Preliminary Study of Different Treatment Responses between Bevacizumab, Aflibercept and Dexamethasone Implant According to Renal Function in Diabetic Macular Edema Patients
Abstract
:1. Introduction
2. Methods
2.1. Ophthalmic Examinations
2.2. Laboratory Examinations
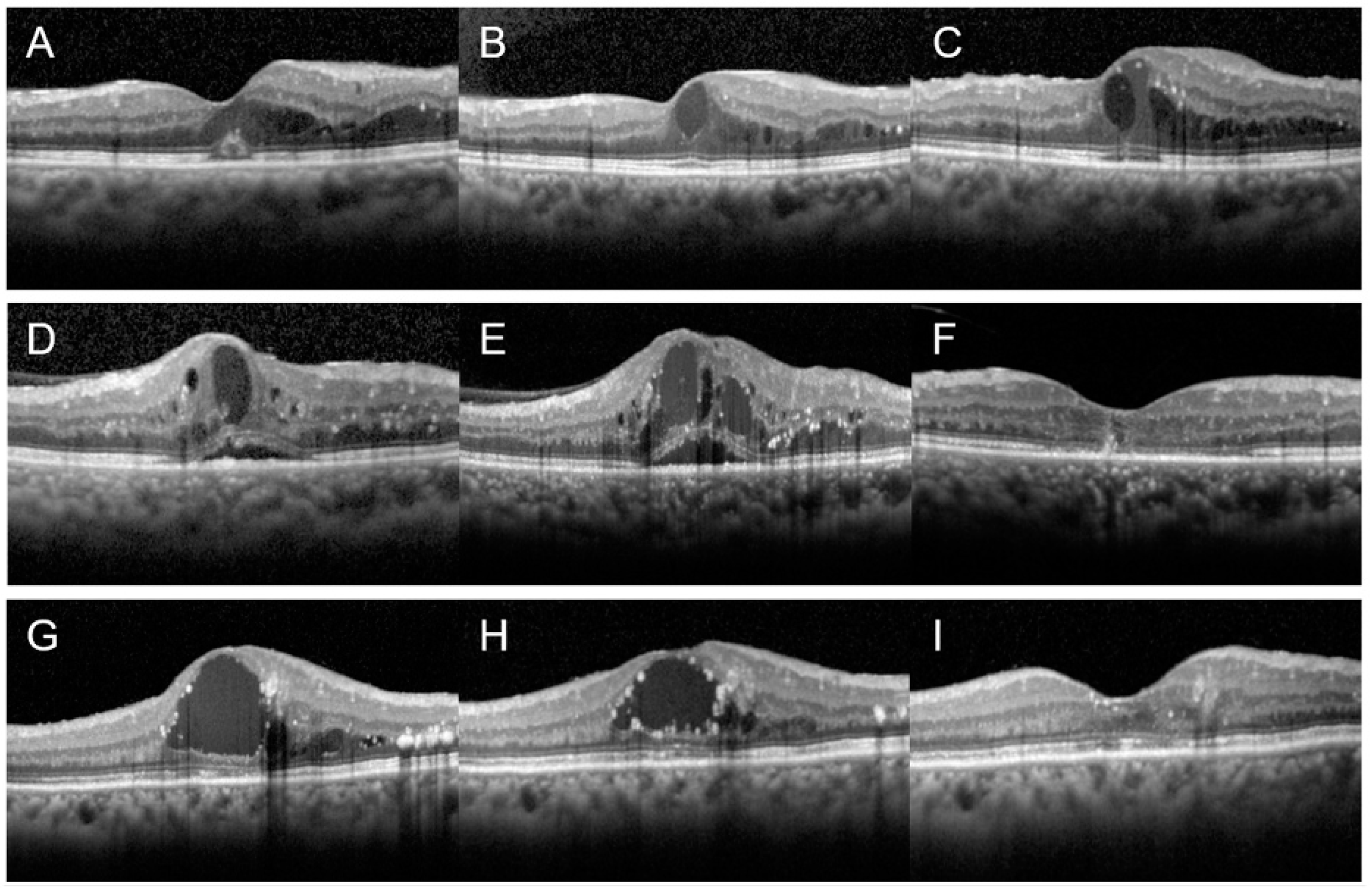
2.3. Measurement of Treatment Responsiveness
2.4. Statistical Analysis
3. Results
3.1. Treatment Responsiveness of DME according to Renal Function
3.2. Response to Further Treatment for DME in Patients with Moderate to Severe CKD
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, T.A.; Amador, A.G.; Zinman, B. Diabetic retinopathy and diabetic macular edema: Pathophysiology, screening, and novel therapies. Diabetes Care 2003, 26, 2653–2664. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017, 237, 185–222. [Google Scholar] [CrossRef] [PubMed]
- Browning, D.J.; Stewart, M.W.; Lee, C. Diabetic macular edema: Evidence-based management. Indian J. Ophthalmol. 2018, 66, 1736–1750. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Elman, M.J.; Ayala, A.; Bressler, N.M.; Browning, D.; Flaxel, C.J.; Glassman, A.R.; Jampol, L.M.; Stone, T.W.; Diabetic Retinopathy Clinical Research Network. Intravitreal Ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology 2015, 122, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Boyer, D.S.; Yoon, Y.H.; Belfort, R., Jr.; Bandello, F.; Maturi, R.K.; Augustin, A.J.; Li, X.Y.; Cui, H.; Hashad, Y.; Whitcup, S.M.; et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014, 121, 1904–1914. [Google Scholar] [CrossRef]
- Diabetic Retinopathy Clinical Research Network; Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Aiello, L.P.; Antoszyk, A.N.; Arnold-Bush, B.; Baker, C.W.; Bressler, N.M.; et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 2015, 372, 1193–1203. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.D.; Brown, D.M.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Gibson, A.; Sy, J.; Rundle, A.C.; Hopkins, J.J.; et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012, 119, 789–801. [Google Scholar] [CrossRef]
- Matsuda, S.; Tam, T.; Singh, R.P.; Kaiser, P.K.; Petkovsek, D.; Carneiro, G.; Zanella, M.T.; Ehlers, J.P. The impact of metabolic parameters on clinical response to VEGF inhibitors for diabetic macular edema. J. Diabetes Complicat. 2014, 28, 166–170. [Google Scholar] [CrossRef]
- Ozturk, B.T.; Kerimoglu, H.; Adam, M.; Gunduz, K.; Okudan, S. Glucose regulation influences treatment outcome in ranibizumab treatment for diabetic macular edema. J. Diabetes Complicat. 2011, 25, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Hsieh, Y.T.; Shen, E.P.; Peng, Y.J. Systemic Associations with Residual Subretinal Fluid after Ranibizumab in Diabetic Macular Edema. J. Ophthalmol. 2017, 2017, 4834201. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.S.; Khurana, R.N.; Wieland, M.R.; Wang, P.W.; Van Everen, S.A.; Tuomi, L. Influence of Glycosylated Hemoglobin on the Efficacy of Ranibizumab for Diabetic Macular Edema: A Post Hoc Analysis of the RIDE/RISE Trials. Ophthalmology 2015, 122, 1573–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.P.; Habbu, K.; Ehlers, J.P.; Lansang, M.C.; Hill, L.; Stoilov, I. The Impact of Systemic Factors on Clinical Response to Ranibizumab for Diabetic Macular Edema. Ophthalmology 2016, 123, 1581–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, H.; Lee, H.; Kim, J.Y.; Lee, S.; Seo, E.J.; Chae, J.B.; Kim, D.Y. Systemic factors and early treatment response to intravitreal injection for diabetic macular edema; the role of renal function. Retina 2020. [Google Scholar] [CrossRef]
- Lai, I.P.; Huang, W.L.; Yang, C.M.; Yang, C.H.; Ho, T.C.; Hsieh, Y.T. Renal Biomarkers for Treatment Effect of Ranibizumab for Diabetic Macular Edema. J. Diabetes Res. 2020, 2020, 7239570. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Barber, A.J.; Hollinger, L.A.; Wolpert, E.B.; Gardner, T.W. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J. Biol. Chem. 1999, 274, 23463–23467. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.J.; Cheng, C.K.; Wang, Y.C. Association of Body Fluid Expansion With Optical Coherence Tomography Measurements in Diabetic Retinopathy and Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3606–3612. [Google Scholar] [CrossRef] [Green Version]
- Ciardella, A.P. Partial resolution of diabetic macular oedema after systemic treatment with furosemide. Br. J. Ophthalmol. 2004, 88, 1224–1225. [Google Scholar] [CrossRef]
- Hwang, H.; Chae, J.B.; Kim, J.Y.; Moon, B.G.; Kim, D.Y. Changes in Optical Coherence Tomography Findings in Patients with Chronic Renal Failure Undergoing Dialysis for the First Time. Retina 2019, 39, 2360–2368. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, I.; Van Noorden, C.J.; Schlingemann, R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Ji, J.F.; Xu, W.; Bu, W.; Zheng, Y.; Ma, A.; Zhao, B.; Fan, Q. Distinct downstream signaling and the roles of VEGF and PlGF in high glucose-mediated injuries of human retinal endothelial cells in culture. Sci. Rep. 2019, 9, 15339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakiyanov, O.; Kalousova, M.; Zima, T.; Tesar, V. Placental growth factor in patients with decreased renal function. Ren. Fail. 2011, 33, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Ando, R.; Noda, K.; Namba, S.; Saito, W.; Kanda, A.; Ishida, S. Aqueous humour levels of placental growth factor in diabetic retinopathy. Acta Ophthalmol. 2014, 92, e245–e246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitamura, Y.; Tashimo, A.; Nakamura, Y.; Tagawa, H.; Ohtsuka, K.; Mizue, Y.; Nishihira, J. Vitreous levels of placenta growth factor and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Diabetes Care 2002, 25, 2352. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, K.; Khosrof, S.; Bursell, S.E.; Moromizato, Y.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1). Am. J. Pathol. 2000, 156, 1733–1739. [Google Scholar] [CrossRef] [Green Version]
