The Prevalence of Periodontitis and Assessment of Oral Micro-Biota in Patients with Hidradenitis Suppurativa: A Descriptive Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Evaluation
2.3. Sample Collection and Processing
2.4. Bacterial Identification
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sabat, R.; Jemec, G.B.E.; Matusiak, Ł.; Kimball, A.B.; Prens, E.; Wolk, K. Hidradenitis suppurativa. Nat. Rev. Dis. Prim. 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Naik, H.B.; Paul, M.; Cohen, S.R.; Alavi, A.; Suàrez-Fariñas, M.; Lowes, M.A. Distribution of Self-reported Hidradenitis Suppurativa Age at Onset. JAMA Dermatol. 2019, 155, 971–973. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.R. The epidemiology of hidradenitis suppurativa. Br. J. Dermatol. 2020, 183, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Goldburg, S.R.; Strober, B.E.; Payette, M.J. Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J Am. Acad. Dermatol. 2020, 82, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.H.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Eke, P.I.; Dye, B.A.; Wei, L.; Thornton-Evans, G.O.; Genco, R.J. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Periodontitis in 1990-2010: A Systematic Review and Meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontology 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontology 2005, 38, 135–187. [Google Scholar] [CrossRef]
- Mohanty, R.; Asopa, S.J.; Joseph, M.D.; Singh, B.; Rajguru, J.P.; Saidath, K.; Sharma, U. Red complex: Polymicrobial conglomerate in oral flora: A review. J. Fam. Med. Prim. Care 2019, 8, 3480–3486. [Google Scholar] [CrossRef]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major periodontopathic pathogen overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef] [PubMed]
- Dalmády, S.; Kemény, L.; Antal, M.; Gyulai, R. Periodontitis: A newly identified comorbidity in psoriasis and psoriatic arthritis. Expert. Rev. Clin. Immunol. 2020, 16, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Wijarnpreecha, K.; Wetter, D.A. Periodontitis and risk of psoriasis: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Potempa, J.; Mydel, P.; Koziel, J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat. Rev. Rheumatol. 2017, 13, 606–620. [Google Scholar] [CrossRef]
- Leech, M.T.; Bartold, P.M. The association between rheumatoid arthritis and periodontitis. Best Pract. Res. Clin. Rheumatol. 2015, 29, 189–201. [Google Scholar] [CrossRef]
- She, Y.Y.; Kong, X.B.; Ge, Y.P.; Liu, Z.-Y.; Chen, J.-Y.; Jiang, J.-W.; Jiang, H.-B.; Fang, S.-L. Periodontitis and inflammatory bowel disease: A meta-analysis. BMC Oral Health 2020, 20, 67. [Google Scholar] [CrossRef]
