A Prospective Validation Study of the Functional Bedside Aspiration Screen with Endoscopy: Is It Clinically Applicable in Acute Stroke?
Abstract
1. Introduction
2. Materials and Methods
2.1. Functional Bedside Aspiration Screen (FBAS)
2.2. Other Swallowing Measures
2.3. Functional Oral Intake Scale (FOIS)
2.4. Flexible Endoscopic Evaluation of Swallowing (FEES)
2.5. Therapy Requirement Scale
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Everton, L.F.; Benfield, J.K.; Hedstrom, A.; Wilkinson, G.; Michou, E.; England, T.J.; Dziewas, R.; Bath, P.M.; Hamdy, S. Psychometric assessment and validation of the dysphagia severity rating scale in stroke patients. Sci. Rep. 2020, 10, 7268. [Google Scholar] [CrossRef] [PubMed]
- Dziewas, R.; Michou, E.; Trapl-Grundschober, M.; Lal, A.; Arsava, E.M.; Bath, P.M.; Clavé, P.; Glahn, J.; Hamdy, S.; Pownall, S.; et al. European Stroke Organisation and European Society for Swallowing Disorders guideline for the diagnosis and treatment of post-stroke dysphagia. Eur. Stroke J. 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Hinchey, J.A.; Shephard, T.; Furie, K.; Smith, D.; Wang, D.; Tonn, S.; Stroke Practice Improvement Network Investigators. Formal dysphagia screening protocols prevent pneumonia. Stroke 2005, 36, 1972–1976. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayan, K.; Tsai, A.W.; Tong, X.; Vazquez, G.; Peacock, J.M.; George, M.G.; Luepker, R.V.; Anderson, D.C. Utility of dysphagia screening results in predicting poststroke pneumonia. Stroke 2010, 41, 2849–2854. [Google Scholar] [CrossRef]
- Bray, B.D.; Smith, C.J.; Cloud, G.C.; Enderby, P.; James, M.; Paley, L.; Tyrrell, P.J.; Wolfe, C.D.; Rudd, A.G. The association between delays in screening for and assessing dysphagia after acute stroke, and the risk of stroke-associated pneumonia. J. Neurol. Neurosurg. Psychiatry 2017, 88, 25–30. [Google Scholar] [CrossRef]
- Al-Khaled, M.; Matthis, C.; Binder, A.; Mudter, J.; Schattschneider, J.; Pulkowski, U.; Strohmaier, T.; Niehoff, T.; Zybur, R.; Eggers, J.; et al. Dysphagia in Patients with Acute Ischemic Stroke: Early Dysphagia Screening May Reduce Stroke-Related Pneumonia and Improve Stroke Outcomes. Cerebrovasc. Dis. 2016, 42, 81–89. [Google Scholar] [CrossRef]
- Palmer, P.M.; Padilla, A.H. Risk of an Adverse Event in Individuals Who Aspirate: A Review of Current Literature on Host Defenses and Individual Differences. Am. J. Speech Lang. Pathol. 2022, 31, 148–162. [Google Scholar] [CrossRef]
