Choice of Glucose-Lowering Drugs as Initial Monotherapy for Type 2 Diabetes Patients with Contraindications or Intolerance to Metformin: A Systematic Review and Meta-Analysis
Abstract
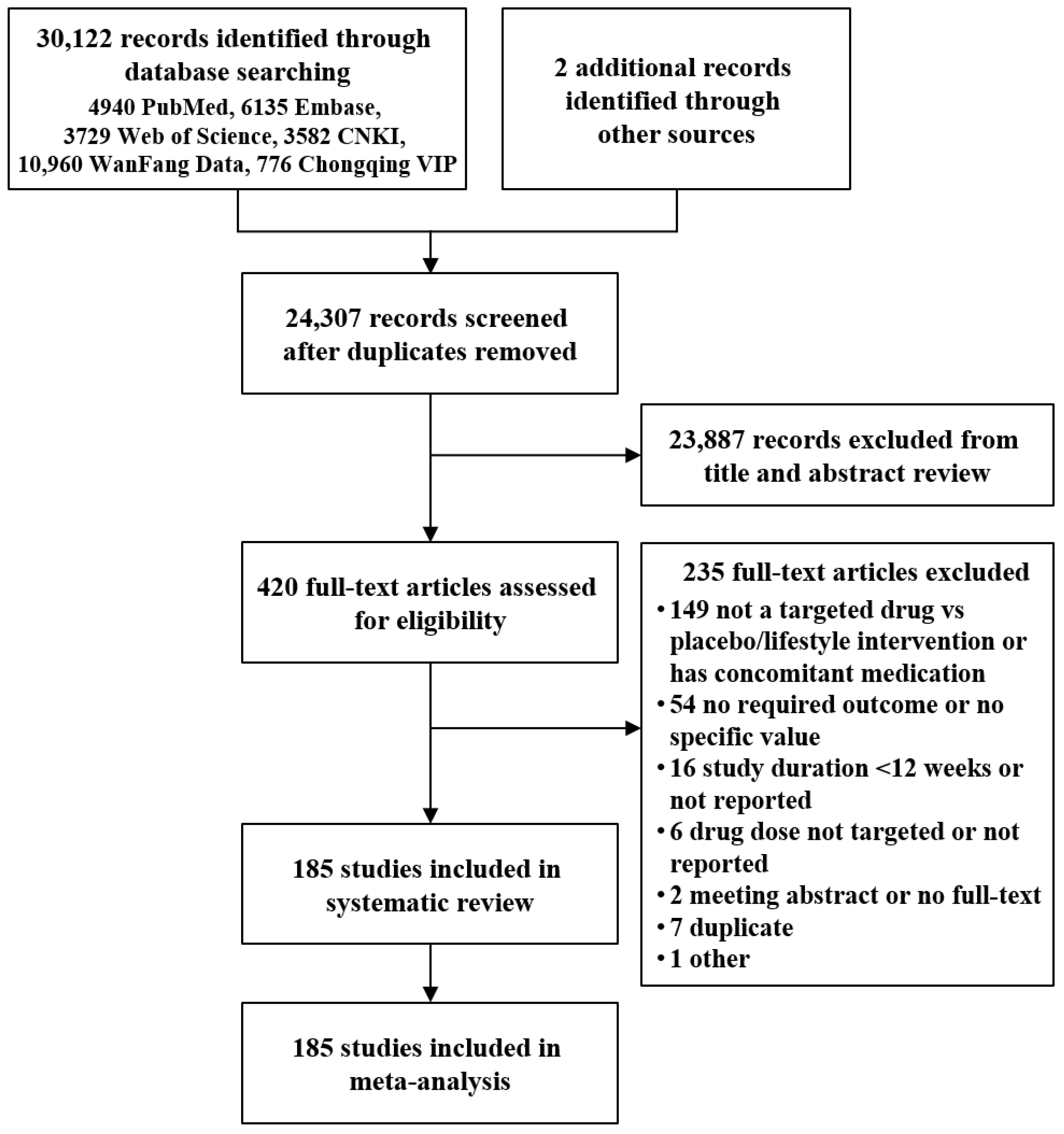
:1. Introduction
2. Methods
2.1. Eligibility Criteria
| Biguanide | Metformin |
| SUs | Glyburide, glimepiride, gliclazide, glipizide, and gliquidone |
| TZDs | Rosiglitazone and pioglitazone |
| NIDEs | Repaglinide, nateglinide, and mitiglinide |
| AGIs | Acarbose, voglibose, and miglitol |
| DPP-4is | Sitagliptin, saxagliptin, vildagliptin, linagliptin, and alogliptin |
| SGLT2is | Dapagliflozin, empagliflozin, and canagliflozin |
| INSs | Insulin and insulin analogs |
| GLP-1RAs | Exenatide, liraglutide, lixisenatide, and beinaglutide |
2.2. Information Sources and Searches
2.3. Study Selection
2.4. Data Extraction and Quality Assessment
2.5. Data Synthesis and Analysis
3. Results
3.1. Intermediate Outcomes
3.1.1. Hemoglobin Alc
3.1.2. Fasting Plasma Glucose
3.1.3. Body Mass Index
3.1.4. Total Cholesterol
3.1.5. High Density Lipoprotein-Cholesterol
3.1.6. Systolic Blood Pressure
3.2. Hypoglycemia
3.3. Mortality and Vascular Outcomes
3.4. Discontinuation
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF DIABETES ATLAS Ninth Edition 2019. Available online: https://diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf (accessed on 3 May 2021).
- International Diabetes Federation. About Diabetes: Types of Diabetes. Available online: https://www.idf.org/aboutdiabetes/what-is-diabetes/types-of-diabetes.html (accessed on 1 March 2018).
