A Combination of Xyloglucan, Pea Protein and Chia Seed Ameliorates Intestinal Barrier Integrity and Mucosa Functionality in a Rat Model of Constipation-Predominant Irritable Bowel Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. In Vivo Model of IBS-C Induction
2.4. Experimental Groups
- Group 1: Sham + vehicle group, rats received an intragastric injection of saline for 14 days.
- Group 2: IBS-C group, rats received gastric instillation of 2 mL of cold water for 14 days.
- Group 3: IBS-C + XP + CS group, rats received gastric instillation of 2 mL of cold water for 14 days and then received XP + CS for 7 days.
2.5. Determination of Constipation-Related Indicators
2.6. Stool Moisture Content and Urine Volume
2.7. Histological Analysis
2.8. Immunohistochemical Localization of ZO-1 and Occludin
2.9. Visceral Sensitivity and Abdominal Pain Tests
2.10. Statistical Analysis
3. Results
3.1. Evaluation of XP + CS Treatment on Constipation-Related Factors after IBS-C Induction
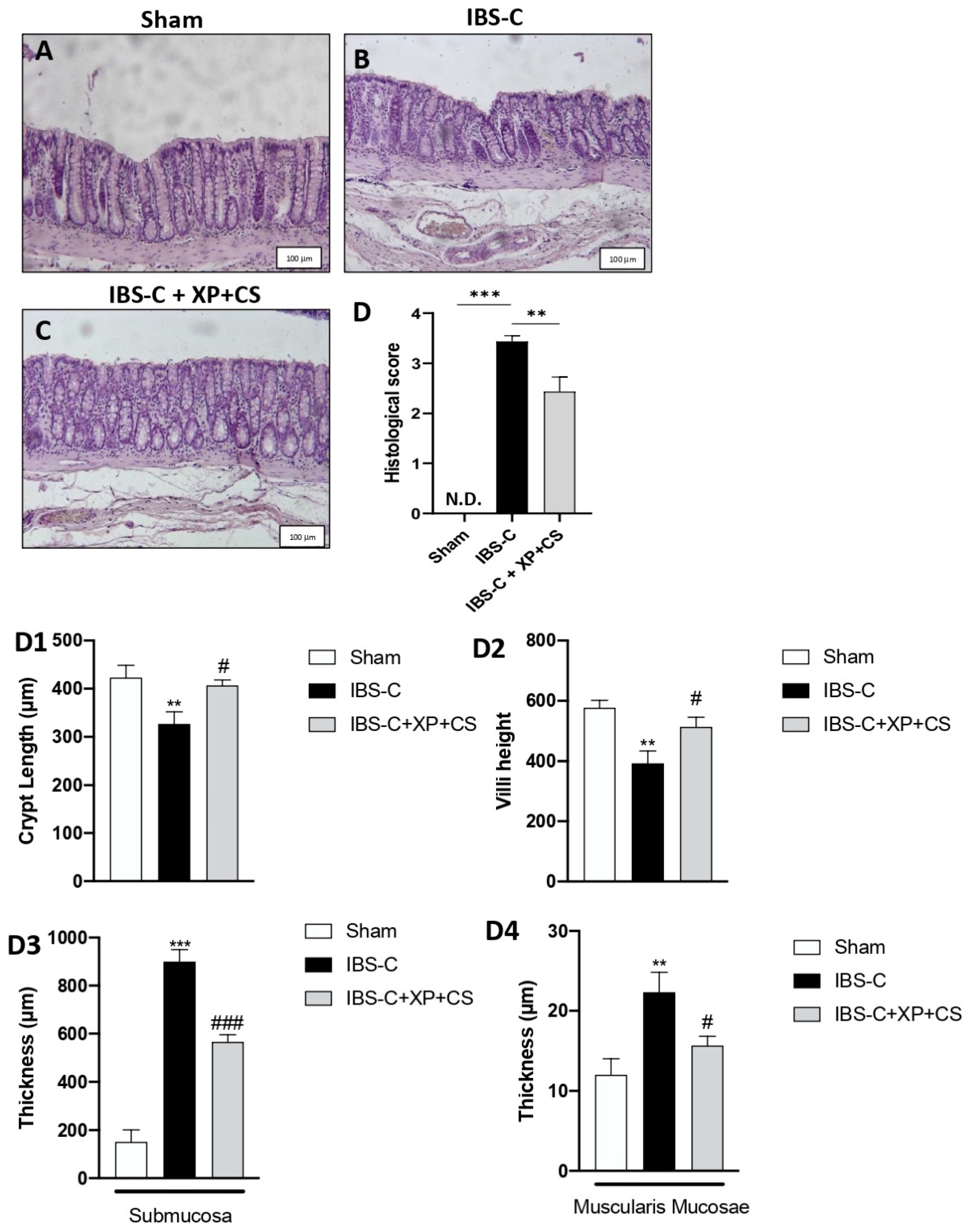
3.2. Efficacy of XP + CS Treatment in Resolving Colonic Damage Induced by IBS-C
3.3. XP + CS Increased Occludin and ZO-1 Expression after IBS-C Induction
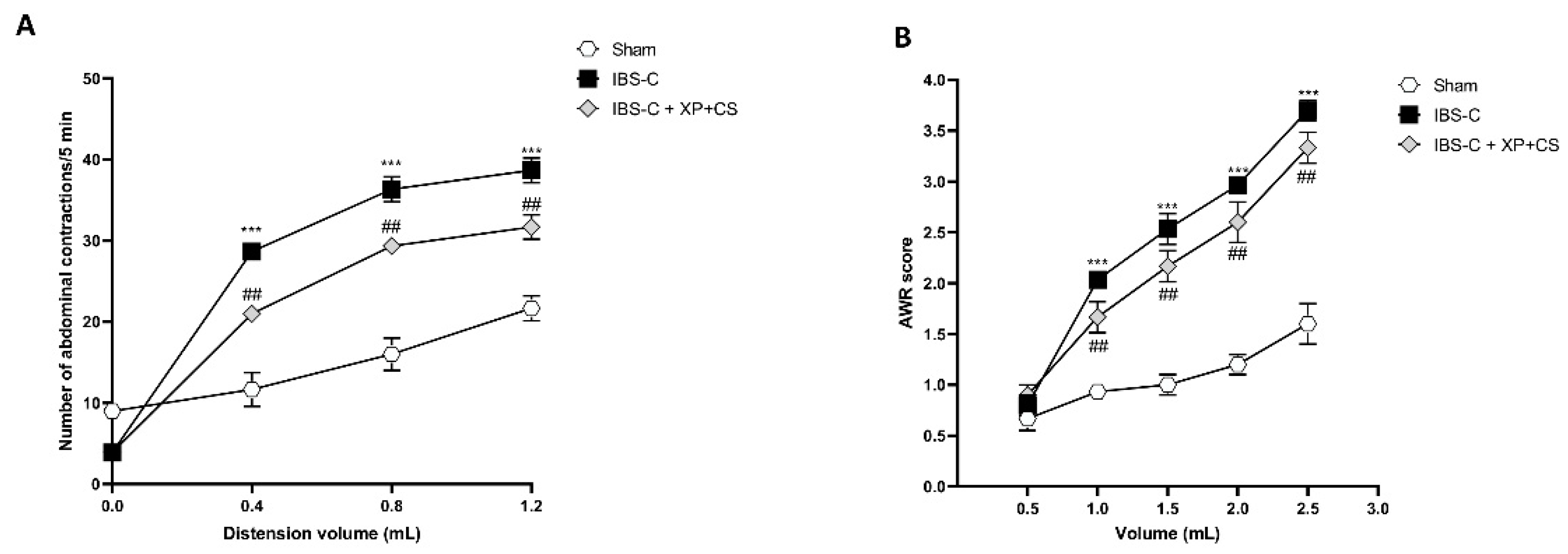
3.4. XP + CS Decreased Abdominal Pain after IBS-C Induction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ardizzone, A.; Filippone, A.; Mannino, D.; Scuderi, S.A.; Casili, G.; Lanza, M.; Cucinotta, L.; Campolo, M.; Esposito, E. Ulva pertusa, a Marine Green Alga, Attenuates DNBS-Induced Colitis Damage via NF-kappaB/Nrf2/SIRT1 Signaling Pathways. J. Clin. Med. 2022, 11, 4301. [Google Scholar] [CrossRef]
- Li, C.; Nie, S.P.; Zhu, K.X.; Xiong, T.; Li, C.; Gong, J.; Xie, M.Y. Effect of Lactobacillus plantarum NCU116 on loperamide-induced constipation in mice. Int. J. Food Sci. Nutr. 2015, 66, 533–538. [Google Scholar] [CrossRef]
- Lacy, B.E.; Patel, N.K. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Kopczynska, M.; Mokros, L.; Pietras, T.; Malecka-Panas, E. Quality of life and depression in patients with irritable bowel syndrome. Przegląd Gastroenterol. 2018, 13, 102–108. [Google Scholar] [CrossRef]