- Eltringham, S.A.; Kilner, K.; Gee, M.; Sage, K.; Bray, B.D.; Pownall, S.; Smith, C.J. Impact of Dysphagia Assessment and Management on Risk of Stroke-Associated Pneumonia: A Systematic Review. Cerebrovasc. Dis. 2018, 46, 99–107. [Google Scholar] [CrossRef]
- Ouyang, M.; Boaden, E.; Arima, H.; Lavados, P.M.; Billot, L.; Hackett, M.L.; Olavarría, V.V.; Muñoz-Venturelli, P.; Song, L.; Rogers, K.; et al. Dysphagia screening and risks of pneumonia and adverse outcomes after acute stroke: An international multicenter study. Int. J. Stroke 2020, 15, 206–215. [Google Scholar] [CrossRef]
- Virvidaki, I.-E.; Giannopoulos, S.; Nasios, G.; Dimakopoulos, G.; Michou, E.; Milionis, H. Predictive value of a novel pragmatic tool for post-stroke aspiration risk: The Functional Bedside Aspiration Screen. Neurogastroenterol. Motil. 2019, 31, e13683. [Google Scholar] [CrossRef]
- Martino, R.; Silver, F.; Teasell, R.; Bayley, M.; Nicholson, G.; Streiner, D.L.; Diamant, N.E. The Toronto Bedside Swallowing Screening Test (TOR-BSST): Development and validation of a dysphagia screening tool for patients with stroke. Stroke 2009, 40, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, M.B.; Suiter, D.M.; González-Fernández, M.; Michtalik, H.J.; Frymark, T.B.; Venediktov, R.; Schooling, T. Screening Accuracy for Aspiration Using Bedside Water Swallow Tests: A Systematic Review and Meta-Analysis. Chest 2016, 150, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Lindner-Pfleghar, B.; Neugebauer, H.; Stösser, S.; Kassubek, J.; Ludolph, A.; Dziewas, R.; Prosiegel, M.; Riecker, A. [Management of dysphagia in acute stroke: A prospective study for validation of current recommendations]. Nervenarzt 2017, 88, 173–179. [Google Scholar] [CrossRef]
- Warnecke, T.; Teismann, I.; Meimann, W.; Olenberg, S.; Zimmermann, J.; Krämer, C.; Ringelstein, E.B.; Schäbitz, W.R.; Dziewas, R. Assessment of aspiration risk in acute ischaemic stroke--evaluation of the simple swallowing provocation test. J. Neurol. Neurosurg. Psychiatry 2008, 79, 312–314. [Google Scholar] [CrossRef][Green Version]
- DePippo, K.L.; Holas, M.A.; Reding, M.J. Validation of the 3-oz water swallow test for aspiration following stroke. Arch. Neurol. 1992, 49, 1259–1261. [Google Scholar] [CrossRef] [PubMed]