- Jia, W.; Weng, J.; Zhu, D.; Ji, L.; Lu, J.; Zhou, Z.; Zou, D.; Guo, L.; Ji, Q.; Chen, L.; et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes/Metab. Res. Rev. 2019, 35, e3158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S111–S124. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Lu, J.; Weng, J.; Jia, W.; Tian, H.; Zhu, D.; Xing, X.; Guo, L. China type 2 diabetes treatment status survey of treatment pattern of oral drugs users. J. Diabetes 2015, 7, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Hu, D.; Pan, C.; Weng, J.; Huo, Y.; Ma, C.; Mu, Y.; Hao, C.; Ji, Q.; Ran, X.; et al. Primacy of the 3B Approach to Control Risk Factors for Cardiovascular Disease in Type 2 Diabetes Patients. Am. J. Med. 2013, 126, 925.e11–925.e22. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. Available online: http://handbook-5-1.cochrane.org/ (accessed on 1 June 2017).
- Bucher, H.C.; Guyatt, G.H.; Griffith, L.E.; Walter, S.D. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J. Clin. Epidemiol. 1997, 50, 683–691. [Google Scholar] [CrossRef]
- Song, F.; Altman, D.G.; Glenny, A.-M.; Deeks, J. Validity of indirect comparison for estimating efficacy of competing interventions: Empirical evidence from published meta-analyses. BMJ 2003, 326, 472. [Google Scholar] [CrossRef] [Green Version]
- Eng, C.; Kramer, C.K.; Zinman, B.; Retnakaran, R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: A systematic review and meta-analysis. Lancet 2014, 384, 2228–2234. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M.; Paschetta, E. Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: Meta-analysis of randomised controlled trials. BMJ 2019, 365, l1328. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, L.; Han, P.; Wang, X.; Liu, J.; Zheng, S.; Jou, Y.; O’Neill, E.A.; Golm, G.T.; Engel, S.S.; Kaufman, K.D.; et al. Randomized clinical trial of the safety and efficacy of sitagliptin and metformin co-administered to Chinese patients with type 2 diabetes mellitus. J. Diabetes Investig. 2016, 7, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Abd-Allah, G.M. Effects of metformin plus gliclazide versus metformin plus glimepiride on cardiovascular risk factors in patients with type 2 diabetes mellitus. Pak. J. Pharm. Sci. 2015, 28, 1723–1730. [Google Scholar] [PubMed]
- Pratley, R.E.; Fleck, P.; Wilson, C. Efficacy and safety of initial combination therapy with alogliptin plus metformin versus either as monotherapy in drug-naïve patients with type 2 diabetes: A randomized, double-blind, 6-month study. Diabetes Obes. Metab. 2014, 16, 613–621. [Google Scholar] [CrossRef]
- Ferrannini, E.; Seman, L.; Seewaldt-Becker, E.; Hantel, S.; Pinnetti, S.; Woerle, H.J. A Phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 721–728. [Google Scholar] [CrossRef]
- Haak, T.; Meinicke, T.; Von Eynatten, M.; Woerle, H.-J.; Jones, R.; Weber, S. Initial combination of linagliptin and metformin improves glycaemic control in type 2 diabetes: A randomized, double-blind, placebo-controlled study. Diabetes Obes. Metab. 2012, 14, 565–574. [Google Scholar] [CrossRef]
- Chakraborty, A.; Chowdhury, S.; Bhattacharyya, M. Effect of metformin on oxidative stress, nitrosative stress and inflammatory biomarkers in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2011, 93, 56–62. [Google Scholar] [CrossRef]
- List, J.F.; Woo, V.; Morales, E.; Tang, W.; Fiedorek, F.T. Sodium-Glucose Cotransport Inhibition with Dapagliflozin in Type 2 Diabetes. Diabetes Care 2009, 32, 650–657. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, B.J.; Feinglos, M.N.; Lunceford, J.K.; Johnson, J.; Williams-Herman, D.E. Effect of Initial Combination Therapy with Sitagliptin, a Dipeptidyl Peptidase-4 Inhibitor, and Metformin on Glycemic Control in Patients with Type 2 Diabetes. Diabetes Care 2007, 30, 1979–1987. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ning, J. Effects of metformin on C-reactive protein and complement factor C3 in patients of obese type 2 diabetes mellitus. Shanxi Med. J. 2006, 35, 888–889. [Google Scholar]
- Fujioka, K.; Brazg, R.L.; Raz, I.; Bruce, S.; Joyal, S.; Swanink, R.; Pans, M. Efficacy, dose-response relationship and safety of once-daily extended-release metformin (GlucophageR XR) in type 2 diabetic patients with inadequate glycaemic control despite prior treatment with diet and exercise: Results from two double-blind, placebo-controlled studies. Diabetes Obes. Metab. 2005, 7, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.K.; Hallsten, K.; Bjornholm, M.; Tsuchida, H.; Chibalin, A.V.; Virtanen, K.A.; Heinonen, O.J.; Lonnqvist, F.; Nuutila, P.; Zierath, J.R. Effects of metformin and rosiglitazone treatment on insulin signaling and glucose uptake in patients with newly diagnosed type 2 diabetes: A randomized controlled study. Diabetes 2005, 54, 1459–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viljanen, A.P.M.; Virtanen, K.A.; Järvisalo, M.J.; Hällsten, K.; Parkkola, R.; Rönnemaa, T.; Lönnqvist, F.; Iozzo, P.; Ferrannini, E.; Nuutila, P. Rosiglitazone Treatment Increases Subcutaneous Adipose Tissue Glucose Uptake in Parallel with Perfusion in Patients with Type 2 Diabetes: A Double-Blind, Randomized Study with Metformin. J. Clin. Endocrinol. Metab. 2005, 90, 6523–6528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horton, E.S.; Foley, J.E.; Shen, S.G.; Baron, M.A. Efficacy and tolerability of initial combination therapy with nateglinide and metformin in treatment-naïve patients with type 2 diabetes. Curr. Med. Res. Opin. 2004, 20, 883–889. [Google Scholar] [CrossRef]
