Robot-Assisted Electrode Insertion in Cochlear Implantation Controlled by Intraoperative Electrocochleography—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Types of Device and Electrode Arrays
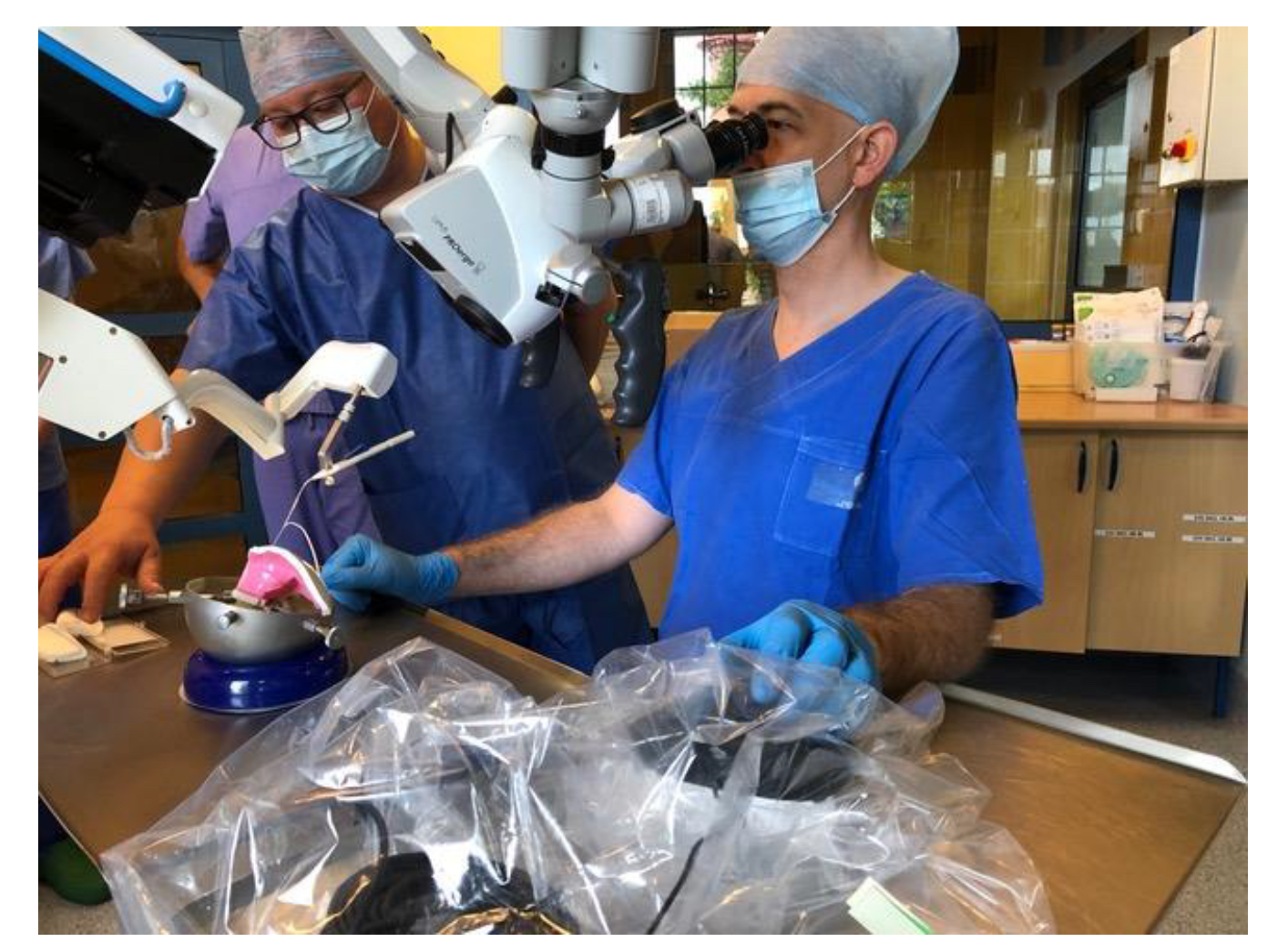
2.3. Robot-Assisted Electrode Array Insertion
2.4. Surgical Technique
2.5. Cochlear Implant System Activation
2.6. Electrophysiological Measurements
2.7. ECochG Measurement
2.8. Imaging
2.9. Pure-Tone Audiometry
3. Results
3.1. Intraoperative Course
3.2. Intraoperative Electrocochleography
3.3. Imaging
3.4. Pure Tone Audiometry and the Estimated Audiogram
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, B.; Gerber, N.; Williamson, T.; Gavaghan, K.; Wimmer, W.; Caversaccio, M.; Weber, S. In vitro accuracy evaluation of image-guided robot system for direct cochlear access. Otol. Neurotol. 2013, 34, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Kratchman, L.B.; Blachon, G.S.; Withrow, T.J.; Balachandran, R.; Labadie, R.F.; Webster, R.J., 3rd. Design of a bone-attached parallel robot for percutaneous cochlear implantation. IEEE Trans. Biomed. Eng. 2011, 58, 2904–2910. [Google Scholar] [CrossRef]
- Riojas, K.E.; Labadie, R.F. Robotic Ear Surgery. Otolaryngol. Clin. N. Am. 2020, 53, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Veleur, M.; Lahlou, G.; Torres, R.; Daoudi, H.; Mosnier, I.; Ferrary, E.; Sterkers, O.; Nguyen, Y. Robot-Assisted Middle Ear Endoscopic Surgery: Preliminary Results on 37 Patients. Front. Surg. 2021, 8, 740935. [Google Scholar] [CrossRef] [PubMed]
- Vittoria, S.; Lahlou, G.; Torres, R.; Daoudi, H.; Mosnier, I.; Mazalaigue, S.; Ferrary, E.; Nguyen, Y.; Sterkers, O. Robot-based assistance in middle ear surgery and cochlear implantation: First clinical report. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 77–85. [Google Scholar] [CrossRef]
- Daoudi, H.; Torres, R.; Mazalaigue, S.; Sterkers, O.; Ferrary, E.; Nguyen, Y. Analysis of forces during robot-assisted and manual manipulations of mobile and fixed footplate in temporal bone specimens. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 4269–4277. [Google Scholar] [CrossRef]
- Nguyen, Y.; Bernardeschi, D.; Sterkers, O. Potential of Robot-Based Surgery for Otosclerosis Surgery. Otolaryngol. Clin. N. Am 2018, 51, 475–485. [Google Scholar] [CrossRef]
- Kazmitcheff, G.; Nguyen, Y.; Miroir, M.; Péan, F.; Ferrary, E.; Cotin, S.; Sterkers, O.; Duriez, C. Middle-ear microsurgery simulation to improve new robotic procedures. BioMed Res. Int. 2014, 2014, 891742. [Google Scholar] [CrossRef]