- Gillman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marlicz, W.; Skonieczna-Zydecka, K.; Krynicka, P.; Loniewski, I.; Rydzewska, G. Probiotics in irritable bowel syndrome—Is the quest for the right strain over? Rapid review of existing guidelines and recommendations. Przegląd Gastroenterol. 2021, 16, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Roudsari, N.M.; Lashgari, N.A.; Momtaz, S.; Farzaei, M.H.; Marques, A.M.; Abdolghaffari, A.H. Natural polyphenols for the prevention of irritable bowel syndrome: Molecular mechanisms and targets; a comprehensive review. Daru 2019, 27, 755–780. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.Q.; Li, X.P.; Ou-Yang, J.; Jiang, R.G.; Huang, F.F.; Wen, B.B.; Zhang, X.N.; Huang, J.A.; Liu, Z.H. The protective effects of yellow tea extract against loperamide-induced constipation in mice. Food Funct. 2021, 12, 5621–5636. [Google Scholar] [CrossRef]
- Lanza, M.; Filippone, A.; Ardizzone, A.; Casili, G.; Paterniti, I.; Esposito, E.; Campolo, M. SCFA Treatment Alleviates Pathological Signs of Migraine and Related Intestinal Alterations in a Mouse Model of NTG-Induced Migraine. Cells 2021, 10, 2756. [Google Scholar] [CrossRef]
- Soltanian, N.; Janghorbani, M. Effect of flaxseed or psyllium vs. placebo on management of constipation, weight, glycemia, and lipids: A randomized trial in constipated patients with type 2 diabetes. Clin. Nutr. ESPEN 2019, 29, 41–48. [Google Scholar] [CrossRef]
- Oh, S.J.; Fuller, G.; Patel, D.; Khalil, C.; Spalding, W.; Nag, A.; Spiegel, B.M.R.; Almario, C.V. Chronic Constipation in the United States: Results From a Population-Based Survey Assessing Healthcare Seeking and Use of Pharmacotherapy. Am. J. Gastroenterol. 2020, 115, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Casili, G.; Paterniti, I.; Filippone, A.; Lanza, M.; Ardizzone, A.; Scuderi, S.A.; Cuzzocrea, S.; Esposito, E. Effect of a Product Containing Xyloglucan and Pea Protein on a Murine Model of Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 3596. [Google Scholar] [CrossRef] [PubMed]
- de Los Rios, C.C.; Falcon, B.S.; Arguelles-Arias, F.; Perez, E.; Teruel, C.; Geijo, F.; Rey, E. Long-term safety and efficacy study of a medical device containing xyloglucan, pea protein reticulated with tannins and xylo-oligosaccharides, in patients with diarrhoea-predominant irritable bowel syndrome. Ther. Adv. Gastroenterol. 2021, 14, 17562848211020570. [Google Scholar] [CrossRef] [PubMed]
- Emkani, M.; Oliete, B.; Saurel, R. Pea Protein Extraction Assisted by Lactic Fermentation: Impact on Protein Profile and Thermal Properties. Foods 2021, 10, 549. [Google Scholar] [CrossRef]