- Manzella, D.; Grella, R.; Esposito, K.; Giugliano, D.; Barbagallo, M.; Paolisso, G. Blood pressure and cardiac autonomic nervous system in obese type 2 diabetic patients: Effect of metformin administration. Am. J. Hypertens. 2004, 17, 223–227. [Google Scholar] [CrossRef]
- Del Prato, S.; Erkelens, D.W.; Leutenegger, M. Six-month efficacy of benfluorex vs. placebo or metformin in diet-failed type 2 diabetic patients. Acta Diabetol. 2003, 40, 20–27. [Google Scholar] [CrossRef]
- Chiasson, J.-L.; Naditch, L.; for the Miglitol Canadian University Investigator Group. The Synergistic Effect of Miglitol Plus Metformin Combination Therapy in the Treatment of Type 2 Diabetes. Diabetes Care 2001, 24, 989–994. [Google Scholar] [CrossRef] [Green Version]
- Mather, K.J.; Verma, S.; Anderson, T.J. Improved endothelial function with metformin in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2001, 37, 1344–1350. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.T.; Han, Y.Q.; Wang, J.Y.; Li, W.J.; Gu, Y.; Shi, Y. Effects of Glucophage on insulin resistance in type 2 diabetes mellitus patients. J. Tongji Univ. (Med. Sci.) 2001, 22, 31–32+35. [Google Scholar]
- Lee, A.; Morley, J.E. Metformin Decreases Food Consumption and Induces Weight Loss in Subjects with Obesity with Type II Non-Insulin-Dependent Diabetes. Obes. Res. 1998, 6, 47–53. [Google Scholar] [CrossRef]
- Hoffmann, J.; Spengler, M. Efficacy of 24-Week Monotherapy with Acarbose, Metformin, or Placebo in Dietary-Treated NIDDM Patients: The Essen-II Study. Am. J. Med. 1997, 103, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.L.; Xian, B. Effect of pioglitazone and metformin on patients with incipient type 2 diabetes and nonalcoholic fatty liver disease. Chin. Baby 2020, 4, 122. [Google Scholar]
- Yang, Y. Application study of metformin combined with individualized diet and exercise therapy in elderly patients with type 2 diabetes. Diet Health 2019, 6, 45–46. [Google Scholar]
- Han, S.Q.; Liu, R.; Liu, J.X.; Geng, Y.Q. Effects of metformin on hepatic inflammation, liver fibrosis and insulin resistance in patients with type 2 diabetes mellitus complicated with nonalcoholic fatty liver disease. Hebei Med. J. 2018, 40, 1769–1773. [Google Scholar]
- Zhou, R.F.; Zhong, S.; Guo, Y.; Sun, Y.J.; Shao, Z.X. Impact of exenatide combined with metformin on islet function in new-onset type 2 diabetes mellitus. Clin. Focus 2017, 32, 965–968. [Google Scholar]
- Bai, N. The efficacy and side effects of metformin in the treatment of type 2 diabetes mellitus complicated with nonalcoholic fatty liver disease. China Health Stand. Manag. 2016, 7, 78–80. [Google Scholar]
- Geng, N.; Qi, H.; Liu, J.Q. Efficacy of rosuvastatin in male patients with newly diagnosed type 2 diabetes and hyperlipidemia and its effect on serum vaspin level. Hebei Med. J. 2016, 38, 2775–2777, 2781. [Google Scholar]
- Guo, D.L. Analysis of the efficacy of metformin in the treatment of type 2 diabetes. Diabetes New World 2014, 11, 1–2. [Google Scholar]
- Guo, W.; Gao, M.S.; Ye, Z.H.; Li, Y.; Tu, J.J.; Lei, W.M. Effects of metformin on the serum nesfatin-1 and liver steatosis in type 2 diabetic patients with nonalcoholic fatty liver disease. Chin. J. Difficult Complicat. Cases 2014, 13, 374–377. [Google Scholar]
- Li, L.; Zhu, K.S.; Qu, J.C.; Xia, A.X.; Zhang, W. Clinical effect of liraglutide and metformin hydrochioride on overweight diabetic patients with poor glycemic control. Clin. Med. China 2014, 30, 67–69. [Google Scholar]
- Esteghamati, A.; Eskandari, D.; Mirmiranpour, H.; Noshad, S.; Mousavizadeh, M.; Hedayati, M.; Nakhjavani, M. Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: A randomized clinical trial. Clin. Nutr. 2013, 32, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Hu, B.; Jian, L.; Li, J.W.; Huang, L. Influence of metformin on the level of serum vaspin in T2DM patients. Chin. J. Diabetes 2013, 21, 705–708. [Google Scholar]
- Xi, Y. Efficacy of liraglutide in patients with new-onset type 2 diabetes and obesity and its impact on patients’ micro-inflammation. Chin. Gen. Pract. 2013, 16, 3339–3340, 3345. [Google Scholar]
- Yang, W.C.; Dang, Y.; Qiao, L.; Wang, H.; Li, C.Q.; Dang, X.Y. Comparison of efficacy of exercise therapy and metformin in patients with newly diagnosed type 2 diabetes. J. Henan Norm. Univ. (Nat. Sci. Ed.) 2013, 41, 122–126. [Google Scholar]
- Qu, J.C.; Zhu, K.S.; Wang, T.; Li, L.; Zhao, L. Effect of rosiglitazone and metformin on lipid metabolism in patients with type 2 diabetes. China Med. 2011, 6, 286–287. [Google Scholar]
- Nar, A.; Gedik, O. The effect of metformin on leptin in obese patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Geol. Rundsch. 2009, 46, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Study on the Plasma Visfatin Level in Newly Diagnosed Obese Type 2 Diabetes Patients and the Influence of Pioglitazone on It; Guangdong Medical University: Zhanjiang, China, 2008. [Google Scholar]
