Safety and Efficacy of the Preserflo® Microshunt in Refractory Glaucoma: A One-Year Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
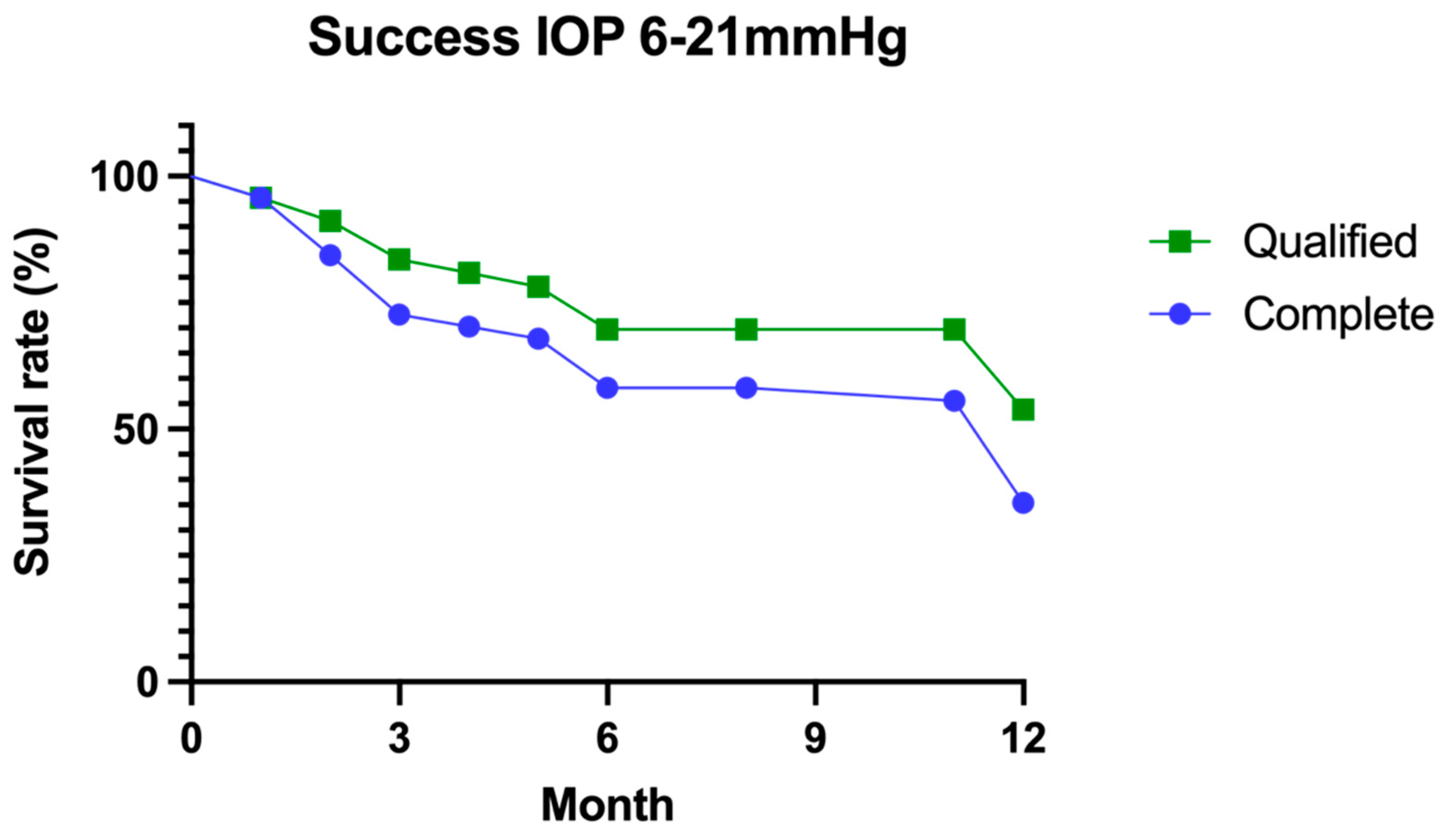
3.1. Success
3.2. Needling or Bleb Revision
3.3. Security
3.4. Failure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Aptel, F. Traitements des glaucomes réfractaires. EMC Ophtalmol. 2015, 12, 1–6. Available online: https://www.em-consulte.com/article/985631/traitements-des-glaucomes-refractaires (accessed on 10 October 2022).