- Edmiaston, J.; Connor, L.T.; Loehr, L.; Nassief, A. Validation of a dysphagia screening tool in acute stroke patients. Am. J. Crit. Care 2010, 19, 357–364. [Google Scholar] [CrossRef]
- Suiter, D.M.; Leder, S.B. Clinical utility of the 3-ounce water swallow test. Dysphagia 2008, 23, 244–250. [Google Scholar] [CrossRef]
- Daniels, S.K.; Brailey, K.; Priestly, D.H.; Herrington, L.R.; Weisberg, L.A.; Foundas, A.L. Aspiration in patients with acute stroke. Arch. Phys. Med. Rehabil. 1998, 79, 14–19. [Google Scholar] [CrossRef]
- John, J.S.; Berger, L. Using the gugging swallowing screen (GUSS) for dysphagia screening in acute stroke patients. J. Contin. Educ. Nurs. 2015, 46, 103–104. [Google Scholar] [CrossRef]
- Trapl, M.; Enderle, P.; Nowotny, M.; Teuschl, Y.; Matz, K.; Dachenhausen, A.; Brainin, M. Dysphagia bedside screening for acute-stroke patients: The Gugging Swallowing Screen. Stroke 2007, 38, 2948–2952. [Google Scholar] [CrossRef]
- Crary, M.A.; Mann, G.D.; Groher, M.E. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch. Phys. Med. Rehabil. 2005, 86, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Langmore, S.E.; Ginsberg, S.; Dostie, A. The significance of accumulated oropharyngeal secretions and swallowing frequency in predicting aspiration. Dysphagia 1996, 11, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Bahia, M.M.; Mourão, L.F.; Chun, R.Y. Dysarthria as a predictor of dysphagia following stroke. NeuroRehabilitation 2016, 38, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Henke, C.; Foerch, C.; Lapa, S. Early Screening Parameters for Dysphagia in Acute Ischemic Stroke. Cerebrovasc. Dis. 2017, 44, 285–290. [Google Scholar] [CrossRef]
- McCullough, G.H.; Rosenbek, J.C.; Wertz, R.T.; McCoy, S.; Mann, G.; McCullough, K. Utility of clinical swallowing examination measures for detecting aspiration post-stroke. J. Speech. Lang. Hear. Res. 2005, 48, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, T.; Im, S.; Kaiser, C.; Hamacher, C.; Oelenberg, S.; Dziewas, R. Aspiration and dysphagia screening in acute stroke—The Gugging Swallowing Screen revisited. Eur. J. Neurol. 2017, 24, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Logemann, J.A.; Veis, S.; Colangelo, L. A screening procedure for oropharyngeal dysphagia. Dysphagia 1999, 14, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Althaus, K.; Dreyhaupt, J.; Hyrenbach, S.; Pinkhardt, E.H.; Kassubek, J.; Ludolph, A.C. MRI as a first-line imaging modality in acute ischemic stroke: A sustainable concept. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211030363. [Google Scholar] [CrossRef]