- Cao, Y. Clinical Study on Multiple Factor Interventions in Improving the Insulin Resistance in Type 2 Diabetes; Shandong University of Traditional Chinese Medicine: Jinan, China, 2007. [Google Scholar]
- Deng, H.O.; Lin, K.; Li, D.F.; Li, Y.L. Effect of metformin on serum testosterone in male patients with type 2 diabetes. Guangdong Med. J. 2007, 28, 601–602. [Google Scholar]
- Li, H.Z.; Zhang, C.; Wang, M.; Liu, X.H. Effect of metformin and rosiglitazone maleate on lipid metabolism in patients with type 2 diabetes. J. Clin. Res. 2007, 24, 319–321. [Google Scholar]
- Mei, Q. Therapeutic effect of metformin on type 2 diabetes mellitus complicated with nonalcoholic fatty liver disease. Pract. Pharm. Clin. Remedies 2006, 9, 346–347. [Google Scholar]
- Hanefeld, M.; Haffner, S.; Menschikowski, M.; Koehler, C.; Temelkova-Kurktschiev, T.; Wildbrett, J.; Fischer, S. Different effects of acarbose and glibenclamide on proinsulin and insulin profiles in people with Type 2 diabetes. Diabetes Res. Clin. Pract. 2002, 55, 221–227. [Google Scholar] [CrossRef]
- Birkeland, K.I.; Furuseth, K.; Melander, A.; Mowinckel, P.; Vaaler, S. Long-Term Randomized Placebo-Controlled Double-Blind Therapeutic Comparison of Glipizide and Glyburide: Glycemic control and insulin secretion during 15 months. Diabetes Care 1994, 17, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Takami, K.; Takeda, N.; Nakashima, K.; Takami, R.; Hayashi, M.; Ozeki, S.; Yamada, A.; Kokubo, Y.; Sato, M.; Kawachi, S.-I.; et al. Effects of Dietary Treatment Alone or Diet with Voglibose or Glyburide on Abdominal Adipose Tissue and Metabolic Abnormalities in Patients with Newly Diagnosed Type 2 Diabetes. Diabetes Care 2002, 25, 658–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaku, K.; Araki, T.; Yoshinaka, R. Randomized, Double-Blind, Dose-Ranging Study of TAK-875, a Novel GPR40 Agonist, in Japanese Patients with Inadequately Controlled Type 2 Diabetes. Diabetes Care 2013, 36, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bautista, J.L.; Bugos, C.; Dirnberger, G.; Atherton, T. Efficacy and safety profile of glimepiride in Mexican American Patients with type 2 diabetes mellitus: A randomized, placebo-controlled study. Clin. Ther. 2003, 25, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.X.; Wu, Q.; Zhou, L.L. Efficacy of simvastatin in treatment of patients with type 2 diabetes and dyslipidemia and its effect on serum vaspin level. Chin. J. Gerontol. 2014, 34, 6588–6589. [Google Scholar]
- Wei, J.C.; Ruan, D.J.; Meng, X.Z.; Qu, S.H.; Zheng, W.W. Effect of glipizide on blood glucose and blood lipids in patients with type 2 diabetes. Prog. Mod. Biomed. 2010, 10, 3067–3069. [Google Scholar]
- Scott, R.; Wu, M.; Sanchez, M.; Stein, P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int. J. Clin. Pract. 2007, 61, 171–180. [Google Scholar] [CrossRef]
- Simonson, D.C.; Kourides, I.A.; Feinglos, M.; Shamoon, H.; Fischette, C.T.; Aronoff, S.; Blonde, L.; Cefalu, W.; Clement, S.; Greenberg, C.; et al. Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemic control and insulin secretion in NIDDM-Results of two multicenter, randomized, placebo-controlled clinical trials. Diabetes Care 1997, 20, 597–606. [Google Scholar] [CrossRef]
- Gul, O.O.; Tuncel, E.; Yilmaz, Y.; Ulukaya, E.; Gul, C.B.; Kiyici, S.; Oral, A.Y.; Guclu, M.; Ersoy, C.; Imamoglu, S. Comparative effects of pioglitazone and rosiglitazone on plasma levels of soluble receptor for advanced glycation end products in type 2 diabetes mellitus patients. Metabolism 2010, 59, 64–69. [Google Scholar] [CrossRef]
- Rahman, S.; Ismail, A.A.S.; Ismail, S.B.; Naing, N.N.; Rahman, A.R.A. Effect of rosiglitazone and ramipril on macrovasculopathy in patients with type 2 diabetes: Needs longer treatment and/or higher doses? Clin. Pharmacol. 2010, 2, 83–87. [Google Scholar] [CrossRef]
- Oz, O.; Tuncel, E.; Eryilmaz, S.; Fazlioglu, M.; Gul, C.B.; Ersoy, C.; Ocak, N.; Dirican, M.; Cangur, S.; Baran, I.; et al. Arterial elasticity and plasma levels of adiponectin and leptin in type 2 diabetic patients treated with thiazolidinediones. Endocrine 2008, 33, 101–105. [Google Scholar] [CrossRef]
- Albertini, J.-P.; McMorn, S.O.; Chen, H.; Mather, R.A.; Valensi, P. Effect of rosiglitazone on factors related to endothelial dysfunction in patients with type 2 diabetes mellitus. Atherosclerosis 2007, 195, e159–e166. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Miyazaki, Y.; Pettiti, M.; Santini, E.; Ciociaro, D.; DeFronzo, R.A.; Ferrannini, E. The Effect of Rosiglitazone on the Liver: Decreased Gluconeogenesis in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 806–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.Y.; Zhang, Y.; Wu, Y. Effects of rosiglitazone on C-reactive protein in patients with type 2 diabetes. J. Jilin Univ. (Med. Ed.) 2004, 30, 465–467. [Google Scholar]