- Bunte, K.; Beikler, T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef]
- Abusleme, L.; Moutsopoulos, N.M. IL-17; overview and role in oral immunity and microbiome. Oral Dis. 2017, 23, 854–865. [Google Scholar] [CrossRef]
- Schlapbach, C.; Hänni, T.; Yawalkar, N.; Hunger, R.E. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J. Am. Acad. Dermatol. 2011, 65, 790–798. [Google Scholar] [CrossRef]
- Song, B.; Zhang, Y.L.; Chen, L.J.; Zhou, T.; Huang, W.K.; Zhou, X.; Shao, L.Q. The role of Toll-like receptors in periodontitis. Oral Dis. 2017, 23, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Plewig, G. Impaired Notch-MKP-1 signalling in hidradenitis suppurativa: An approach to pathogenesis by evidence from translational biology. Exp. Dermatol. 2013, 22, 172–177. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Del Marmol, V.; Mrowietz, U.; Prens, E.P.; Tzellos, T.; Jemec, G.B.E. Hidradenitis Suppurativa/Acne Inversa: Criteria for Diagnosis, Severity Assessment, Classification and Disease Evaluation. Dermatology 2015, 231, 184–190. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Tzellos, T.; Kyrgidis, A.; Jemec, G.B.E.; Bechara, F.G.; Giamarellos-Bourboulis, E.J.; Ingram, J.R.; Kanni, T.; Karagiannidis, I.; Martorell, A.; et al. Development and Validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a Novel Dynamic Scoring System to Assess HS Severity. Br. J. Dermatol. 2017, 177, 1401–1409. [Google Scholar] [CrossRef]
- Włodarek, K.; Stefaniak, A.; Matusiak, Ł.; Szepietowski, J.C. Could Residents Adequately Assess the Severity of Hidradenitis Suppurativa? Interrater and Intrarater Reliability Assessment of Major Scoring Systems. Dermatology 2020, 236, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89, 159–172. [Google Scholar] [CrossRef]
- Azizi, A.; Rezaee, M. Comparison of Periodontal Status in Gingival Oral Lichen Planus Patients and Healthy Subjects. Dermatol. Res. Pract. 2012, 2012, 561232. [Google Scholar] [CrossRef] [PubMed]
- Thorat, M.S.; Raju, A.; Pradeep, A.R. Pemphigus Vulgaris: Effects on Periodontal Health. J. Oral Sci. 2010, 52, 449–454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tricamo, M.B.; Rees, T.D.; Hallmon, W.W.; Wright, J.M.; Cueva, M.A.; Plemons, J.M. Periodontal Status in Patients with Gingival Mucous Membrane Pemphigoid. J. Periodontol. 2006, 77, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, S.; Ying, S.; Tang, S.; Ding, Y.; Li, Y.; Qiao, J.; Fang, H. The IL-23/IL-17 Pathway in Inflammatory Skin Diseases: From Bench to Bedside. Front. Immunol. 