- Khedr, E.M.; Abbass, M.A.; Soliman, R.K.; Zaki, A.F.; Gamea, A. Post-stroke dysphagia: Frequency, risk factors, and topographic representation: Hospital-based study. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 23. [Google Scholar] [CrossRef]
- Paciaroni, M.; Mazzotta, G.; Corea, F.; Caso, V.; Venti, M.; Milia, P.; Silvestrelli, G.; Palmerini, F.; Parnetti, L.; Gallai, V. Dysphagia following Stroke. Eur. Neurol. 2004, 51, 162–167. [Google Scholar] [CrossRef]
- Andersen, K.K.; Olsen, T.S.; Dehlendorff, C.; Kammersgaard, L.P. Hemorrhagic and ischemic strokes compared: Stroke severity, mortality, and risk factors. Stroke 2009, 40, 2068–2072. [Google Scholar] [CrossRef] [PubMed]
- Joundi, R.A.; Martino, R.; Saposnik, G.; Giannakeas, V.; Fang, J.; Kapral, M.K. Dysphagia screening after intracerebral hemorrhage. Int. J. Stroke 2018, 13, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Joundi, R.A.; Martino, R.; Saposnik, G.; Giannakeas, V.; Fang, J.; Kapral, M.K. Predictors and Outcomes of Dysphagia Screening After Acute Ischemic Stroke. Stroke 2017, 48, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.K.; McAdam, C.P.; Brailey, B.; Foundas, A.L. Clinical Assessment of Swallowing and Prediction of Dysphagia Severity. Am. J. Speech Lang. Pathol. 1997, 6, 17–24. [Google Scholar] [CrossRef]
- Ickenstein, G.; Hofmayer, A.; Lindner-Pfleghar, B.; Pluschinski, P.; Riecker, A.; Schelling, A.; Prosiegel, M. Standardisation of diagnostic and therapeutic procedures for neurogenic oropharyngeal dysphagia (NOD). Neurol. Rehabil. 2009, 15, 290–300. [Google Scholar]
- Rosenbek, J.C.; Robbins, J.A.; Roecker, E.B.; Coyle, J.L.; Wood, J.L. A penetration-aspiration scale. Dysphagia 1996, 11, 93–98. [Google Scholar] [CrossRef]
- Hey, C.; Pluschinski, P.; Zaretsky, Y.; Almahameed, A.; Hirth, D.; Vaerst, B.; Wagenblast, J.; Stöver, T. [Penetration-Aspiration Scale according to Rosenbek. Validation of the German version for endoscopic dysphagia diagnostics]. HNO 2014, 62, 276–281. [Google Scholar] [CrossRef]