- Juhl, C.B.; Hollingdal, M.; Pørksen, N.; Prange, A.; Lönnqvist, F.; Schmitz, O. Influence of Rosiglitazone Treatment on β-Cell Function in Type 2 Diabetes: Evidence of an Increased Ability of Glucose to Entrain High-Frequency Insulin Pulsatility. J. Clin. Endocrinol. Metab. 2003, 88, 3794–3800. [Google Scholar] [CrossRef] [Green Version]
- Carey, D.G.; Cowin, G.J.; Galloway, G.J.; Jones, N.P.; Richards, J.C.; Biswas, N.; Doddrell, D.M. Effect of rosiglitazone on insulin sensitivity and body composition in type 2 diabetic patients [corrected]. Obes. Res. 2002, 10, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Greenberg, A.S.; Weston, W.M.; Chen, H.; Williams, K.; Freed, M.I. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation 2002, 106, 679–684. [Google Scholar] [CrossRef] [Green Version]
- Lebovitz, H.E.; Dole, J.F.; Patwardhan, R.; Rappaport, E.B.; Freed, M.I. Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2001, 86, 280–288. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Glass, L.; Triplitt, C.; Matsuda, M.; Cusi, K.; Mahankali, A.; Mahankali, S.; Mandarino, L.J.; DeFronzo, R.A. Effect of rosiglitazone on glucose and non-esterified fatty acid metabolism in Type II diabetic patients. Diabetologia 2001, 44, 2210–2219. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.; Anderson, R.J.; Rappaport, E.B. Rosiglitazone monotherapy improves glycaemic control in patients with type 2 diabetes: A twelve-week, randomized, placebo-controlled study. Diabetes Obes. Metab. 1999, 1, 165–172. [Google Scholar] [CrossRef]
- Huang, D.; Wei, X.; Li, Z.Y.; Qu, W.J.; Wang, C.P.; Chen, Y.M.; Chen, D.Z. Effect of rosiglitazone on serum SPARC in patients with newly diagnosed type 2 diabetes mellitus. Mod. Med. Health 2016, 32, 1284–1286, 1289. [Google Scholar]
- Berberoglu, Z.; Yazici, A.C.; Demirag, N.G. ORIGINAL ARTICLE: Effects of rosiglitazone on bone mineral density and remodelling parameters in Postmenopausal diabetic women: A 2-year follow-up study. Clin. Endocrinol. 2010, 73, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.H.; Feng, Y.X.; Wu, X.M.; Bian, Q.X.; Zhang, J.H. Effect of aerobic exercise on blood glucose and blood lipids in obese patients with type 2 diabetes. China Foreign Med. Treat. 2009, 28, 53–54. [Google Scholar]
- Zhu, K.S.; Wang, P. Effect of rosiglitazone on type 2 diabetes with postprandial hypoglycemia as the first symptom. J. Pract. Diabetol. 2009, 5, 46–47. [Google Scholar]
- Berberoglu, Z.; Gursoy, A.; Bayraktar, N.; Yazici, A.C.; Tutuncu, N.B.; Demirag, N.G. Rosiglitazone Decreases Serum Bone-Specific Alkaline Phosphatase Activity in Postmenopausal Diabetic Women. J. Clin. Endocrinol. Metab. 2007, 92, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Clinical comparison of pioglitazone hydrochloride in the treatment of patients with incipient type 2 diabetes. China Health Vis. 2020, 1, 100–101. [Google Scholar]
- Bajpeyi, S.; Pasarica, M.; Conley, K.E.; Newcomer, B.R.; Jubrias, S.A.; Gamboa, C.; Murray, K.; Sereda, O.; Sparks, L.M.; Smith, S.R. Pioglitazone-induced improvements in insulin sensitivity occur without concomitant changes in muscle mitochondrial function. Metabolism 2017, 69, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Q. Observation on the effect of pioglitazone hydrochloride monotherapy in the treatment of elderly patients with incipient type 2 diabetes. Chin. J. Clin. Ration. Drug Use 2017, 10, 71–72. [Google Scholar]
- Sykes, A.P.; Kemp, G.L.; Dobbins, R.; O’Connor-Semmes, R.; Almond, S.R.; Wilkison, W.O.; Walker, S.; Kler, L. Randomized efficacy and safety trial of once-daily remogliflozin etabonate for the treatment of type 2 diabetes. Diabetes Obes. Metab. 2015, 17, 98–101. [Google Scholar] [CrossRef]
- Sykes, A.P.; O’Connor-Semmes, R.; Dobbins, R.; Dorey, D.J.; Lorimer, J.D.; Walker, S.; Wilkison, W.O.; Kler, L. Randomized trial showing efficacy and safety of twice-daily remogliflozin etabonate for the treatment of type 2 diabetes. Diabetes Obes. Metab. 2015, 17, 94–97. [Google Scholar] [CrossRef]
- Chou, H.S.; Truitt, K.E.; Moberly, J.B.; Merante, D.; Choi, Y.; Mun, Y.; Pfützner, A. A 26-week, placebo- and pioglitazone-controlled monotherapy study of rivoglitazone in subjects with type 2 diabetes mellitus. Diabetes Obes. Metab. 2012, 14, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.P.S.; Yamasaki, A.; Ozaki, R.; Saito, H.; Asami, T.; Ohwada, S.; Ko, G.T.C.; Wong, C.K.; Leung, G.T.C.; Lee, K.F.; et al. A randomized-controlled trial to investigate the effects of rivoglitazone, a novel PPAR gamma agonist on glucose-lipid control in type 2 diabetes. Diabetes Obes. Metab. 2011, 13, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Clinical observation of pioglitazone hydrochloride on 136 patients with incipient type 2 diabetes. China Medical Herald. 2009, 6, 58–59. [Google Scholar]
- Gastaldelli, A.; Casolaro, A.; Pettiti, M.; Nannipieri, M.; Ciociaro, D.; Frascerra, S.; Buzzigoli, E.; Baldi, S.; Mari, A.; Ferrannini, E. Effect of Pioglitazone on the Metabolic and Hormonal Response to a Mixed Meal in Type II Diabetes. Clin. Pharmacol. Ther. 2007, 81, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.J.; Rosenstock, J.; Anzalone, D.; Tou, C.; Ohman, K.P. Effect of tesaglitazar, a dual PPAR alpha/gamma agonist, on glucose and lipid abnormalities in patients with type 2 diabetes: A 12-week dose-ranging trial. Curr. Med. Res. Opin. 2006, 22, 2575–2590. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.R.; Han, Q.W.; Yuan, W.T.; He, X.J. Effects of pioglitazone on elderly patients with type 2 diabetes mellitus. Med. J. Wuhan Univ. 2006, 27, 104–107. [Google Scholar]
