Surg. 2013, 145, S85–S90. [Google Scholar] [CrossRef]
- Vallejo, N.; Rodriguez-Lopez, J.A.; Heidari, P.; Wheatley, G.; Caparrelli, D.; Ramaiah, V.; Diethrich, E.B. Hybrid repair of thoracic aortic lesions for zone 0 and 1 in high-risk patients. J. Vasc. Surg. 2012, 55, 318–325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Milewski, R.K.; Szeto, W.Y.; Pochettino, A.; Moser, G.W.; Moeller, P.; Bavaria, J.E. Have hybrid procedures replaced open aortic arch reconstruction in high-risk patients? A comparative study of elective open arch debranching with endovascular stent graft placement and conventional elective open total and distal aortic arch reconstruction. J. Thorac. Cardiovasc. Surg. 2010, 140, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.D.; Appoo, J.J.; Bavaria, J.E.; Herget, E.J.; Moeller, P.; Pochettino, A.; Wong, J.K. Results of type II hybrid arch repair with zone 0 stent graft deployment for complex aortic arch pathology. J. Thorac. Cardiovasc. Surg. 2014, 148, 2951–2955. [Google Scholar] [CrossRef] [PubMed]
- Narita, H.; Komori, K.; Usui, A.; Yamamoto, K.; Banno, H.; Kodama, A.; Sugimoto, M. Postoperative Outcomes of Hybrid Repair in the Treatment of Aortic Arch Aneurysms. Ann. Vasc. Surg. 2016, 34, 55–61. [Google Scholar] [CrossRef]
- Czerny, M.; Weigang, E.; Sodeck, G.; Schmidli, J.; Antona, C.; Gelpi, G.; Friess, T.; Klocker, J.; Szeto, W.Y.; Moeller, P.; et al. Targeting landing zone 0 by total arch rerouting and TEVAR: Midterm results of a transcontinental registry. Ann. Thorac. Surg. 2012, 94, 84–89. [Google Scholar] [CrossRef]
- Tokuda, Y.; Oshima, H.; Narita, Y.; Abe, T.; Araki, Y.; Mutsuga, M.; Fujimoto, K.; Terazawa, S.; Yagami, K.; Ito, H.; et al. Hybrid versus open repair of aortic arch aneurysms: Comparison of postoperative and mid-term outcomes with a propensity score-matching analysis. Eur. J. Cardiothorac. Surg. 2016, 49, 149–156. [Google Scholar] [CrossRef]
- Maeda, K.; Ohki, T.; Kanaoka, Y.; Shukuzawa, K.; Baba, T.; Momose, M. A Novel Shaggy Aorta Scoring System to Predict Embolic Complications Following Thoracic Endovascular Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 57–66. [Google Scholar] [CrossRef]
- He, X.; Liu, W.; Li, Z.; Liu, X.; Wang, T.; Ding, C.; Zeng, H. Hybrid Approach to Management of Complex Aortic Arch Pathologies: A Single-Center Experience in China. Ann. Vasc. Surg. 2016, 31, 23–29. [Google Scholar] [CrossRef]
- Melissano, G.; Tshomba, Y.; Bertoglio, L.; Rinaldi, E.; Chiesa, R. Analysis of stroke after TEVAR involving the aortic arch. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 269–275. [Google Scholar] [CrossRef]
- Gandet, T.; Canaud, L.; Ozdemir, B.A.; Ziza, V.; Demaria, R.; Albat, B.; Alric, P. Factors favoring retrograde aortic dissection after endovascular aortic arch repair. J. Thorac. Cardiovasc. Surg. 2015, 150, 136–142. [Google Scholar] [CrossRef]