- Bellini, M.; Berti, G.; Bonfrate, L.; Ciranni, F.; Di Ciaula, A.; Di Ruscio, M.; Dell’Era, A.; Lambiase, C.; Noto, A.; Pancetti, A.; et al. Use of GELSECTAN((R)) in Patients with Irritable Bowel Syndrome (IBS): An Italian Experience. Patient Prefer. Adherence 2021, 15, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Garland, V.; Herlitz, L.; Regunathan-Shenk, R. Diet-induced oxalate nephropathy from excessive nut and seed consumption. BMJ Case Rep. 2020, 13, e237212. [Google Scholar] [CrossRef] [PubMed]
- Kulczynski, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michalowska, A. The Chemical Composition and Nutritional Value of Chia Seeds-Current State of Knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef] [Green Version]
- Ardizzone, A.; Lanza, M.; Casili, G.; Campolo, M.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Efficacy of a Novel Therapeutic, Based on Natural Ingredients and Probiotics, in a Murine Model of Multiple Food Intolerance and Maldigestion. Nutrients 2022, 14, 2251. [Google Scholar] [CrossRef]
- Xu, J.R.; Luo, J.Y.; Shang, L.; Kong, W.M. Effect of change in an inhibitory neurotransmitter of the myenteric plexus on the pathogenetic mechanism of irritable bowel syndrome subgroups in rat models. Chin. J. Dig. Dis. 2006, 7, 89–96. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, J.H.; Jeung, H.W.; Lee, C.U.; Kim, D.S.; Li, B.; Lee, G.H.; Sung, M.S.; Ha, K.C.; Back, H.I.; et al. Effects of Ficus carica paste on loperamide-induced constipation in rats. Food Chem. Toxicol. 2012, 50, 895–902. [Google Scholar] [CrossRef]
- Xie, N.; Cui, Y.; Yin, Y.N.; Zhao, X.; Yang, J.W.; Wang, Z.G.; Fu, N.; Tang, Y.; Wang, X.H.; Liu, X.W.; et al. Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complement. Altern. Med. 2011, 11, 53. [Google Scholar] [CrossRef] [Green Version]
- Campolo, M.; Crupi, R.; Cordaro, M.; Cardali, S.M.; Ardizzone, A.; Casili, G.; Scuderi, S.A.; Siracusa, R.; Esposito, E.; Conti, A.; et al. Co-Ultra PEALut Enhances Endogenous Repair Response Following Moderate Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 8717. [Google Scholar] [CrossRef] [PubMed]
- Filippone, A.; Casili, G.; Ardizzone, A.; Lanza, M.; Mannino, D.; Paterniti, I.; Esposito, E.; Campolo, M. Inhibition of Prolyl Oligopeptidase Prevents Consequences of Reperfusion following Intestinal Ischemia. Biomedicines 2021, 9, 1354. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, S.A.; Casili, G.; Lanza, M.; Ardizzone, A.; Pantaleo, L.; Campolo, M.; Paterniti, I.; Cucinotta, L.; Cuzzocrea, S.; Esposito, E. Efficacy of a Product Containing Xyloglucan and Pea Protein on Intestinal Barrier Function in a Partial Restraint Stress Animal Model. Int. J. Mol. Sci. 2022, 23, 2269. [Google Scholar] [CrossRef]
