Myocarditis Related to COVID-19 and SARS-CoV-2 Vaccination
Abstract
1. Introduction
2. Materials and Methods
3. Etiopathogenic Bases
3.1. Etiopathogenic Bases of COVID-19-Related Myocarditis
3.2. Etiopathogenic Bases of Myocarditis Related to SARS-CoV-2 Vaccination
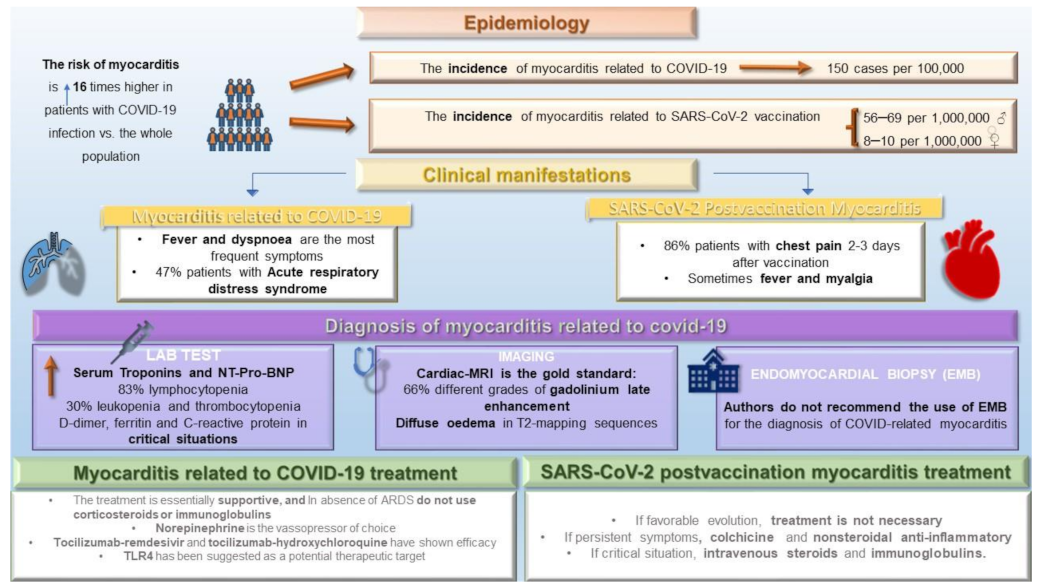
4. Epidemiology: Frequency and Risk Factors
4.1. Epidemiology of COVID-19-Related Myocarditis
4.2. Epidemiology of Myocarditis Related to SARS-CoV-2 Vaccination
5. Clinical Manifestations
6. Diagnosis
6.1. Diagnosis of COVID-19-Related Myocarditis
6.1.1. Lab Test
6.1.2. Electrocardiogram and Image Data
6.1.3. Endomyocardial Biopsy and Autopsy
6.2. Diagnosis of Myocarditis Related to SARS-CoV-2 Vaccination
7. Treatment
7.1. Basis for Treatment for COVID-Related Myocarditis
7.2. Immunosuppressive Therapy for COVID-19-Related Myocraditis
7.3. Treatment of Myocarditis Related to SARS-CoV-2 Vaccination
8. Evolution
9. Prognosis
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Definition: An inflammatory infiltrate of the myocardium with necrosis and/or degeneration of adjacent myocytes not typical of the ischemic damage associated with coronary artery disease |
First biopsy
|
Subsequent biopsy
|
References
- Castiello, T.; Georgiopoulos, G.; Finocchiaro, G.; Claudia, M.; Gianatti, A.; Delialis, D.; Aimo, A.; Prasad, S. COVID-19 and myocarditis: A systematic review and overview of current challenges. Heart Fail. Rev. 2022, 27, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Aboudounya, M.M.; Heads, R.J. COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 May Bind and Activate TLR4 to Increase ACE2 Expression, Facilitating Entry and Causing Hyperinflammation. Mediat. Inflamm. 2021, 2021, 8874339. [Google Scholar] [CrossRef]
- Chilazi, M.; Duffy, E.Y.; Thakkar, A.; Michos, E.D. COVID and Cardiovascular Disease: What We Know in 2021. Curr. Atheroscler. Rep. 2021, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Shchendrygina, A.; Nagel, E.; Puntmann, V.O.; Valbuena-Lopez, S. COVID-19 myocarditis and prospective heart failure burden. Expert Rev. Cardiovasc. Ther. 2021, 19, 5–14. [Google Scholar] [CrossRef]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef] [PubMed]
- McLean, K.; Johnson, T.J. Myopericarditis in a previously healthy adolescent male following COVID-19 vaccination: A case report. Acad. Emerg. Med. 2021, 28, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.F.; Ammirati, E.; Adler, E.D.; Cooper, L.T.; Hong, K.N.; Saponara, G.; Couri, D.; Cereda, A.; Procopio, A.; Cavalotti, C.; et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation 2021, 144, 507–509. [Google Scholar] [CrossRef]