- Khan, M.; Murray, F.T.; Karunaratne, M.; Perez, A. Pioglitazone and reductions in post-challenge glucose levels in patients with type 2 diabetes. Diabetes Obes. Metab. 2006, 8, 31–38. [Google Scholar] [CrossRef]
- Pang, W.Y.; Liu, Y.; Zhao, C.Y. Pioglitazone monotherapy for elderly patients with new-onset type 2 diabetes mellitus. Cent. Plains Med. J. 2006, 33, 6–7. [Google Scholar]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. An increase in insulin sensitivity and basal beta-cell fuction in diabetic subjects treated with pioglitazone in a placebo-controlled randomized study. Diabet. Med. 2004, 21, 568–576. [Google Scholar] [CrossRef]
- Liu, C.M. Observation of clinical effects of pioglitazone hydrochlodde in treatment of type 2 diabetic patients. J. Med. Forum. 2003, 24, 5–6. [Google Scholar]
- Miyazaki, Y.; Matsuda, M.; DeFronzo, R.A. Dose-Response Effect of Pioglitazone on Insulin Sensitivity and Insulin Secretion in Type 2 Diabetes. Diabetes Care 2002, 25, 517–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherbaum, W.A.; Göke, B.; and the German Pioglitazone Study Group. Metabolic Efficacy and Safety of Once-Daily Pioglitazone Monotherapy in Patients with Type 2 Diabetes: A Double-Blind, Placebo-Controlled Study. Horm. Metab. Res. 2002, 34, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Aronoff, S.; Rosenblatt, S.; Braithwaite, S.; Egan, J.W.; Mathisen, A.L.; Schneider, R.L. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: A 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care 2000, 23, 1605–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirmiranpour, H.; Mousavizadeh, K.; Noshad, S.; Ghavami, M.; Ebadi, M.; Ghasemiesfe, M.; Nakhjavani, M.; Esteghamati, A. Comparative effects of pioglitazone and metformin on oxidative stress markers in newly diagnosed type 2 diabetes patients: A randomized clinical trial. J. Diabetes Its Complicat. 2013, 27, 501–507. [Google Scholar] [CrossRef]
- Bech, P.; Moses, R.; Gomis, R. The effect of prandial glucose regulation with repaglinide on treatment satisfaction, wellbeing and health status in patients with pharmacotherapy-naïve type 2 diabetes: A placebo-controlled, multicentre study. Qual. Life Res. 2003, 12, 413–425. [Google Scholar] [CrossRef]
- Xu, L. Efficacy of repaglinide on 31 patients with type 2 diabetes. J. Aerosp. Med. 2011, 22, 458–459. [Google Scholar]
- Zhong, X.Y.; Lv, L.L.; Chen, Z.H.; Xu, Z.H. The effect of repaglinide on 31 patients with type 2 diabetes. Guide China Med. 2010, 8, 200–201. [Google Scholar]
- Guo, X.D. Efficacy of repaglinide on 31 patients with type 2 diabetes. Guide China Med. 2009, 7, 83–84. [Google Scholar]
- Zhao, W.; Zhuang, L.F.; Peng, L.Y.; Luo, J.L. Observation on the efficacy of repaglinide in treatment of type 2 diabetes. World Health Dig. 2008, 5, 71–73. [Google Scholar]
- González-Clemente, J.-M.; on behalf of the Spanish Nateglinide Study Group. Improvement of glycaemic control by nateglinide decreases systolic blood pressure in drug-naive patients with type 2 diabetes. Eur. J. Clin. Investig. 2008, 38, 174–179. [Google Scholar] [CrossRef]
- Schwarz, S.L.; Gerich, J.E.; Marcellari, A.; Jean-louis, L.; Purkayastha, D.; Baron, M.A. Nateglinide, alone or in combination with metformin, is effective and well tolerated in treatment-naïve elderly patients with type 2 diabetes. Diabetes Obes. Metab. 2008, 10, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Gastaldelli, A.; Foley, J.E.; Pratley, R.E.; Ferrannini, E. β-Cell function in mild type 2 diabetic patients: Effects of 6-month glucose lowering with nateglinide. Diabetes Care 2005, 28, 1132–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Inoue, T.; Node, K. Postprandial endothelial dysfunction in subjects with new-onset type 2 diabetes: An acarbose and nateglinide comparative study. Cardiovasc. Diabetol. 2010, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Kirkman, M.S.; Shankar, R.R.; Shankar, S.; Shen, C.; Brizendine, E.; Baron, A.; McGill, J. Treating postprandial hyperglycemia does not appear to delay progression of early type 2 diabetes: The Early Diabetes Intervention Program. Diabetes Care 2006, 29, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Josse, R.G.; Chiasson, J.-L.; Ryan, E.A.; Lau, D.C.W.; Ross, S.A.; Yale, J.-F.; Leiter, L.A.; Maheux, P.; Tessier, D.; Wolever, T.M.S.; et al. Acarbose in the treatment of elderly patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2003, 59, 37–42. [Google Scholar] [CrossRef]
- Wu, G.T.; Han, Y.Q.; Yu, Y.C.; Teng, X.H.; Zhang, J.C.; Qu, F.J. Influence of acarbose on insulin resistant of patients with type 2 diabetes mellitus. Chin. J. New Drugs Clin. Remedies 2003, 22, 535–538. [Google Scholar]