- Panara, K.; Shahal, D.; Mittal, R.; Eshraghi, A.A. Robotics for Cochlear Implantation Surgery: Challenges and Opportunities. Otol. Neurotol. 2021, 42, e825–e835. [Google Scholar] [CrossRef]
- De Seta, D.; Daoudi, H.; Torres, R.; Ferrary, E.; Sterkers, O.; Nguyen, Y. Robotics, automation, active electrode arrays, and new devices for cochlear implantation: A contemporary review. Hear. Res. 2022, 414, 108425. [Google Scholar] [CrossRef]
- Loth, A.; Vazzana, C.; Leinung, M.; Guderian, D.; Issing, C.; Baumann, U.; Stöver, T. Quality control in cochlear implant therapy: Clinical practice guidelines and registries in European countries. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 4779–4786. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Pan, J.X.; Li, Y.; Zhang, Z.H.; Tan, H.Y.; Wang, Z.Y.; Wu, H. Preliminary application of robot-assisted electrode insertion in cochlear implantation. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi Chin. J. Otorhinolaryngol. Head Neck Surg. 2020, 55, 952–956. (In Chinese) [Google Scholar] [CrossRef]
- Torres, R.; Daoudi, H.; Lahlou, G.; Sterkers, O.; Ferrary, E.; Mosnier, I.; Nguyen, Y. Restoration of High Frequency Auditory Perception After Robot-Assisted or Manual Cochlear Implantation in Profoundly Deaf Adults Improves Speech Recognition. Front. Surg. 2021, 8, 729736. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.; Hochet, B.; Daoudi, H.; Carré, F.; Mosnier, I.; Sterkers, O.; Ferrary, E.; Nguyen, Y. Atraumatic Insertion of a Cochlear Implant Pre-Curved Electrode Array by a Robot-Automated Alignment with the Coiling Direction of the Scala Tympani. Audiol. Neurootol. 2022, 27, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Giardina, C.K.; Brown, K.D.; Adunka, O.F.; Buchman, C.A.; Hutson, K.A.; Pillsbury, H.C.; Fitzpatrick, D.C. Intracochlear Electrocochleography: Response Patterns During Cochlear Implantation and Hearing Preservation. Ear Hear. 2019, 40, 833–848. [Google Scholar] [CrossRef]
- Buechner, A.; Bardt, M.; Haumann, S.; Geissler, G.; Salcher, R.; Lenarz, T. Clinical experiences with intraoperative electrocochleography in cochlear implant recipients and its potential to reduce insertion trauma and improve postoperative hearing preservation. PLoS ONE 2022, 17, e0266077. [Google Scholar] [CrossRef] [PubMed]
- Koka, K.; Riggs, W.J.; Dwyer, R.; Holder, J.T.; Noble, J.H.; Dawant, B.M.; Ortmann, A.; Valenzuela, C.V.; Mattingly, J.K.; Harris, M.M.; et al. Intra-Cochlear Electrocochleography During Cochear Implant Electrode Insertion Is Predictive of Final Scalar Location. Otol. Neurotol. 2018, 39, e654–e659. [Google Scholar] [CrossRef] [PubMed]