- Bautista García, J.; Peña Ortega, P.; Bonilla Fernández, J.A.; Cárdenes León, A.; Ramírez Burgos, L.; Caballero Dorta, E. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19. Rev. Esp. Cardiol. 2021, 74, 812–814. [Google Scholar] [CrossRef]
- Muthukumar, A.; Narasimhan, M.; Li, Q.Z.; Mahimainathan, L.; Hitto, I.; Fuda, F.; Batra, K.; Jiang, X.; Zhu, C.; Schoggins, J.; et al. In-depth evaluation of a case of presumed myocarditis after the second dose of COVID-19 mRNA vaccine. Circulation 2021, 144, 487–498. [Google Scholar] [CrossRef]
- Choi, S.; Lee, S.; Seo, J.W.; Kim, M.J.; Jeon, Y.H.; Park, J.H.; Lee, J.K.; Yeo, N.S. Myocarditis-induced Sudden Death after BNT162b2 mRNA COVID-19 Vaccination in Korea: Case Report Focusing on Histopathological Findings. J. Korean Med. Sci. 2021, 36, e286. [Google Scholar] [CrossRef]
- Hu, H.; Ma, F.; Wei, X.; Fang, Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur. Heart J. 2021, 42, 206, Erratum in: Eur. Heart J. 2021, 42, 191. [Google Scholar] [CrossRef]
- Dabbagh, M.F.; Aurora, L.; D’Souza, P.; Weinmann, A.J.; Bhargava, P.; Basir, M.B. Cardiac Tamponade Secondary to COVID-19. JACC Case Rep. 2020, 2, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Szarpak, L.; Pruc, M.; Filipiak, K.J.; Popieluch, J.; Bielski, A.; Jaguszewski, M.j.; Gilis-Malinowska, N.; Chirico, F.; Rafique, Z.; Peacock, F.W. Myocarditis: A complication of COVID-19 and LONG-COVID-19 syndrome as a serious threat in modern cardiology. Cardiol. J. 2022, 29, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Cavalotti, C.; Milazzo, A.; Pedrotti, P.; Soriano, F.; Schroeder, J.W.; Morici, N.; Giannattasio, C.; Frigerio, M.; Metra, M.; et al. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection. Int. J. Cardiol. Heart Vasc. 2021, 34, 100774. [Google Scholar] [CrossRef] [PubMed]
- Yu, X. Potential of Heparin in the Treatment of COVID-19-Associated Myocarditis. Pediatr. Emerg. Care 2022, 38, e504. [Google Scholar] [CrossRef]
- Abrams, J.Y.; Oster, M.E.; Godfred-Cato, S.E.; Bryant, B.; Datta, S.D.; Campbell, A.P.; Leung, J.W.; Tsang, C.A.; Pierce, T.J.; Kennedy, J.L.; et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: A retrospective surveillance study. Lancet Child. Adolesc. Health 2021, 5, 323–331. [Google Scholar] [CrossRef]
- Hajjo, R.; Sabbah, D.A.; Bardaweel, S.K.; Tropsha, A. Shedding the Light on Post-Vaccine Myocarditis and Pericarditis in COVID-19 and Non-COVID-19 Vaccine Recipients. Vaccines 2021, 9, 1186. [Google Scholar] [CrossRef]
- Boehmer, T.K.; Kompaniyets, L.; Lavery, A.M.; Hsu, J.; Ko, J.Y.; Yusuf, H.; Romano, S.D.; Gundlapalli, A.V.; Oster, M.E.; Harris, A.M. Association Between COVID-19 and Myocarditis Using Hospital-Based Administrative Data—United States, March 2020-January 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1228–1232. [Google Scholar] [CrossRef]