- Meneilly, G.S.; Ryan, E.A.; Radziuk, J.; Lau, D.C.; Yale, J.F.; Morais, J.; Chiasson, J.L.; Rabasa-Lhoret, R.; Maheux, P.; Tessier, D.; et al. Effect of acarbose on insulin sensitivity in elderly patients with diabetes. Diabetes Care 2000, 23, 1162–1167. [Google Scholar] [CrossRef] [Green Version]
- Scott, R.; Lintott, C.J.; Zimmet, P.; Campbell, L.; Bowen, K.; Welborn, T. Will acarbose improve the metabolic abnormalities of insulin-resistant type 2 diabetes mellitus? Diabetes Res. Clin. Pract. 1999, 43, 179–185. [Google Scholar] [CrossRef]
- Chan, J.C.; Chan, K.-W.A.; Ho, L.L.; MC Fuh, M.; Horn, L.C.; Sheaves, R.; Panelo, A.A.; Kim, D.-K.; Embong, M. For the Asian Acarbose Study Group An Asian Multicenter Clinical Trial to Assess the Efficacy and Tolerability of Acarbose Compared with Placebo in Type 2 Diabetic Patients Previously Treated with Diet. Diabetes Care 1998, 21, 1058–1061. [Google Scholar] [CrossRef]
- Fischer, S.; Hanefeld, M.; Spengler, M.; Boehme, K.; Temelkova-Kurktschiev, T. European study on dose-response relationship of acarbose as a first-line drug in non-insulin-dependent diabetes mellitus: Efficacy and safety of low and high doses. Geol. Rundsch. 1998, 35, 34–40. [Google Scholar] [CrossRef]
- Braun, D.; Schönherr, U.; Mitzkat, H.J. Efficacy of acarbose monotherapy in patients with type 2 diabetes: A double-blind study conducted in general practice. Endocrinol. Metab. Suppl. 1996, 3, 275–280. [Google Scholar]
- Hou, W.K.; Zhang, B.Z.; Xu, J.; Jiang, B.; Shi, Z.C. A double-blind controlled study of glucobay in the treatment of NIDDM. Chin. J. Diabetes 1996, 4, 56–59. [Google Scholar]
- Zheng, G.F.; Wang, J.P.; Zhang, H.; Hu, Z.X.; Liu, J.; Xiao, J.Z.; Chen, S.M.; Cao, H.B.; Li, G.W.; Hu, Y.H.; et al. The clinical investigation of the effect of acarbose on non-insulin-dependent diabetics. Chin. J. Endocrinol. Metab. 1995, 11, 163–164, 192. [Google Scholar]
- Hotta, N.; Kakuta, H.; Sano, T.; Matsumae, H.; Yamada, H.; Kitazawa, S.; Sakamoto, N. Long-term Effect of Acarbose on Glycaemic Control in Non-insulin-dependent Diabetes Mellitus: A Placebo-controlled Double-blind Study. Diabet. Med. 1993, 10, 134–138. [Google Scholar] [CrossRef]
- Santeusanio, F.; Ventura, M.M.; Contadini, S.; Compagnucci, P.; Moriconi, V.; Zaccarini, P.; Marra, G.; Amigoni, S.; Bianchi, W.; Brunetti, P. Efficacy and safety of two different dosages of acarbose in non-insulin dependent diabetic patients treated by diet alone. Diabetes Nutr. Metab.-Clin. Exp. 1993, 6, 147–154. [Google Scholar]
- Hanefeld, M.; Fischer, S.; Schulze, J.; Spengler, M.; Wargenau, M.; Schollberg, K.; Fücker, K. Therapeutic Potentials of Acarbose as First-Line Drug in NIDDM Insufficiently Treated with Diet Alone. Diabetes Care 1991, 14, 732–737. [Google Scholar] [CrossRef]
- Chen, D.L.; Han, C.K.; Zhang, H.J. Evaluation of clinical effect of acarbose in the treatment of type 2 diabetes complicated with nonalcoholic fatty liver disease. Chin. Foreign Med. Res. 2017, 15, 123–124. [Google Scholar]
- Yang, L.H. Clinical efficacy of acarbose in the treatment of patients with type 2 diabetes combined with nonalcoholic fatty liver disease. Guide China Med. 2015, 13, 17–18. [Google Scholar]
- Wu, H.X.; Li, J.X. Efficacy of acarbose on type 2 diabetes complicated with nonalcoholic fatty liver disease. J. Pract. Diabetol. 2014, 10, 32–33. [Google Scholar]
- Yang, Y.; Xing, L.F.; Wang, A.M. Clinical study for the influence of acarbose on TNF-α and IL-6 on type 2 diabetes mellitus. Chin. J. Pract. Med. 2014, 41, 8–10. [Google Scholar]
- Hao, H.R.; Hu, W.; Yu, W.N. Platelet CD62P expression in early type 2 diabetes and the effect of acarbose. Shandong Med. J. 2012, 52, 50–52. [Google Scholar]
- Ye, X.H.; Yan, X.D.; Liang, S.; Lao, D.H. Efficacy of domestic acarbose on new-onset type 2 diabetes. Guangxi Med. J. 2009, 31, 963–964. [Google Scholar]
- Seino, Y.; Fujita, T.; Hiroi, S.; Hirayama, M.; Kaku, K. Efficacy and safety of alogliptin in Japanese patients with type 2 diabetes mellitus: A randomized, double-blind, dose-ranging comparison with placebo, followed by a long-term extension study. Curr. Med. Res. Opin. 2011, 27, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.G.; Ling, H.W. Clinical effect of voglibose in the treatment of type 2 diabetes mellitus. Acta Acad. Med. Xuzhou 2001, 21, 311–313. [Google Scholar]
- Drent, M.L.; Tollefsen, A.T.M.; Van Heusden, F.H.J.A.; Hoenderdos, E.B.M.; Jonker, J.J.C.; Van Der Veen, E.A. Dose-dependent efficacy of miglitol, an alpha-glucosidase inhibitor, in type 2 diabetic patients on diet alone: Results of a 24-week double-blind placebo-controlled study. Diabetes Nutr. Metab.-Clin. Exp. 2002, 15, 152–159. [Google Scholar]
- Gantz, I.; Okamoto, T.; Ito, Y.; Okuyama, K.; O’Neill, E.A.; Kaufman, K.D.; Engel, S.S.; Lai, E.; and the Omarigliptin Study 020 Group. A randomized, placebo- and sitagliptin-controlled trial of the safety and efficacy of omarigliptin, a once-weekly dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes. Diabetes Obes. Metab. 2017, 19, 1602–1609. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Liang, Z.; Liu, R.; Li, K.; Tan, X.; Luo, Y.; Yang, M.; Gu, H.F.; Liu, H.; Li, L.; et al. Effects of sitagliptin on circulating zinc-α2-glycoprotein levels in newly diagnosed type 2 diabetes patients: A randomized trial. Eur. J. Endocrinol. 2016, 174, 147–155. [Google Scholar] [CrossRef]