- Sugimoto, Y.; Mochizuki, H.; Ohkubo, S.; Higashide, T.; Sugiyama, K.; Kiuchi, Y. Intraocular Pressure Outcomes and Risk Factors for Failure in the Collaborative Bleb-Related Infection Incidence and Treatment Study. Ophthalmology 2015, 122, 2223–2233. [Google Scholar] [CrossRef] [PubMed]
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L.; Tube versus Trabeculectomy Study Group. Treatment Outcomes in the Tube Versus Trabeculectomy (TVT) Study After Five Years of Follow-up. Am. J. Ophthalmol. 2012, 153, 789–803.e2. [Google Scholar] [CrossRef] [Green Version]
- Budenz, D.L.; Feuer, W.J.; Barton, K.; Schiffman, J.; Costa, V.P.; Godfrey, D.G.; Buys, Y.M.; Budenz, D.; Gedde, S.J.; El Sayyad, F.; et al. Postoperative Complications in the Ahmed Baerveldt Comparison Study During Five Years of Follow-up. Am. J. Ophthalmol. 2016, 163, 75–82.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luzu, J.; Baudouin, C.; Hamard, P. The role of Ahmed glaucoma valve in the management of refractory glaucoma: Long-term outcomes and complications. Eur. J. Ophthalmol. 2020, 31, 1120672120968733. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Ma, K.T.; Hong, Y.J.; Kim, C.Y. Long-term clinical outcomes of Ahmed valve implantation in patients with refractory glaucoma. PLoS ONE 2017, 12, e0187533. [Google Scholar] [CrossRef] [Green Version]
- Christakis, P.G.; Kalenak, J.W.; Zurakowski, D.; Tsai, J.C.; Kammer, J.A.; Harasymowycz, P.J.; Ahmed, I.I. The Ahmed Versus Baerveldt Study: One-Year Treatment Outcomes. Ophthalmology 2011, 118, 2180–2189. [Google Scholar] [CrossRef]
- Souissi, S.; Le Mer, Y.; Metge, F.; Portmann, A.; Baudouin, C.; Labbé, A.; Hamard, P. An update on continuous-wave cyclophotocoagulation (CW-CPC) and micropulse transscleral laser treatment (MP-TLT) for adult and paediatric refractory glaucoma. Acta Ophthalmol. 2020, 99, e621–e653. [Google Scholar] [CrossRef]
- Saheb, H.; Ahmed, I.I.K. Micro-invasive glaucoma surgery: Current perspectives and future directions. Curr. Opin. Ophthalmol. 2012, 23, 96–104. [Google Scholar] [CrossRef]
- Durr, G.M.; Schlenker, M.B.; Samet, S.; Ahmed, I.I.K. One-year outcomes of stand-alone ab externo SIBS microshunt implantation in refractory glaucoma. Br. J. Ophthalmol. 2020, 106, 71–79. [Google Scholar] [CrossRef]
- Quaranta, L.; Micheletti, E.; Carassa, R.; Bruttini, C.; Fausto, R.; Katsanos, A.; Riva, I. Efficacy and Safety of PreserFlo® MicroShunt After a Failed Trabeculectomy in Eyes with Primary Open-Angle Glaucoma: A Retrospective Study. Adv. Ther. 2021, 38, 4403–4412. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.P.; Budenz, D.L.; Lee, P.P.; Noecker, R.J.; Walt, J.G.; Siegartel, L.R.; Evans, S.J.; Doyle, J.J. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am. J. Ophthalmol. 2006, 141, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Law, S.K.; Shih, K.; Tran, D.H.; Coleman, A.L.; Caprioli, J. Long-term outcomes of repeat vs. initial trabeculectomy in open-angle glaucoma. Am. J. Ophthalmol. 2009, 148, 685–695.e1. [Google Scholar] [CrossRef] [PubMed]