- Pienkowski, M.; Adunka, O.F.; Lichtenhan, J.T. Editorial: New Advances in Electrocochleography for Clinical and Basic Investigation. Front. Neurosci. 2018, 12, 310. [Google Scholar] [CrossRef] [PubMed]
- Riggs, W.J.; Roche, J.P.; Giardina, C.K.; Harris, M.S.; Bastian, Z.J.; Fontenot, T.E.; Buchman, C.A.; Brown, K.D.; Adunka, O.F.; Fitzpatrick, D.C. Intraoperative Electrocochleographic Characteristics of Auditory Neuropathy Spectrum Disorder in Cochlear Implant Subjects. Front. Neurosci. 2017, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.S.; Riggs, W.J.; Giardina, C.K.; O’Connell, B.P.; Holder, J.T.; Dwyer, R.T.; Koka, K.; Labadie, R.F.; Fitzpatrick, D.C.; Adunka, O.F. Patterns Seen During Electrode Insertion Using Intracochlear Electrocochleography Obtained Directly Through a Cochlear Implant. Otol. Neurotol. 2017, 38, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Attias, J.; Ulanovski, D.; Hilly, O.; Greenstein, T.; Sokolov, M.; HabibAllah, S.; Mormer, H.; Raveh, E. Postoperative Intracochlear Electrocochleography in Pediatric Cochlear Implant Recipients: Association to Audiometric Thresholds and Auditory Performance. Ear Hear. 2020, 41, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, B.P.; Holder, J.T.; Dwyer, R.T.; Gifford, R.H.; Noble, J.H.; Bennett, M.L.; Rivas, A.; Wanna, G.B.; Haynes, D.S.; Labadie, R.F. Intra- and Postoperative Electrocochleography May Be Predictive of Final Electrode Position and Postoperative Hearing Preservation. Front. Neurosci. 2017, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Caversaccio, M.; Wimmer, W.; Anso, J.; Mantokoudis, G.; Gerber, N.; Rathgeb, C.; Schneider, D.; Hermann, J.; Wagner, F.; Scheidegger, O.; et al. Robotic middle ear access for cochlear implantation: First in man. PLoS ONE 2019, 14, e0220543. [Google Scholar] [CrossRef] [PubMed]
- Labadie, R.F.; Balachandran, R.; Noble, J.H.; Blachon, G.S.; Mitchell, J.E.; Reda, F.A.; Dawant, B.M.; Fitzpatrick, J.M. Minimally invasive image-guided cochlear implantation surgery: First report of clinical implementation. Laryngoscope 2014, 124, 1915–1922. [Google Scholar] [CrossRef]
- Labadie, R.F.; Riojas, K.; Von Wahlde, K.; Mitchell, J.; Bruns, T.; Webster, R., 3rd; Dawant, B.; Fitzpatrick, J.M.; Noble, J. Clinical Implementation of Second-generation Minimally Invasive Image-guided Cochlear Implantation Surgery. Otol. Neurotol. 2021, 42, 702–705. [Google Scholar] [CrossRef]
- Klopp-Dutote, N.; Lefranc, M.; Strunski, V.; Page, C. Minimally invasive fully ROBOT-assisted cochlear implantation in humans: Preliminary results in five consecutive patients. Clin. Otolaryngol. 2021, 46, 1326–1330. [Google Scholar] [CrossRef]