- Power, M.L.; Hamdy, S.; Goulermas, J.Y.; Tyrrell, P.J.; Turnbull, I.; Thompson, D.G. Predicting aspiration after hemispheric stroke from timing measures of oropharyngeal bolus flow and laryngeal closure. Dysphagia 2009, 24, 257–264. [Google Scholar] [CrossRef]
- Suiter, D.M.; Sloggy, J.; Leder, S.B. Validation of the Yale Swallow Protocol: A prospective double-blinded videofluoroscopic study. Dysphagia 2014, 29, 199–203. [Google Scholar] [CrossRef]
- Warnecke, T.; Ritter, M.A.; Kröger, B.; Oelenberg, S.; Teismann, I.; Heuschmann, P.U.; Ringelstein, E.B.; Nabavi, D.G.; Dziewas, R. Fiberoptic Endoscopic Dysphagia Severity Scale Predicts Outcome after Acute Stroke. Cerebrovasc. Dis. 2009, 28, 283–289. [Google Scholar] [CrossRef]
- Suntrup-Krueger, S.; Minnerup, J.; Muhle, P.; Claus, I.; Schröder, J.B.; Marian, T.; Warnecke, T.; Kalic, M.; Berger, K.; Dziewas, R. The Effect of Improved Dysphagia Care on Outcome in Patients with Acute Stroke: Trends from 8-Year Data of a Large Stroke Register. Cerebrovasc. Dis. 2018, 45, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Jannini, T.B.; Ruggiero, M.; Viganò, A.; Comanducci, A.; Maestrini, I.; Giuliani, G.; Vicenzini, E.; Fattapposta, F.; Pauri, F.; Ruoppolo, G.; et al. The role of the Sapienza GLObal Bedside Evaluation of Swallowing after Stroke (GLOBE-3S) in the prevention of stroke-associated pneumonia (SAP). Neurol. Sci. 2022, 43, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; Viganò, A.; Rea, A.; Verzina, A.; Sasso D’Elia, T.; Puledda, F.; Longo, L.; Mancini, V.; Ruggiero, M.; Jannini, T.B.; et al. Sapienza Global Bedside Evaluation of Swallowing after Stroke: The GLOBE-3S study. Eur. J. Neurol. 2019, 26, 596–602. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, D.K.; Seo, K.M.; Kang, S.H. Usefulness of the simplified cough test in evaluating cough reflex sensitivity as a screening test for silent aspiration. Ann. Rehabil. Med. 2014, 38, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Wakasugi, Y.; Tohara, H.; Hattori, F.; Motohashi, Y.; Nakane, A.; Goto, S.; Ouchi, Y.; Mikushi, S.; Takeuchi, S.; Uematsu, H. Screening test for silent aspiration at the bedside. Dysphagia 2008, 23, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Nakajoh, K.; Nakagawa, T.; Sekizawa, K.; Matsui, T.; Arai, H.; Sasaki, H. Relation between incidence of pneumonia and protective reflexes in post-stroke patients with oral or tube feeding. J. Intern. Med. 2000, 247, 39–42. [Google Scholar] [CrossRef]