- Bois, M.C.; Boire, N.A.; Layman, A.J.; Aubry, M.C.; Alexander, M.P.; Roden, A.C.; Hagen, C.E.; Quinton, R.A.; Larsen, C.; Erben, Y.; et al. COVID-19-Associated Nonocclusive Fibrin Microthrombi in the Heart. Circulation 2021, 143, 230–243. [Google Scholar] [CrossRef]
- Luo, P.; Liu, Y.; Qiu, L.; Liu, X.; Liu, D.; Li, J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020, 92, 814–818. [Google Scholar] [CrossRef]
- Blagova, O.V.; Kogan, E.A.; Lutokhina, Y.A.; Kukleva, A.D.; Ainetdinova, D.H.; Novosadov, V.M.; Rud, R.S.; Zaitsev, A.Y.; Zaidenov, V.A.; Kupriyanova, A.G.; et al. Subacute and chronic post-covid myoendocarditis: Clinical presentation, role of coronavirus persistence and autoimmune mechanisms. Kardiologiia 2021, 61, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Paiardi, G.; Richter, S.; Oreste, P.; Urbinati, C.; Rusnati, M.; Wade, R.C. The binding of heparin to spike glycoprotein inhibits SARS-CoV-2 infection by three mechanisms. J. Biol. Chem. 2021, 298, 101507. [Google Scholar] [CrossRef]
- Talasaz, A.H.; Kakavand, H.; Van Tassell, B.; Aghakouchakzadeh, M.; Sadeghipour, P.; Dunn, S.; Geraiely, B. Cardiovascular Complications of COVID-19: Pharmacotherapy Perspective. Cardiovasc. Drugs Ther. 2021, 35, 249–259. [Google Scholar] [CrossRef]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T., Jr.; Chahal, C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020, 17, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P.; Connor, J.H.; Jain, A. Acute and Chronic Cardiovascular Manifestations of COVID-19: Role for Endotheliopathy. Methodist. Debakey Cardiovasc. J. 2021, 17, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Haussner, W.; DeRosa, A.P.; Haussner, D.; Tran, J.; Torres-Lavoro, J.; Kamler, J.; Shah, K. COVID-19 associated myocarditis: A systematic review. Am. J. Emerg. Med. 2022, 51, 150–155. [Google Scholar] [CrossRef]
- Pillai, A.; Lawson, B. Coronavirus disease 2019 and cardiovascular diseases: Collateral damage? Curr. Opin. Anaesthesiol. 2022, 35, 5–11. [Google Scholar] [CrossRef]
- Sawalha, K.; Abozenah, M.; Kadado, A.J.; Battisha, A.; Al-Akchar, M.; Salerno, C.; Hernandez-Montfort, J.; Islam, A.M. Systematic Review of COVID-19 Related Myocarditis: Insights on Management and Outcome. Cardiovasc. Revasc. Med. 2021, 23, 107–113. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, X.; Jian, J.; Chen, X.; Yin, K. Cardiovascular disease in patients with COVID-19: Evidence from cardiovascular pathology to treatment. Acta Biochim. Biophys. Sin. 2021, 53, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, R.; Sakamoto, A.; Kawai, K.; Gianatti, A.; Pellegrini, D.; Nasr, A.; Kutys, B.; Guo, L.; Cornelissen, A.; Mori, M.; et al. Pathological Evidence for SARS-CoV-2 as a Cause of Myocarditis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 314–325. [Google Scholar] [CrossRef]