- Hirunpatravong, P.; Reza, A.; Romero, P.; Kim, E.A.; Nouri-Mahdavi, K.; Law, S.K.; Morales, E.; Caprioli, J. Same-site Trabeculectomy Revision for Failed Trabeculectomy: Outcomes and Risk Factors for Failure. Am. J. Ophthalmol. 2016, 170, 110–118. [Google Scholar] [CrossRef]
- Schlenker, M.B.; Gulamhusein, H.; Conrad-Hengerer, I.; Somers, A.; Lenzhofer, M.; Stalmans, I.; Reitsamer, H.; Hengerer, F.H.; Ahmed, I.I.K. Efficacy, Safety, and Risk Factors for Failure of Standalone Ab Interno Gelatin Microstent Implantation versus Standalone Trabeculectomy. Ophthalmology 2017, 124, 1579–1588. [Google Scholar] [CrossRef]
- Tham, C.C.Y.; Leung, D.Y.L.; Kwong, Y.Y.Y.; Liang, Y.; Peng, A.Y.; Li, F.C.H.; Lai, J.S.M.; Lam, D.S.C. Factors correlating with failure to control intraocular pressure in primary angle-closure glaucoma eyes with coexisting cataract treated by phacoemulsification or combined phacotrabeculectomy. Asia-Pac. J. Ophthalmol. 2015, 4, 56–59. [Google Scholar] [CrossRef]
- Hassanein, D.H.; Awadein, A.; Elhilali, H. Factors associated with early and late failure after goniotomy for primary pediatric glaucoma. Eur. J. Ophthalmol. 2020, 30, 162–167. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Han, Y.; Shi, Y.; Xin, C.; Yin, P.; Li, M.; Cao, K.; Wang, N. Outcomes of gonioscopy-assisted transluminal trabeculotomy in juvenile-onset primary open-angle glaucoma. Eye 2021, 35, 2848–2854. [Google Scholar] [CrossRef]
- Schlenker, M.B.; Durr, G.M.; Michaelov, E.; Ahmed, I.I.K. Intermediate Outcomes of a Novel Standalone Ab Externo SIBS Microshunt With Mitomycin C. Am. J. Ophthalmol. 2020, 215, 141–153. [Google Scholar] [CrossRef]
- Bell, K.; de Padua Soares Bezerra, B.; Mofokeng, M.; Montesano, G.; Nongpiur, M.E.; Marti, M.V.; Lawlor, M. Learning from the past: Mitomycin C use in trabeculectomy and its application in bleb-forming minimally invasive glaucoma surgery. Surv. Ophthalmol. 2021, 66, 109–123. [Google Scholar] [CrossRef]
- Baker, N.D.; Barnebey, H.S.; Moster, M.R.; Stiles, M.C.; Vold, S.D.; Khatana, A.K.; Flowers, B.E.; Grover, D.S.; Strouthidis, N.G.; Panarelli, J.F.; et al. Ab-externo MicroShunt versus Trabeculectomy in Primary Open-Angle Glaucoma: 1-year Results from a 2-year Randomized, Multicenter Study. Ophthalmology 2021, 12, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Michaels, L.; Holland, L.; Mercieca, K. Trans-conjunctival Erosion of a Novel SIBS Microshunt After Revision Surgery Using Mitomycin C. J. Glaucoma 2021, 30, e349–e351. [Google Scholar] [CrossRef] [PubMed]
- Bunod, R.; Robin, M.; Buffault, J.; Keilani, C.; Labbé, A.; Baudouin, C. PreserFlo MicroShunt® exposure: A case series. BMC Ophthalmol. 2021, 21, 273. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Huang, H.T.; Bair, J.S.; Lee, C.C. Trabeculectomy with simultaneous topical application of mitomycin-C in refractory glaucoma. J. Ocul. Pharmacol. 1990, 6, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, N.; Syeda, S.; Tokko, H.; Thipparthi, M.; Cohen, M.I.; Kim, C.; Al-Timimi, F.R.; Tannir, J.R.; Goyal, A.; Juzych, M.S.; et al. Three-year outcomes of trabeculectomy and Ahmed valve implant in patients with prior failed filtering surgeries. Int. Ophthalmol. 2020, 40, 3377–3391. [Google Scholar] [CrossRef]