- Williams, J.B.; Andersen, N.D.; Bhattacharya, S.D.; Scheer, E.; Piccini, J.P.; McCann, R.L.; Hughes, G.C. Retrograde ascending aortic dissection as an early complication of thoracic endovascular aortic repair. J. Vasc. Surg. 2012, 55, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Yammine, H.; Briggs, C.S.; Stanley, G.A.; Ballast, J.K.; Anderson, W.E.; Nussbaum, T.; Madjarov, J.; Frederick, J.R.; Arko, F.R., 3rd. Retrograde type A dissection after thoracic endovascular aortic repair for type B aortic dissection. J. Vasc. Surg. 2019, 69, 24–33. [Google Scholar] [CrossRef] [PubMed]



| All n = 54 | bTEVAR n = 25 (46.3%) | hTEVAR n = 29 (53.7%) | p-Value | |
|---|---|---|---|---|
| Patients’ characteristics | ||||
| Age (years) | 78 (73–82) | 81 (76–84) | 77 (69–80) | 0.023 |
| Age ≥ 80 years, n (%) | 22 (40.7) | 15 (60.0) | 7 (24.1) | 0.007 |
| Female, n (%) | 12 (22.2) | 9 (36.0) | 3 (10.3) | 0.046 |
| Emergency, n (%) | 0 | 0 | 0 | 1.00 |
| Aortic pathologies | ||||
| Degenerative aortic aneurysm, n (%) | 49 (90.7) | 22 (88.0) | 27 (93.1) | 0.653 |
| Dissecting aortic aneurysm, n (%) | 5 (9.3) | 3 (12.0) | 2 (6.9) | 0.653 |
| Dissection with patent false lumen, n (%) | 0 | 0 | 0 | 1.00 |
| Medical history | ||||
| Cerebrovascular disease, n (%) | 12 (22.2) | 7 (28.0) | 5 (7.2) | 0.513 |
| Coronary artery disease, n (%) | 11 (20.4) | 7 (28.0) | 4 (13.8) | 0.310 |
| CKD stage ≥ 4, n (%) | 12 (22.2) | 6 (24.0) | 6 (20.7) | 0.771 |
| COPD, n (%) | 14 (25.9) | 12 (48.0) | 2 (6.9) | 0.001 |
| EF (%) | 66 (60–73) | 65 (59–72) | 68 (60–74) | 0.419 |
| Previous cardiovascular surgery, n (%) | 13 (24.1) | 10 (40.0) | 3 (10.3) | 0.023 |
| Prior median sternotomy, n (%) | 0 | 0 | 0 | 1.00 |
| Logistic Euro SCORE (%) | 32 (20–40) | 38 (34–56) | 21 (13–30) | <0.001 |
| All n = 54 | bTEVAR n = 25 (46.3%) | hTEVAR n = 29 (53.7%) | p Value | |
|---|---|---|---|---|
| Preoperative measurements | ||||
| Maximum aneurysm diameter (mm) | 58 (53–65) | 57 (54–62) | 60 (52–74) | 0.335 |
| Length of proximal LZ (mm) | 33.6 ± 6.8 | 35.6 ± 1.3 | 31.9 ± 5.4 | 0.049 |
| Diameter of proximal LZ (mm) | 33.6 ± 3.0 | 34.9 ± 3.5 | 32.5 ± 2.0 | 0.003 |
| Diameter of distal LZ (mm) | 28.5 ±3.2 | 29.3 ± 3.4 | 27.8 ± 3.0 | 0.109 |
| Atheroma grade | ||||
| Ascending aorta ≥2, n (%) | 13 (24.1) | 9 (36.0) | 4 (13.8) | 0.109 |
| Aortic arch ≥3, n (%) | 47 (87.0) | 21 (84.0) | 26 (89.7) | 0.692 |
| Descending aorta ≥3, n (%) | 9 (16.7) | 7 (28.0) | 2 (6.9) | 0.065 |
| BCA ≥2, n (%) | 14 (25.9) | 6 (24.0) | 8 (27.6) | 0.764 |
| Left CCA ≥2, n (%) | 5 (9.3) | 4 (16.0) | 1 (3.5) | 0.170 |
| Stent-grafts | ||||
| Number of stent-graft, n (%) | 1.5 ± 0.5 | 1.1 ± 0.3 | 1.8 ± 0.4 | <0.001 |
| Type of proximal stent-grafts | ||||
| Bolton Relay NBS, n (%) | 25 (46.3) | 25 (100) | 0 | |
| Bolton Relay Plus, n (%) | 2 (3.7) | 0 | 2 (6.9) | |
| Gore TAG, n (%) | 10 (18.5) | 0 | 10 (34.5) | |
| Gore CTAG, n (%) | 16 (29.6) | 0 | 16 (55.2) | |
| Cook Zenith TX2, n (%) | 1 (1.9) | 0 | 1 (3.4) | |
| Proximal stent-grafts | ||||
| Diameter (mm) | 39.2 ± 3.8 | 41.9 ± 3.3 | 36.9 ± 2.4 | <0.001 |
| Oversizing rate (%) | 16.9 ± 8.1 | 20.7 ± 8.1 | 13.7 ± 6.6 | 0.001 |