- Halushka, M.K.; Vander Heide, R.S. Myocarditis is rare in COVID-19 autopsies: Cardiovascular findings across 277 postmortem examinations. Cardiovasc. Pathol. 2021, 50, 107300. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.; Alam, F.; Saeed, A.; Butt, F.; Khaliq, M.A.; Malik, A.; Chaudhry, M.; Abdullah, M. Multisystem Inflammatory Syndrome in Children and Adolescents (MIS-C) under the Setting of COVID-19: A Review of Clinical Presentation, Workup and Management. Infect. Dis. 2021, 14, 11786337211026642. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Deswal, A.; Khalid, U. COVID-19 myocarditis and long-term heart failure sequelae. Curr. Opin. Cardiol. 2021, 36, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, Y.; Su, G.; Li, Y.; Shuai, X. Intravenous immunoglobulin therapy for acute myocarditis in children and adults. Int. Heart J. 2019, 60, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yun, K.W.; Kim, K.R.; Song, S.H.; Ahn, B.; Kim, D.R.; Kim, G.B.; Huh, J.; Choi, E.H.; Kim, Y.J. Epidemiology and Clinical Features of Myocarditis/Pericarditis before the Introduction of mRNA COVID-19 Vaccine in Korean Children: A Multicenter Study. J. Korean Med. Sci. 2021, 36, e232. [Google Scholar] [CrossRef]
- Nygaard, U.; Holm, M.; Bohnstedt, C.; Chai, Q.; Schmidt, L.S.; Hartling, U.B.; Petersen, J.J.H.; Thaarup, J.; Bjerre, J.; Vejlstrup, N.G.; et al. Population-based Incidence of Myopericarditis After COVID-19 Vaccination in Danish Adolescents. Pediatr. Infect. Dis. J. 2022, 41, e25–e28. [Google Scholar] [CrossRef] [PubMed]
- Deftereos, S.G.; Giannopoulos, G.; Vrachatis, D.A.; Siasos, G.D.; Giotaki, S.G.; Gargalianos, P.; Metallidis, S.; Sianos, G.; Baltagiannis, S.; Panagopoulos, P.; et al. GRECCO-19 investigators. Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized with Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2013136. [Google Scholar] [CrossRef]
- Sarhan, R.M.; Harb, H.S.; Abou Warda, A.E.; Salem-Bekhit, M.M.; Shakeel, F.; Alzahrani, S.A.; Madney, Y.M.; Boshra, M.S. Efficacy of the early treatment with tocilizumab-hydroxy.ychloroquine and tocilizumab-remdesivir in severe COVID-19 Patients. J. Infect. Public Health 2022, 15, 116–122. [Google Scholar] [CrossRef]
- Basso, C.; Aguilera, B.; Banner, J.; Cohle, S.; d’Amati, G.; de Gouveia, R.H.; di Gioia, C.; Fabre, A.; Gallagher, P.J.; Leone, O.; et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Archiv. 2017, 471, 691–705. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 46, 854–887. [Google Scholar] [CrossRef] [PubMed]
- Centre for Disease Control. Interim Clinical Considerations for Use of COVID-19 Vaccines. Available online: https://www.cdc.gov/vaccines/covid-19/clinical (accessed on 15 August 2022).