- Lucarini, E.; Nocentini, A.; Bonardi, A.; Chiaramonte, N.; Parisio, C.; Micheli, L.; Toti, A.; Ferrara, V.; Carrino, D.; Pacini, A.; et al. Carbonic Anhydrase IV Selective Inhibitors Counteract the Development of Colitis-Associated Visceral Pain in Rats. Cells 2021, 10, 2540. [Google Scholar] [CrossRef]
- Saha, L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 6759–6773. [Google Scholar] [CrossRef]
- Qian, Y.; Suo, H.; Du, M.; Zhao, X.; Li, J.; Li, G.J.; Song, J.L.; Liu, Z. Preventive effect of Lactobacillus fermentum Lee on activated carbon-induced constipation in mice. Exp. Ther. Med. 2015, 9, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Yu, H.; Ma, J.; Wang, J.; Banerjee, S.; Charboneau, R.; Barke, R.A.; Roy, S. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS ONE 2013, 8, e54040. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Rao, S. Constipation: Pathophysiology and Current Therapeutic Approaches. Handb. Exp. Pharmacol. 2017, 239, 59–74. [Google Scholar] [CrossRef]
- Vriesman, M.H.; Koppen, I.J.N.; Camilleri, M.; Di Lorenzo, C.; Benninga, M.A. Management of functional constipation in children and adults. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 21–39. [Google Scholar] [CrossRef]
- Hossen, I.; Hua, W.; Ting, L.; Mehmood, A.; Jingyi, S.; Duoxia, X.; Yanping, C.; Hongqing, W.; Zhipeng, G.; Kaiqi, Z.; et al. Phytochemicals and inflammatory bowel disease: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1321–1345. [Google Scholar] [CrossRef] [PubMed]
- Hagan, M.; Hayee, B.H.; Rodriguez-Mateos, A. (Poly)phenols in Inflammatory Bowel Disease and Irritable Bowel Syndrome: A Review. Molecules 2021, 26, 1843. [Google Scholar] [CrossRef] [PubMed]
- Pique, N.; Gomez-Guillen, M.D.C.; Montero, M.P. Xyloglucan, a Plant Polymer with Barrier Protective Properties over the Mucous Membranes: An Overview. Int. J. Mol. Sci. 2018, 19, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campolo, M.; Lanza, M.; Filippone, A.; Paterniti, I.; Casili, G.; Scuderi, S.A.; Ardizzone, A.; Cuzzocrea, S.; Esposito, E. Evaluation of a Product Containing Xyloglucan and Pea Protein on Skin Barrier Permeability. Skin Pharmacol. Physiol. 