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L.; Tube Versus Trabeculectomy Study Group. Treatment Outcomes in the Tube Versus Trabeculectomy Study After One Year of Follow-up. Am. J. Ophthalmol. 2007, 143, 9–22.e2. [Google Scholar] [CrossRef]
- Grover, D.S.; Flynn, W.J.; Bashford, K.P.; Lewis, R.A.; Duh, Y.-J.; Nangia, R.S.; Niksch, B. Performance and Safety of a New Ab Interno Gelatin Stent in Refractory Glaucoma at 12 Months. Am. J. Ophthalmol. 2017, 183, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Lewczuk, K.; Konopińska, J.; Jabłońska, J.; Rudowicz, J.; Laszewicz, P.; Mariak, Z.; Rękas, M. XEN Glaucoma Implant for the Management of Operated Uncontrolled Glaucoma: Results and Complications during a Long-Term Follow-Up. J. Ophthalmol. 2021, 2021, 2321922. [Google Scholar] [CrossRef]


| Population | Numbers |
|---|---|
| Mean age (years) | 64 |
| Women | 38% |
| Mean BCVA (logMar) | 0.84 ± 0.7 |
| Mean pre-op IOP (mm Hg) | 30.11 ± 7.08 |
| Mean pre-op number of medications | 3.40 ± 0.95 |
| Glaucoma etiology | |
| Primary | 25 (54%) |
| Pseudoexfoliation | 4 (9%) |
| Pigmentary | 4 (9%) |
| Juvenile | 1 (2%) |
| Congenital | 3 (6%) |
| Uveitic | 3 (6%) |
| Silicone oil | 2 (4%) |
| Other | 5 (10%) |
| Glaucoma severity | |
| Mild | 5 (11%) |
| Moderate | 7 (15%) |
| Advanced | 35 (74%) |
| Cup/disc (mean) | 0.9 |
| Mean pre-op. MD (dB) | −19.2 ± 7.4 |
| Surgical history | |
| Mean number of glaucoma surgeries | 2.3 ± 1.3 |
| Trabeculectomy or Deep Sclerectomy (nb) | 45 (96%) |
| Xen® | 8 (17%) |
| Ahmed | 2 (4%) |
| Starflo® | 3 (6%) |
| Cypass® | 1 (2%) |
| Diode | 13 (28%) |
| Cataract | 42 (89%) |
| Retinal-Detachment—vitrectomy | 4 (8%) |
| Corneal transplant | 2 (4%) |
| Other | 2 (4%) |
| Mean intraoperative MMC (min) | 2.13 |
| Complication | Early (<3 Months) | Late (≥3 Months) |
|---|---|---|
| Choroidal detachment | 4 | |
| Dellen effect | 1 | |
| Tube exposure | 1 | |
| Tube transection | 1 | |
| Macular edema | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majoulet, A.; Scemla, B.; Hamard, P.; Brasnu, E.; Hage, A.; Baudouin, C.; Labbé, A. Safety and Efficacy of the Preserflo® Microshunt in Refractory Glaucoma: A One-Year Study. J. Clin. Med. 2022, 11, 7086. https://doi.org/10.3390/jcm11237086
Majoulet A, Scemla B, Hamard P, Brasnu E, Hage A, Baudouin C, Labbé A. Safety and Efficacy of the Preserflo® Microshunt in Refractory Glaucoma: A One-Year Study. Journal of Clinical Medicine. 2022; 11(23):7086. https://doi.org/10.3390/jcm11237086
Chicago/Turabian StyleMajoulet, Alexandre, Benjamin Scemla, Pascale Hamard, Emmanuelle Brasnu, Alexandre Hage, Christophe Baudouin, and Antoine Labbé. 2022. "Safety and Efficacy of the Preserflo® Microshunt in Refractory Glaucoma: A One-Year Study" Journal of Clinical Medicine 11, no. 23: 7086. https://doi.org/10.3390/jcm11237086