2020, 11, 594735. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immunomicrobial Pathogenesis of Periodontitis: Keystones, Pathobionts, and Host Response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zenobia, C.; Hajishengallis, G. Basic Biology and Role of Interleukin-17 in Immunity and Inflammation. Periodontology 2000 2015, 69, 142–159. [Google Scholar] [CrossRef]
- Kotake, S.; Yago, T.; Kawamoto, M.; Nanke, Y. Role of Osteoclasts and Interleukin-17 in the Pathogenesis of Rheumatoid Arthritis: Crucial “Human Osteoclastology. ” J. Bone Miner. Metab. 2012, 30, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and TH17 Cells in Chronic Inflammation. Nat. Rev. Drug Discov. 2012, 11, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, H.; Kato-Kogoe, N.; Kuhara, A.; Nishimura, F.; Nakasho, K.; Yamanegi, K.; Yamada, N.; Hata, M.; Yamane, J.; Terada, N. The Involvement of IL-23 and the Th17 Pathway in Periodontitis. J. Dent. Res. 2009, 88, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, H.; Wu, Y.; Sun, Z.; Wu, Y. Clinical Significance of IL-23 Regulating IL-17A and/or IL-17F Positive Th17 Cells in Chronic Periodontitis. Mediat. Inflamm. 2014, 2014, 627959. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Moran, B.; Petrasca, A.; Smith, C.M. IL-17 in Inflammatory Skin Diseases Psoriasis and Hidradenitis Suppurativa. Clin Exp Immunol 2020, 201, 121–134. [Google Scholar] [CrossRef]
- Matusiak, Ł.; Szczęch, J.; Bieniek, A.; Nowicka-Suszko, D.; Szepietowski, J.C. Increased Interleukin (IL)-17 Serum Levels in Patients with Hidradenitis Suppurativa: Implications for Treatment with Anti-IL-17 Agents. J. Am. Acad. Dermatol. 2017, 76, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Moran, B.; Sweeney, C.M.; Hughes, R.; Malara, A.; Kirthi, S.; Tobin, A.-M.; Kirby, B.; Fletcher, J.M. Hidradenitis Suppurativa Is Characterized by Dysregulation of the Th17:Treg Cell Axis, Which Is Corrected by Anti-TNF Therapy. J. Investig. Dermatol. 2017, 137, 2389–2395. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Wark, K.J.L.; Cains, G.D. The Microbiome in Hidradenitis Suppurativa: A Review. Dermatol. Ther. 2021, 11, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Hunger, R.E.; Surovy, A.M.; Hassan, A.S.; Braathen, L.R.; Yawalkar, N. Toll-like Receptor 2 Is Highly Expressed in Lesions of Acne Inversa and Colocalizes with C-Type Lectin Receptor. Br. J. Dermatol. 2008, 158, 691–697. [Google Scholar] [CrossRef]