| Distal stent-grafts | ||||
| Diameter (mm) | 34.0 ± 3.9 | 34.4 ± 4.7 | 33.7 ± 3.1 | 0.531 |
| Oversizing rate (%) | 19.7 ± 8.6 | 17.6 ± 9.4 | 21.5 ± 7.4 | 0.097 |
| All n = 54 | bTEVAR n = 25 (46.3%) | hTEVAR n = 29 (53.7%) | p-Value | |
|---|---|---|---|---|
| Procedure success (%) | 100 | 100 | 100 | 1.00 |
| Operative time (minutes) | 255 (217–290) | 220 (193–257) | 279 (246–328) | <0.001 |
| Postoperative hospital stay (days) | 16 (12–25) | 12 (9–22) | 17 (14–26) | 0.013 |
| In-hospital mortality | ||||
| RTAD, n (%) | 1 (1.9) | 0 | 1 (3.4) * | 1.00 |
| Aortic complication, n (%) | ||||
| PND, n (%) | 2 (3.7) | 2 (8.0) | 0 | 0.210 |
| Spinal cord injury, n (%) | 0 | 0 | 0 | 1.00 |
| Abdominal embolic event, n (%) | 0 | 0 | 0 | 1.00 |
| New dialysis, n (%) | 1 (1.9) | 0 | 1 (3.4) | 1.00 |
| RTAD, n (%) | 1 (1.9) | 0 | 1 (3.4) * | 1.00 |
| Aneurysm enlargement, n (%) | 0 | 0 | 0 | 1.00 |
| Aneurysm rupture, n (%) | 0 | 0 | 0 | 1.00 |
| Endoleaks, n (%) | ||||
| Type 1a, n (%) | 0 | 0 | 0 | 1.00 |
| Type 1b, n (%) | 0 | 0 | 0 | 1.00 |
| Type 1c, n (%) | 0 | 0 | 0 | 1.00 |
| Type 2, n (%) | 0 | 0 | 0 | 1.00 |
| Type 3, n (%) | 0 | 0 | 0 | 1.00 |
| All n = 54 | bTEVAR n = 25 (46.3%) | hTEVAR n = 29 (53.7%) | p-Value | |
|---|---|---|---|---|
| Aortic complication, n (%) | ||||
| PND, n (%) | 0 | 0 | 0 | 1.00 |
| RTAD, n (%) | 0 | 0 | 0 | 1.00 |
| Aneurysm enlargement, n (%) | 0 | 0 | 0 | 1.00 |
| Aneurysm rupture, n (%) | 2 (3.7) | 2 (8.0) *,+ | 0 | 0.210 |
| Distal SINE, n (%) | 0 | 0 | 0 | 1.00 |
| Prosthetic infection, n (%) | 2 (3.7) | 0 | 2 (6.9) | 0.493 |
| Branched endograft occlusion, n (%) | 0 | 0 | 0 | 1.00 |
| Bypass graft occlusion, n (%) | 0 | 0 | 0 | 1.00 |
| Endoleaks, n (%) | ||||
| Type 1a, n (%) | 0 | 0 | 0 | 1.00 |
| Type 1b, n (%) | 1 (1.9) | 1 (4.0) * | 0 | 0.463 |
| Type 1c, n (%) | 0 | 0 | 0 | 1.00 |
| Type 2, n (%) | 0 | 0 | 0 | 1.00 |
| Type 3, n (%) | 1 (1.9) | 1 (4.0) + | 0 | 0.463 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudo, T.; Kuratani, T.; Sawa, Y.; Miyagawa, S. Effectiveness and Minimal-Invasiveness of Zone 0 Landing Thoracic Endovascular Aortic Repair Using Branched Endograft. J. Clin. Med. 2022, 11, 6981. https://doi.org/10.3390/jcm11236981
Kudo T, Kuratani T, Sawa Y, Miyagawa S. Effectiveness and Minimal-Invasiveness of Zone 0 Landing Thoracic Endovascular Aortic Repair Using Branched Endograft. Journal of Clinical Medicine. 2022; 11(23):6981. https://doi.org/10.3390/jcm11236981
Chicago/Turabian StyleKudo, Tomoaki, Toru Kuratani, Yoshiki Sawa, and Shigeru Miyagawa. 2022. "Effectiveness and Minimal-Invasiveness of Zone 0 Landing Thoracic Endovascular Aortic Repair Using Branched Endograft" Journal of Clinical Medicine 11, no. 23: 6981. https://doi.org/10.3390/jcm11236981
APA StyleKudo, T., Kuratani, T., Sawa, Y., & Miyagawa, S. (2022). Effectiveness and Minimal-Invasiveness of Zone 0 Landing Thoracic Endovascular Aortic Repair Using Branched Endograft. Journal of Clinical Medicine, 11(23), 6981. https://doi.org/10.3390/jcm11236981