2020, 33, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Gnessi, L.; Bacarea, V.; Marusteri, M.; Pique, N. Xyloglucan for the treatment of acute diarrhea: Results of a randomized, controlled, open-label, parallel group, multicentre, national clinical trial. BMC Gastroenterol. 2015, 15, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, L.; Ma, J.T.; Jin, Z.J.; Duan, L.W.; Su, W.P.; Zheng, J.; Zhang, L.J.; Xu, J.; Li, D.F. Efficacy and Safety of High Specific Volume Polysaccharide-A New Type of Dietary Fiber for Treatment of Functional Constipation and IBS-C. J. Nutr. Sci. Vitaminol. (Tokyo) 2015, 61, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Tamargo, A.; Cueva, C.; Laguna, L.; Moreno-Arribas, M.V.; Muñoz, L.A. Understanding the impact of chia seed mucilage on human gut microbiota by using the dynamic gastrointestinal model simgi®. J. Funct. Foods 2018, 50, 104–111. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippone, A.; Ardizzone, A.; Bova, V.; Lanza, M.; Casili, G.; Cuzzocrea, S.; Esposito, E.; Campolo, M.; Paterniti, I. A Combination of Xyloglucan, Pea Protein and Chia Seed Ameliorates Intestinal Barrier Integrity and Mucosa Functionality in a Rat Model of Constipation-Predominant Irritable Bowel Syndrome. J. Clin. Med. 2022, 11, 7073. https://doi.org/10.3390/jcm11237073
Filippone A, Ardizzone A, Bova V, Lanza M, Casili G, Cuzzocrea S, Esposito E, Campolo M, Paterniti I. A Combination of Xyloglucan, Pea Protein and Chia Seed Ameliorates Intestinal Barrier Integrity and Mucosa Functionality in a Rat Model of Constipation-Predominant Irritable Bowel Syndrome. Journal of Clinical Medicine. 2022; 11(23):7073. https://doi.org/10.3390/jcm11237073
Chicago/Turabian StyleFilippone, Alessia, Alessio Ardizzone, Valentina Bova, Marika Lanza, Giovanna Casili, Salvatore Cuzzocrea, Emanuela Esposito, Michela Campolo, and Irene Paterniti. 2022. "A Combination of Xyloglucan, Pea Protein and Chia Seed Ameliorates Intestinal Barrier Integrity and Mucosa Functionality in a Rat Model of Constipation-Predominant Irritable Bowel Syndrome" Journal of Clinical Medicine 11, no. 23: 7073. https://doi.org/10.3390/jcm11237073
APA StyleFilippone, A., Ardizzone, A., Bova, V., Lanza, M., Casili, G., Cuzzocrea, S., Esposito, E., Campolo, M., & Paterniti, I. (2022). A Combination of Xyloglucan, Pea Protein and Chia Seed Ameliorates Intestinal Barrier Integrity and Mucosa Functionality in a Rat Model of Constipation-Predominant Irritable Bowel Syndrome. Journal of Clinical Medicine, 11(23), 7073. https://doi.org/10.3390/jcm11237073