- Acharya, P.; Mathur, M. Hidradenitis Suppurativa and Smoking: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2020, 82, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.R.M.; Nascimento, G.G.; Scheutz, F.; López, R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-Regression. Am. J. Prev. Med. 2018, 54, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, A.; Susin, C.; Gustafsson, A. Smoking and Inflammation: Evidence for a Synergistic Role in Chronic Disease. Periodontology 2000 2014, 64, 111–126. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Socransky, S.S. Relationship of Cigarette Smoking to the Subgingival Microbiota. J. Clin. Periodontol. 2001, 28, 377–388. [Google Scholar] [CrossRef]
- Gomes, S.C.; Piccinin, F.B.; Oppermann, R.V.; Susin, C.; Nonnenmacher, C.I.; Mutters, R.; Marcantonio, R.A.C. Periodontal Status in Smokers and Never-Smokers: Clinical Findings and Real-Time Polymerase Chain Reaction Quantification of Putative Periodontal Pathogens. J. Periodontol. 2006, 77, 1483–1490. [Google Scholar] [CrossRef]
- van Winkelhoff, A.J.; Bosch-Tijhof, C.J.; Winkel, E.G.; van der Reijden, W.A. Smoking Affects the Subgingival Microflora in Periodontitis. J. Periodontol. 2001, 72, 666–671. [Google Scholar] [CrossRef]
- Apatzidou, D.A.; Riggio, M.P.; Kinane, D.F. Impact of Smoking on the Clinical, Microbiological and Immunological Parameters of Adult Patients with Periodontitis. J. Clin. Periodontol. 2005, 32, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Ramseier, C.A.; Kandylaki, M.; Sigrist, L.; Awedowa, E.; Lang, N.P. Experimental Gingivitis in Cigarette Smokers: A Clinical and Microbiological Study. J. Clin. Periodontol. 2005, 32, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, Ł.; Bieniek, A.; Szepietowski, J.C. Bacteriology of Hidradenitis Suppurativa—Which Antibiotics Are the Treatment of Choice? Acta Derm. Venereol. 2014, 94, 699–702. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, Y.; Xu, T.; Zhang, Q.-Z.; Bai, J.; Wang, J.; Zhu, T.; Lou, Q.; Götz, F.; Qu, D.; et al. Nicotine Enhances Staphylococcus Epidermidis Biofilm Formation by Altering the Bacterial Autolysis, Extracellular DNA Releasing, and Polysaccharide Intercellular Adhesin Production. Front. Microbiol. 2018, 9, 2575. [Google Scholar] [CrossRef]
- Jiang, S.W.; Whitley, M.J.; Mariottoni, P.; Jaleel, T.; MacLeod, A.S. Hidradenitis Suppurativa: Host-Microbe and Immune Pathogenesis Underlie Important Future Directions. JID Innov. 2021, 1, 100001. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.K.; Nicholson, C.L.; Parks-Miller, A.; Hamzavi, I.H. Hidradenitis Suppurativa: An Update on Connecting the Tracts. F1000Research 2017, 6, 1272. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.; Lewis, M. The Size of a Pilot Study for a Clinical Trial Should Be Calculated in Relation to Considerations of Precision and Efficiency. J. Clin. Epidemiol. 2012, 65, 301–308. [Google Scholar] [CrossRef] [PubMed]