- Kaneoka, A.; Pisegna, J.M.; Inokuchi, H.; Ueha, R.; Goto, T.; Nito, T.; Stepp, C.E.; LaValley, M.P.; Haga, N.; Langmore, S.E. Relationship Between Laryngeal Sensory Deficits, Aspiration, and Pneumonia in Patients with Dysphagia. Dysphagia 2018, 33, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; Cecconi, E.; Capiluppi, E.; Viganò, A.; Bertora, P.; Campiglio, L.; Mariani, C.; Petolicchio, B.; Sasso D’Elia, T.; Verzina, A.; et al. Neuroanatomical, Clinical and Cognitive Correlates of Post-Stroke Dysphagia. Eur. Neurol. 2015, 74, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.K.; Anderson, J.A.; Willson, P.C. Valid items for screening dysphagia risk in patients with stroke: A systematic review. Stroke 2012, 43, 892–897. [Google Scholar] [CrossRef]
- Khorsan, R.; Crawford, C. How to assess the external validity and model validity of therapeutic trials: A conceptual approach to systematic review methodology. Evid. Based Complement. Altern. Med. 2014, 2014, 694804. [Google Scholar] [CrossRef]

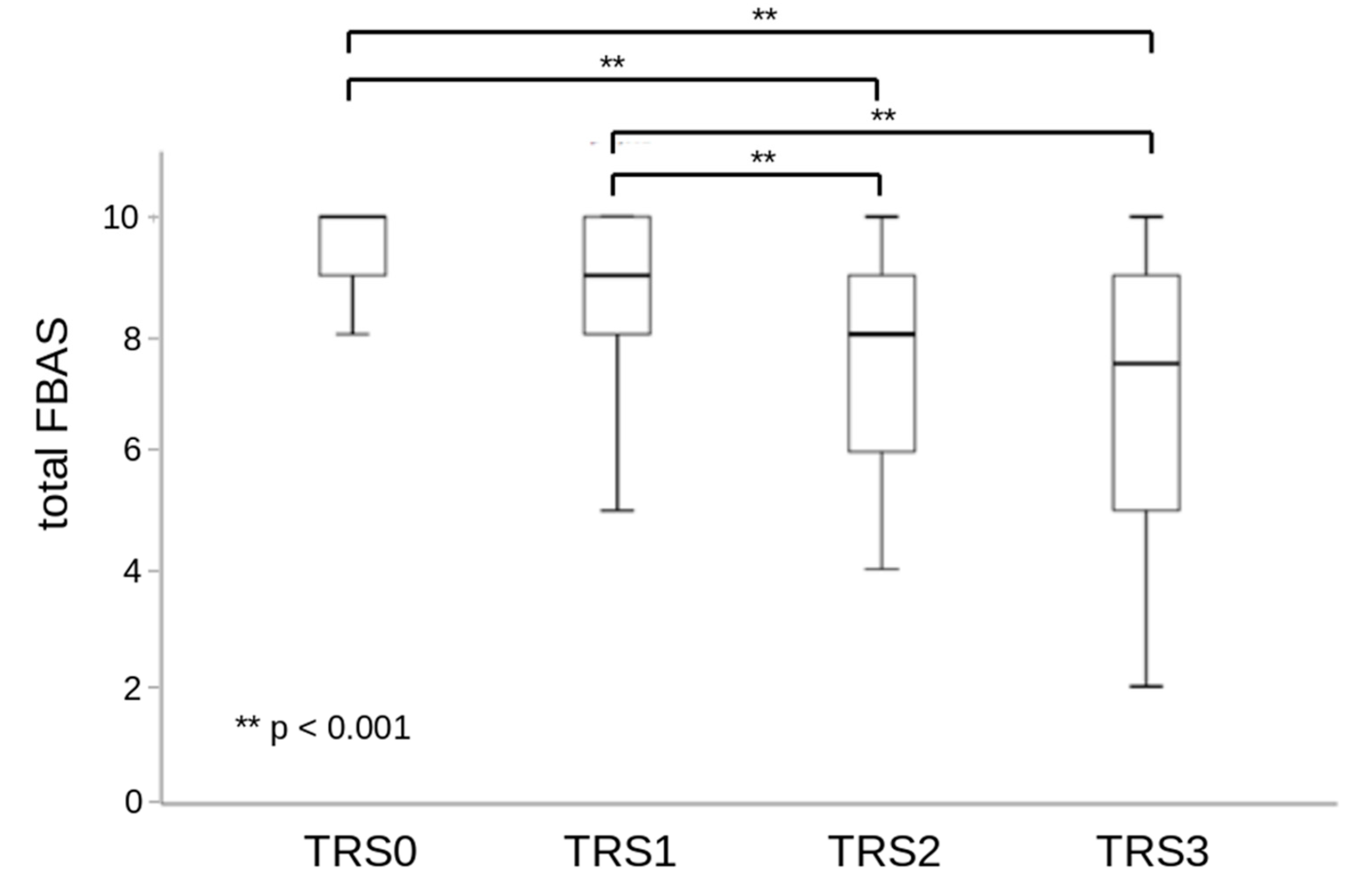
| Grade | Therapy Requirement Scale |
|---|---|
| TRS 0 | no dysphagia, no counseling or diet modification required |
| TRS 1 | education and guidance with regard to the necessary compensatory strategies or diet changes, as-needed occasional dysphagia therapy |
| TRS 2 | compensatory strategies or diet changes required, intensive dysphagia therapy, education and guidance |
| TRS 3 | profound diet changes required, full or partial tube feeding, intensive dysphagia therapy, education and guidance |
| Total n = 101 | PAS 1–2 n = 63 | PAS 3–5 n = 23 | PAS 6–8 n = 15 | p-Value | ||
|---|---|---|---|---|---|---|
| Sex | Female | 53 | 32 | 16 | 5 | 0.083 |
| Male | 48 | 31 | 7 | 10 | ||
| NIHSS at admission | Mild (0–4) | 66 | 46 | 10 | 10 | 0.018 |
| Moderate (5–15) | 32 | 15 | 13 | 4 | ||
| Moderate to severe (16–20) | 1 | 0 | 0 | 1 | ||
| Severe (21–42) | 1 | 1 | 0 | 0 | ||
| mRS atentry | 0—No symptoms | 5 | 4 | 1 | 0 | 0.007 |
| 1—No significant disability | 28 | 22 | 3 | 3 | ||
| 2—Slight disability | 11 | 9 | 1 | 1 | ||
| 3—Moderate disability | 24 | 14 | 5 | 5 | ||
| 4—Moderate to severe disability | 13 | 5 | 5 | 3 | ||
| 5—Severe disability | 20 | 9 | 8 | 3 | ||
| Hemisphere | Right | 41 | 26 | 10 | 5 | 0.201 |
| Left | 39 | 26 | 5 | 8 | ||
| Bilateral | 21 | 10 | 8 | 2 | ||