- Daoudi, H.; Lahlou, G.; Torres, R.; Sterkers, O.; Lefeuvre, V.; Ferrary, E.; Mosnier, I.; Nguyen, Y. Robot-assisted cochlear implant electrode array insertion in adults: A comparative study with manual insertion. Otol. Neurotol. 2021, 42, 438–444. [Google Scholar] [CrossRef]
- Barriat, S.; Peigneux, N.; Duran, U.; Camby, S.; Lefebvre, P.P. The Use of a Robot to Insert an Electrode Array of Cochlear Implants in the Cochlea: A Feasibility Study and Preliminary Results. Audiol. Neurootol. 2021, 26, 361–367. [Google Scholar] [CrossRef]
- Jia, H.; Pan, J.; Gu, W.; Tan, H.; Chen, Y.; Zhang, Z.; Jiang, M.; Li, Y.; Sterkers, O.; Wu, H. Robot-Assisted Electrode Array Insertion Becomes Available in Pediatric Cochlear Implant Recipients: First Report and an Intra-Individual Study. Front. Surg. 2021, 8, 695728. [Google Scholar] [CrossRef]
- Gifford, R.H.; Dorman, M.F.; Skarzynski, H.; Lorens, A.; Polak, M.; Driscoll, C.L.W.; Peter Roland, P.; Buchman, C.A. Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear Hear. 2013, 34, 413–425. [Google Scholar] [CrossRef]
- Henslee, A.M.; Kaufmann, C.R.; Andrick, M.D.; Reineke, P.T.; Tejani, V.D.; Hansen, M.R. Development and Characterization of an Electrocochleography-Guided Robotics-Assisted Cochlear Implant Array Insertion System. Otolaryngol. Head Neck Surg. 2022, 167, 334–340. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawęcki, W.; Balcerowiak, A.; Podlawska, P.; Borowska, P.; Gibasiewicz, R.; Szyfter, W.; Wierzbicka, M. Robot-Assisted Electrode Insertion in Cochlear Implantation Controlled by Intraoperative Electrocochleography—A Pilot Study. J. Clin. Med. 2022, 11, 7045. https://doi.org/10.3390/jcm11237045
Gawęcki W, Balcerowiak A, Podlawska P, Borowska P, Gibasiewicz R, Szyfter W, Wierzbicka M. Robot-Assisted Electrode Insertion in Cochlear Implantation Controlled by Intraoperative Electrocochleography—A Pilot Study. Journal of Clinical Medicine. 2022; 11(23):7045. https://doi.org/10.3390/jcm11237045
Chicago/Turabian StyleGawęcki, Wojciech, Andrzej Balcerowiak, Paulina Podlawska, Patrycja Borowska, Renata Gibasiewicz, Witold Szyfter, and Małgorzata Wierzbicka. 2022. "Robot-Assisted Electrode Insertion in Cochlear Implantation Controlled by Intraoperative Electrocochleography—A Pilot Study" Journal of Clinical Medicine 11, no. 23: 7045. https://doi.org/10.3390/jcm11237045
APA StyleGawęcki, W., Balcerowiak, A., Podlawska, P., Borowska, P., Gibasiewicz, R., Szyfter, W., & Wierzbicka, M. (2022). Robot-Assisted Electrode Insertion in Cochlear Implantation Controlled by Intraoperative Electrocochleography—A Pilot Study. Journal of Clinical Medicine, 11(23), 7045. https://doi.org/10.3390/jcm11237045