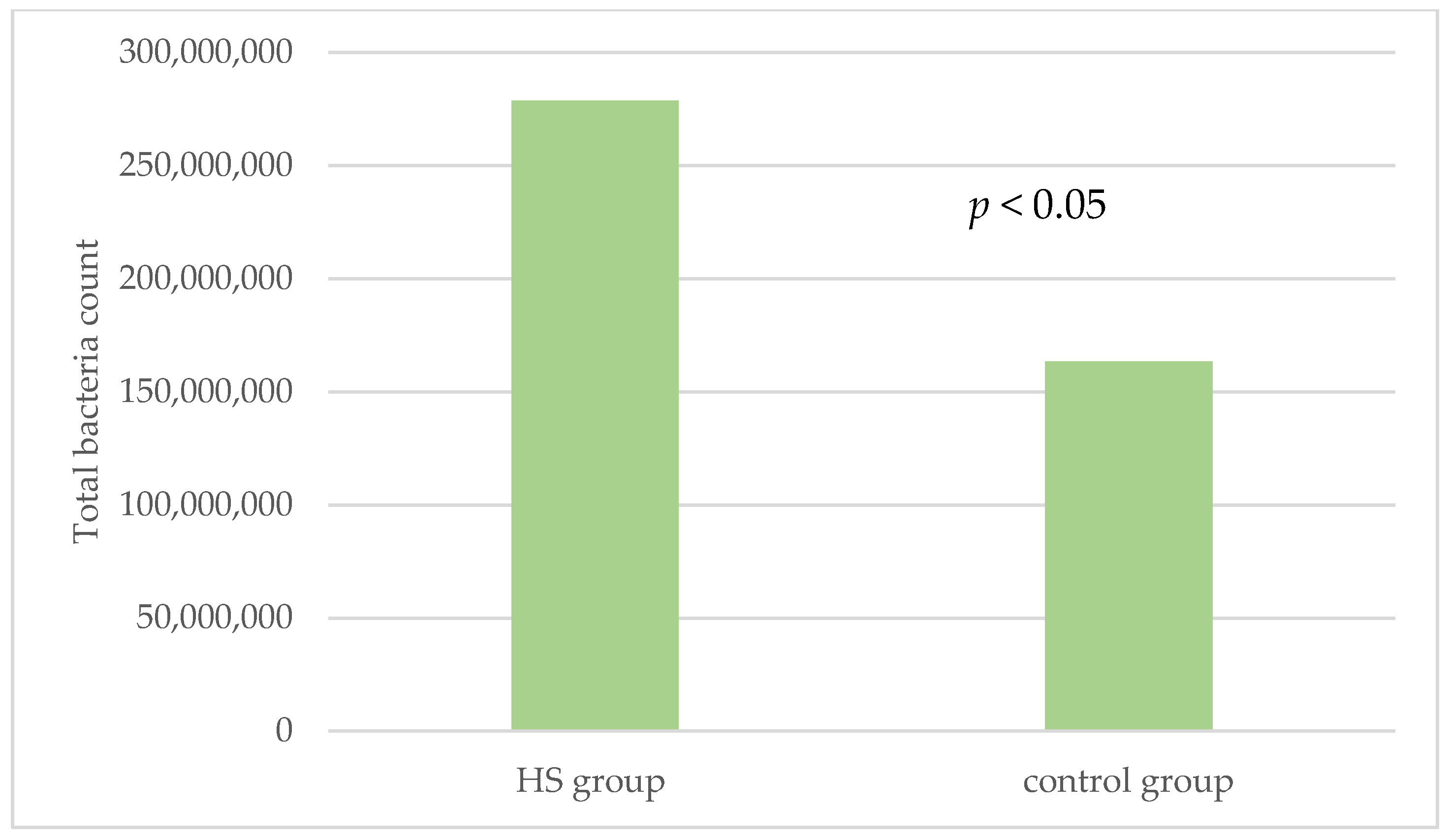
| HS Group (n = 55) | Control Group (n = 55) | |
|---|---|---|
| Age in years (mean ± SD) | 36.22 ± 10.97 (19–61) | 34.45 ± 10.29 (20–58) |
| Sex (Male, %) Smokers (%) | 28 (50.91) 39 (70.91) | 26 (47.27) 7 (12.73) |
| No (%) of Patients | ||
|---|---|---|
| HS Patients with Periodontitis (n = 25) | Controls with Periodontitis (n = 8) | |
| Stage I | 2 (8%) | 0 |
| Stage II | 10 (40%) | 3 (37.5%) |
| Stage III | 7 (28%) | 4 (50%) |
| Stage IV | 6 (24%) | 1 (12.5%) |
| Grade A | 6 (24%) | 4 (50%) |
| Grade B | 2 (8%) | 2 (25%) |
| Grade C | 17 (68%) | 2 (25%) |
| Mean ± SD | No (%) of Infected Patients | |||||
|---|---|---|---|---|---|---|
| Periopathogens Tested | Subjects with HS | Control Group | p | Subjects with HS | Control Group | p |
| A. actinomycetemcomitans | 2.7 × 102 ± 1.5 × 103 | 2.3 × 103 ± 9.4 × 103 | 0.332 | 4 (7.3%) | 7(12.7%) | 0.340 |
| P.gingivalis | 3.3 ×104 ± 1.2 × 105 | 2.7 × 104 ± 1.3 × 105 | 0.635 | 6 (10.9%) | 8 (14.5%) | 0.567 |
| T. denticola | 1.8 × 104 ± 3.0 × 104 | 8.3 × 103 ± 3.1 × 104 | <0.001 * | 39 (70.9%) | 11 (20.0%) | <0.001 * |
| T. forsythia | 7.0 × 105 ± 5.0 × 106 | 6.2 × 103 ± 4.3 × 104 | 0.001 * | 22 (40.0%) | 6 (10.9%) | <0.001 * |
| P. intermedia | 2.7 × 104 ± 9.9 × 104 | 3.7 × 104 ± 1.4 × 105 | 0.381 | 12 (21.8%) | 8 (14.5%) | 0.323 |
| P. micros | 1.8 × 103 ± 8.9 × 103 | 1.0 × 103 ± 4.5 × 103 | 0.010 * | 23 (41.8%) | 10 (18.2%) | 0.007 * |
| F. nucleatum | 5.9 × 102 ± 3.0 × 103 | 10 ± 55 | 0.007 * | 11 (20.0%) | 2 (3.6%) | 0.008 * |
| E. nodatum | 1.5 × 103 ± 1.1 × 104 | 54 ± 3.1 × 102 | 0.625 | 3 (5.5%) | 2 2 (3.6%) | 0.647 |
| C. gingivalis | 2.9 × 103 ± 8.0 × 103 | 3.8 × 103 ± 1.2 × 104 | 0.003 * | 37 (67.3%) | 19 (34.5%) | 0.001 * |
| Total bacteria count | 2.8 × 108 ± 4.3 × 108 | 1.6 × 108 ± 2.6 × 108 | 0.023 * | 55(100%) | 55(100%) | NA |
| HS Group | Control Group | |||||
|---|---|---|---|---|---|---|
| Periopathogens Tested | No (%) of Infected Smokers (n = 39) | No (%) of Infected Non-smokers (n = 16) | p | No (%) of Infected Smokers (n = 7) | No (%) of Infected Non-smokers (n = 48) | p |
| A. actinomycetemcomitans | 3 (7.69) % | 1 (6.25) % | NS | 0% | 7 (14.58) % | NS |
| P.gingivalis | 5 (12.82) % | 1 (6.25) % | NS | 2 (28.57) % | 6 (12.5) % | NS |
| T. denticola | 31 (79.49) % | 8 (50.0) % | 0.029 | 3 (42.85) % | 8 (16.67) % | NS |