| Arterial supply area of stroke | ACA | 0 | 0 | 0 | 0 | 0.252 |
| MCA | 51 | 33 | 12 | 5 | ||
| PCA | 7 | 4 | 0 | 2 | ||
| VBA | 21 | 13 | 3 | 5 | ||
| Multiple vascular territories | 22 | 11 | 8 | 2 | ||
| Screening | FBAS positive (score 0–8) | 44 | 19 | 14 | 11 | 0.002 |
| FBAS negative (score 9–10) | 57 | 44 | 9 | 4 | ||
| pneumonia | 0 | 0 | 0 | 0 | ||
| Binary PAS × FBASrisk | ||||
|---|---|---|---|---|
| FBASrisk | Total | |||
| Low | High | |||
| Binary PAS | Low | TN = 44 | FP = 19 | 63 |
| High | FN = 13 | TP = 25 | 38 | |
| Total | 57 | 44 | 101 | |
| Correlations | ||
|---|---|---|
| TRS | ||
| PAS | Pearson Correlation | 0.687 |
| Sig. (2-tailed) | 0.000 | |
| N | 101 | |
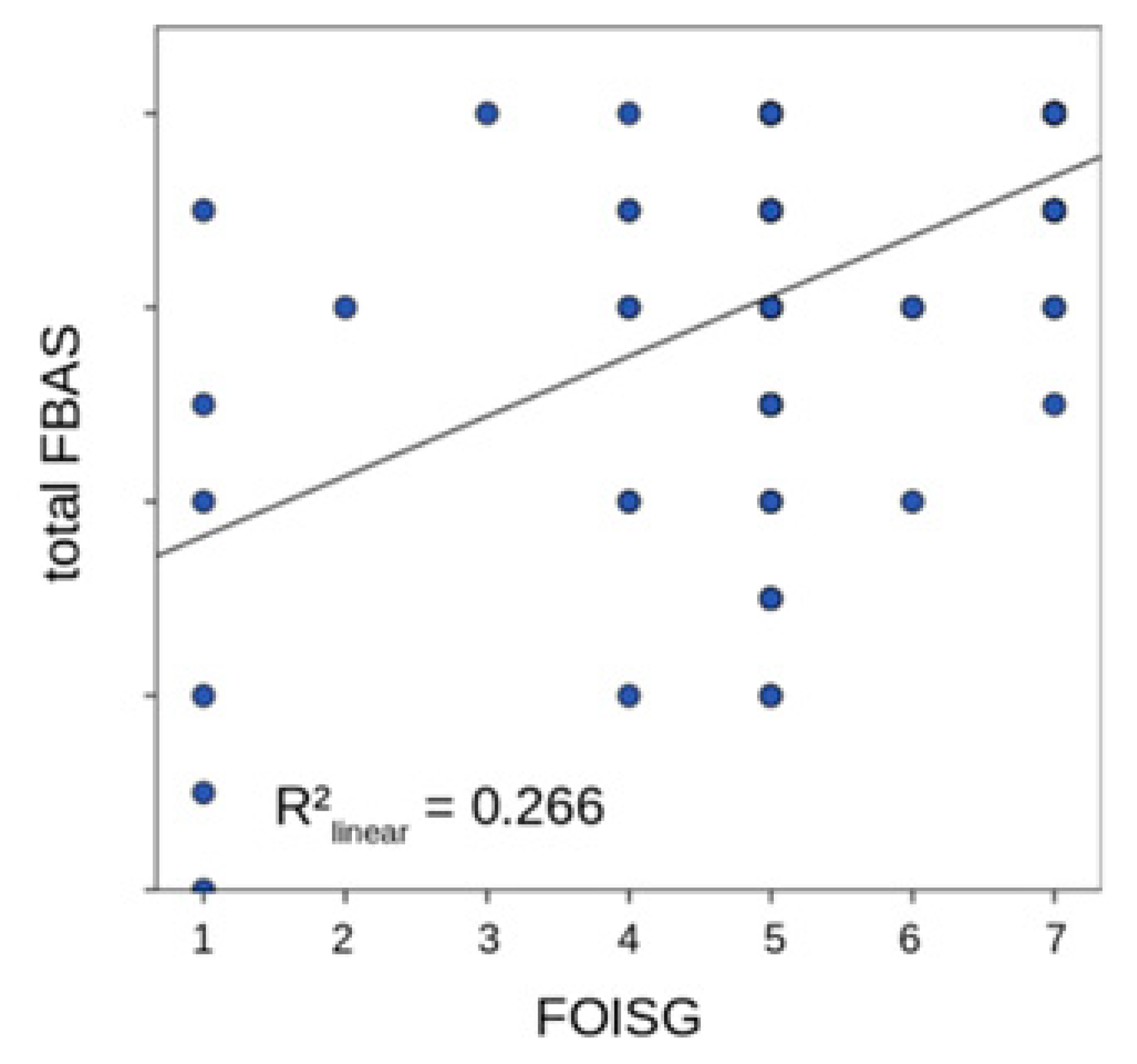
| FOISG | Pearson Correlation | −0.849 |
| Sig. (2-tailed) | 0.000 | |
| N | 101 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassubek, R.; Lindner-Pfleghar, B.; Virvidaki, I.E.; Kassubek, J.; Althaus, K.; Weber, A.M.; Dimakopoulos, G.; Milionis, H.; Nasios, G. A Prospective Validation Study of the Functional Bedside Aspiration Screen with Endoscopy: Is It Clinically Applicable in Acute Stroke? J. Clin. Med. 2022, 11, 7087. https://doi.org/10.3390/jcm11237087
Kassubek R, Lindner-Pfleghar B, Virvidaki IE, Kassubek J, Althaus K, Weber AM, Dimakopoulos G, Milionis H, Nasios G. A Prospective Validation Study of the Functional Bedside Aspiration Screen with Endoscopy: Is It Clinically Applicable in Acute Stroke? Journal of Clinical Medicine. 2022; 11(23):7087. https://doi.org/10.3390/jcm11237087
Chicago/Turabian StyleKassubek, Rebecca, Beate Lindner-Pfleghar, Ioanna Eleni Virvidaki, Jan Kassubek, Katharina Althaus, Antonia Maria Weber, Georgios Dimakopoulos, Haralampos Milionis, and Grigorios Nasios. 2022. "A Prospective Validation Study of the Functional Bedside Aspiration Screen with Endoscopy: Is It Clinically Applicable in Acute Stroke?" Journal of Clinical Medicine 11, no. 23: 7087. https://doi.org/10.3390/jcm11237087
APA StyleKassubek, R., Lindner-Pfleghar, B., Virvidaki, I. E., Kassubek, J., Althaus, K., Weber, A. M., Dimakopoulos, G., Milionis, H., & Nasios, G. (2022). A Prospective Validation Study of the Functional Bedside Aspiration Screen with Endoscopy: Is It Clinically Applicable in Acute Stroke? Journal of Clinical Medicine, 11(23), 7087. https://doi.org/10.3390/jcm11237087