- Barham, R.; El Rami, H.; Sun, J.K.; Silva, P.S. Evidence-Based Treatment of Diabetic Macular Edema. Semin. Ophthalmol. 2017, 32, 56–66. [Google Scholar] [CrossRef]
- Burton, J.L.; Kehrli, M.E., Jr.; Kapil, S.; Horst, R.L. Regulation of L-selectin and CD18 on bovine neutrophils by glucocorticoids: Effects of cortisol and dexamethasone. J. Leukoc. Biol. 1995, 57, 317–325. [Google Scholar] [CrossRef]
- Stewart, M.W. Corticosteroid use for diabetic macular edema: Old fad or new trend? Curr. Diab. Rep. 2012, 12, 364–375. [Google Scholar] [CrossRef]

| eGFR ≥ 60 (n = 60) | 30 ≤ eGFR < 60 (n = 25) | eGFR < 30 (n = 19) | p-Value | |
|---|---|---|---|---|
| Age (years) | 54.70 ± 13.09 | 57.60 ± 12.73 | 60.11 ± 8.79 | 0.219 * |
| Sex (Male/Female) | 32/28 | 17/8 | 9/10 | 0.973 # |
| Right/Left | 33/27 | 13/12 | 13/6 | 0.409 # |
| Type of Diabetes(1/2) | 5/55 | 2/23 | 0/19 | 0.262 # |
| Diabetes duration (years) | 10.36 ± 7.66 | 13.04 ± 6.93 | 16.94 ± 9.51 | 0.008 * |
| PDR/NPDR | 29/31 | 12/13 | 8/10 | 0.795 # |
| eGFR (mL/min/1.73 m2) | 110.51 ± 42.70 | 46.16 ± 7.55 | 14.56 ± 7.69 | <0.001 * |
| Refractive error (S.E.) | −0.91 ± 2.06 | −1.65 ± 2.54 | −0.54 ± 1.86 | 0.214 * |
| HbA1C (%) | 8.26 ± 2.15 | 8.11 ± 1.75 | 6.74 ± 0.92 | 0.045 * |
| Urine albumin to creatinine ratio (mg/g) | 416.35 ± 1219.49 | 2855.89 ± 3095.72 | 3358.78 ± 2729.46 | <0.001 * |
| Urine microalbumin (μg/mgCr) | 382.45 ± 934.05 | 1718.22 ± 1321.17 | 2896.26 ± 2781.10 | <0.001 * |
| BUN (mg/dL) | 15.72 ± 4.19 | 25.56 ± 7.36 | 51.26 ± 20.43 | <0.001 * |
| Serum creatinine (mg/dL) | 0.75 ± 0.20 | 1.56 ± 0.29 | 4.68 ± 2.59 | <0.001 * |
| Proteinuria/Normal | 28/21 | 21/1 | 15/1 | <0.001 # |
| Maintain Bevacizumab (n = 10) | Switch to Aflibercept (n = 5) | Switch to Dexamethasone Implant (n = 7) | p-Value | |
|---|---|---|---|---|
| BCVA change (LogMAR) | 0.15 ± 0.36 | −0.29 ± 0.22 | −0.17 ± 0.41 | 0.043 * |
| CST change (µm) | 32.30 ± 63.87 | 312.20 ± 51.76 | 193.86 ± 101.33 | 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, T.H.; Jo, G.H.; Seo, E.J.; Kim, K.T.; Ku, E.J.; Kwon, S.K.; Kim, J.Y.; Chae, J.B.; Kim, D.Y. Preliminary Study of Different Treatment Responses between Bevacizumab, Aflibercept and Dexamethasone Implant According to Renal Function in Diabetic Macular Edema Patients. J. Clin. Med. 2022, 11, 7047. https://doi.org/10.3390/jcm11237047
Moon TH, Jo GH, Seo EJ, Kim KT, Ku EJ, Kwon SK, Kim JY, Chae JB, Kim DY. Preliminary Study of Different Treatment Responses between Bevacizumab, Aflibercept and Dexamethasone Implant According to Renal Function in Diabetic Macular Edema Patients. Journal of Clinical Medicine. 2022; 11(23):7047. https://doi.org/10.3390/jcm11237047
Chicago/Turabian StyleMoon, Tae Hwan, Gwon Hui Jo, Eoi Jong Seo, Kyung Tae Kim, Eu Jeong Ku, Soon Kil Kwon, Jin Young Kim, Ju Byung Chae, and Dong Yoon Kim. 2022. "Preliminary Study of Different Treatment Responses between Bevacizumab, Aflibercept and Dexamethasone Implant According to Renal Function in Diabetic Macular Edema Patients" Journal of Clinical Medicine 11, no. 23: 7047. https://doi.org/10.3390/jcm11237047