| Type | Total References | First Wave | Second Wave | Third Wave | Fourth Wave | Fifth Wave | Sixth Wave |
|---|---|---|---|---|---|---|---|
| Case report | [6,7,8,9,10,11,12] | - | [12] | [11] | - | [6,7,8,9,10] | - |
| Free full text | [13,14,15] | - | - | [13] | - | [14] | [15] |
| Observational study | [5,16] | - | - | - | [16] | - | [5] |
| Clinical study | [17,18,19,20,21,22] | [20] | [19] | - | [17,18,21] | [22] | |
| Systematic review | [1,2,3,4,23,24,25,26,27,28,29,30,31,32,33,34] | - | [24] | [2,3,29,30,31,32,34] | [4,23,33] | [26] | [1,25,27,28] |
| Meta-analysis | [35] | [35] | - | - | - | - | - |
| Multicenter study | [36,37,38,39] | - | [38] | - | - | [36] | [37,39] |
| Practice Guideline | [40,41] | [40,41] | - | - | - | - | - |
| Article | Inclusion Period | Myocarditis Related to COVID-19 | Pericarditis Related to COVID-19 | Myocarditis Related to SARS-CoV-2 Vaccination | Pericarditis Related to SARS-CoV-2 Vaccination |
|---|---|---|---|---|---|
| Castiello et al. [1] | December 2019 to September 2020 | 38 patients (12 patients confirmed by BEM, 25 patients confirmed by CMR) | |||
| Oster et al [5] | December 2020 to August 2021 | 1626 patients (223 patients confirmed by CMR) | |||
| Hajjo et al. [17] | From 1990 to September 2021 | 1579 patients | 1063 patients | ||
| Bozkurt et al. [26] | From 1990 to June 2021 | 484 patients with myocarditis/pericarditis | |||
| Haussner et al. [27] | February 2020 to November 2020 | 51 patients (5 patients confirmed by BEM, 25 patients by CMR) | |||
| Nygaard et al. [37] | May 2021 to September 2021 | 15 patients | |||
| Sawalha et al. [29] | December 2019 to June 2020 | 14 patients with myocarditis/pericarditis (7 patients confirmed by CMR) | |||
| Halushka et al. [32] | January to September 2020 | 277 autopsied hearts. 5 patients with definitive myocarditis, | |||
| Larson et al. [7] | - | 8 patients (8 patients confirmed by CMR) | |||
| Blagova et al. [21] | March 2020 to March 2021 | 15 patients (6 patients confirmed by BEM, 10 patients by CMR) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Ramos, A.I.; Gómez-Moyano, E.; Rodríguez-Capitán, J.; Angullo-Gómez, M.; Gallardo-Jiménez, P.; Pérez de Pedro, I.; Valiente de Santis, L.; Pérez-Villardón, B.; Piñero-Uribe, I.; Mora-Robles, J.; et al. Myocarditis Related to COVID-19 and SARS-CoV-2 Vaccination. J. Clin. Med. 2022, 11, 6999. https://doi.org/10.3390/jcm11236999
Molina-Ramos AI, Gómez-Moyano E, Rodríguez-Capitán J, Angullo-Gómez M, Gallardo-Jiménez P, Pérez de Pedro I, Valiente de Santis L, Pérez-Villardón B, Piñero-Uribe I, Mora-Robles J, et al. Myocarditis Related to COVID-19 and SARS-CoV-2 Vaccination. Journal of Clinical Medicine. 2022; 11(23):6999. https://doi.org/10.3390/jcm11236999
Chicago/Turabian StyleMolina-Ramos, Ana I., Elisabeth Gómez-Moyano, Jorge Rodríguez-Capitán, María Angullo-Gómez, Patricia Gallardo-Jiménez, Iván Pérez de Pedro, Lucía Valiente de Santis, Beatriz Pérez-Villardón, Isabel Piñero-Uribe, Javier Mora-Robles, and et al. 2022. "Myocarditis Related to COVID-19 and SARS-CoV-2 Vaccination" Journal of Clinical Medicine 11, no. 23: 6999. https://doi.org/10.3390/jcm11236999
APA StyleMolina-Ramos, A. I., Gómez-Moyano, E., Rodríguez-Capitán, J., Angullo-Gómez, M., Gallardo-Jiménez, P., Pérez de Pedro, I., Valiente de Santis, L., Pérez-Villardón, B., Piñero-Uribe, I., Mora-Robles, J., Becerra-Muñoz, V. M., & Jiménez-Navarro, M. (2022). Myocarditis Related to COVID-19 and SARS-CoV-2 Vaccination. Journal of Clinical Medicine, 11(23), 6999. https://doi.org/10.3390/jcm11236999