| T. forsythia | 19 (48.72) % | 3 (18.75) % | 0.039 | 1 (14.29) % | 5 (10.42) % | NS |
| P. intermedia | 10 (25.64) % | 2 (12.5) % | NS | 1 (14.29) % | 7 (14.58) % | NS |
| P. micros | 21 (53.85) % | 2 (12.5) % | 0.005 | 2 (28.57) % | 8 (16.67) % | NS |
| F. nucleatum | 7 (17.95) % | 4 (25.0) % | NS | 1 (14.29) % | 1 (2.08) % | NS |
| E. nodatum | 1 (2.56) % | 2 (12.5) % | NS | 1 (14.29) % | 1 (2.08) % | NS |
| C. gingivalis | 23 (58.97) % | 14 (87.5) % | NS | 5 (71.43) % | 14 (29.17) % | 0.028 |
| Periopathogens Tested | Hurley Score | IHS4 | |
|---|---|---|---|
| A. actinomycetemcomitans | rS | 0.024 | 0.058 |
| p | 0.866 | 0.685 | |
| P.gingivalis | rS | 0.116 | 0.076 |
| p | 0.412 | 0.596 | |
| T. denticola | rS | 0.230 | 0.211 |
| p | 0.101 | 0.136 | |
| T. forsythia | rS | 0.257 | 0.212 |
| p | 0.066 | 0.135 | |
| P. intermedia | rS | −0.057 | −0.090 |
| p | 0.686 | 0.530 | |
| P. micros | rS | 0.229 | 0.162 |
| p | 0.102 | 0.257 | |
| F. nucleatum | rS | −0.049 | 0.039 |
| p | 0.729 | 0.784 | |
| E. nodatum | rS | 0.152 | −0.026 |
| p | 0.282 | 0.854 | |
| C. gingivalis | rS | −0.199 | −0.186 |
| p | 0.158 | 0.191 | |
| Total bacteria count | rS | 0.030 | −0.025 |
| p | 0.833 | 0.861 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jastrząb, B.; Paśnik-Chwalik, B.; Konopka, T.; Krajewski, P.K.; Szepietowski, J.C.; Matusiak, Ł. The Prevalence of Periodontitis and Assessment of Oral Micro-Biota in Patients with Hidradenitis Suppurativa: A Descriptive Cross-Sectional Study. J. Clin. Med. 2022, 11, 7065. https://doi.org/10.3390/jcm11237065
Jastrząb B, Paśnik-Chwalik B, Konopka T, Krajewski PK, Szepietowski JC, Matusiak Ł. The Prevalence of Periodontitis and Assessment of Oral Micro-Biota in Patients with Hidradenitis Suppurativa: A Descriptive Cross-Sectional Study. Journal of Clinical Medicine. 2022; 11(23):7065. https://doi.org/10.3390/jcm11237065
Chicago/Turabian StyleJastrząb, Beata, Barbara Paśnik-Chwalik, Tomasz Konopka, Piotr K. Krajewski, Jacek C. Szepietowski, and Łukasz Matusiak. 2022. "The Prevalence of Periodontitis and Assessment of Oral Micro-Biota in Patients with Hidradenitis Suppurativa: A Descriptive Cross-Sectional Study" Journal of Clinical Medicine 11, no. 23: 7065. https://doi.org/10.3390/jcm11237065
APA StyleJastrząb, B., Paśnik-Chwalik, B., Konopka, T., Krajewski, P. K., Szepietowski, J. C., & Matusiak, Ł. (2022). The Prevalence of Periodontitis and Assessment of Oral Micro-Biota in Patients with Hidradenitis Suppurativa: A Descriptive Cross-Sectional Study. Journal of Clinical Medicine, 11(23), 7065. https://doi.org/10.3390/jcm11237065