- Roden, M.; on behalf of the EMPA-REG EXTEND™ MONO investigators; Merker, L.; Christiansen, A.V.; Roux, F.; Salsali, A.; Kim, G.; Stella, P.; Woerle, H.J.; Broedl, U.C. Safety, tolerability and effects on cardiometabolic risk factors of empagliflozin monotherapy in drug-naïve patients with type 2 diabetes: A double-blind extension of a Phase III randomized controlled trial. Cardiovasc. Diabetol. 2015, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Roden, M.; Weng, J.; Eilbracht, J.; Delafont, B.; Kim, G.; Woerle, H.J.; Broedl, U.C. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013, 1, 208–219. [Google Scholar] [CrossRef]
- Sun, J.; Li, W. Effect of sitagliptin on insulin resistance in patients with type 2 diabetes. Chin. J. Gerontol. 2013, 33, 1886–1887. [Google Scholar]
- Barzilai, N.; Guo, H.; Mahoney, E.M.; Caporossi, S.; Golm, G.T.; Langdon, R.B.; Williams-Herman, D.; Kaufman, K.D.; Amatruda, J.M.; Goldstein, B.J.; et al. Efficacy and tolerability of sitagliptin monotherapy in elderly patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Curr. Med. Res. Opin. 2011, 27, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Taniguchi, T.; Nonaka, K.; Okamoto, T.; Okuyama, K.; Arjona, F.J.C.; Amatruda, J. Dose-ranging efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Endocr. J. 2010, 57, 383–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, V.; Yang, W.; Son, H.-Y.; Xu, L.; Noble, L.; Langdon, R.B.; Amatruda, J.M.; Stein, P.P.; Kaufman, K.D. Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes Res. Clin. Pract. 2009, 83, 106–116. [Google Scholar] [CrossRef]
- Nonaka, K.; Kakikawa, T.; Sato, A.; Okuyama, K.; Fujimoto, G.; Kato, N.; Suzuki, H.; Hirayama, Y.; Ahmed, T.; Davies, M.J.; et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2008, 79, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, M.; Herman, G.A.; Wu, M.; Mickel, C.; Sanchez, M.; Stein, P.P. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr. Med. Res. Opin. 2007, 23, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Aschner, P.; Kipnes, M.S.; Lunceford, J.K.; Sanchez, M.; Mickel, C.; Williams-Herman, D.E. Effect of the Dipeptidyl Peptidase-4 Inhibitor Sitagliptin as Monotherapy on Glycemic Control in Patients with Type 2 Diabetes. Diabetes Care 2006, 29, 2632–2637. [Google Scholar] [CrossRef] [Green Version]
- Raz, I.; Hanefeld, M.; Xu, L.; Caria, C.; Williams-Herman, D.; Khatami, H.; Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006, 49, 2564–2571. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Ma, R.; Zhu, H.; Zhu, J. A randomized-controlled study of sitagliptin for treating diabetes mellitus complicated by nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2017, 29, 297–301. [Google Scholar] [CrossRef]
- Zhang, J.B.; Lu, Y.Z.; Fan, M.S. Efficacy analysis of saxagliptin treatment of new-onset type 2 diabetes. Guide China Med. 2016, 14, 9+12. [Google Scholar]
- Xiu, S.L.; Wang, L. Efficacy and safety of saxagliptin in newly diagnosed type 2 diabetes mellitus patients. China Med. 2015, 10, 352–354. [Google Scholar]
- Han, B.Y.; Lu, L.M.; Zhang, L.P.; Fan, Z.Y. Effect of DPP-4 inhibitor saxagliptin on endothelium-dependent vasodilation in patients with type 2 diabetes. J. Shanxi Med. Univ. 2014, 45, 291–294. [Google Scholar]
- Prasanna Kumar, K.M.; Jain, S.M.; Tou, C.; Schutzer, K.-M. Saxagliptin as initial therapy in treatment-naive Indian adults with type 2 diabetes mellitus inadequately controlled with diet and exercise alone: A randomized, double-blind, placebo-controlled, phase IIIb clinical study. Int. J. Diabetes Dev. Ctries. 2014, 34, 201–209. [Google Scholar] [CrossRef]
- Wu, Y.F. Evaluation of the Efficacy of Saxagliptin Monotherapy in Incipient Type 2 Diabetes; Zhejiang University: Hangzhou, China, 2013. [Google Scholar]
- Frederich, R.; McNeill, R.; Berglind, N.; Fleming, D.; Chen, R. The efficacy and safety of the dipeptidyl peptidase-4 inhibitor saxagliptin in treatment-naïve patients with type 2 diabetes mellitus: A randomized controlled trial. Diabetol. Metab. Syndr. 2012, 4, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, C.Y.; Tou, C.; Gause-Nilsson, I.; Yang, W.; Zhao, J. Efficacy and safety of saxagliptin in drug-naïve Asian patients with type 2 diabetes mellitus: A randomized controlled trial. Diabetes/Metab. Res. Rev. 2012, 28, 268–275. [Google Scholar] [CrossRef]
- Rosenstock, J.; Aguilar-Salinas, C.; Klein, E.; Nepal, S.; List, J.; Chen, R.; for the CV181-011 Study Investigators. Effect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetes. Curr. Med. Res. Opin. 2009, 25, 2401–2411. [Google Scholar] [CrossRef]
- Rosenstock, J.; Sankoh, S.; List, J.F. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes. Metab. 2008, 10, 376–386. [Google Scholar] [CrossRef]
- Mari, A.; Scherbaum, W.A.; Nilsson, P.M.; Lalanne, G.; Schweizer, A.; Dunning, B.E.; Jauffret, S.; Foley, J.E. Characterization of the Influence of Vildagliptin on Model-Assessed β-Cell Function in Patients with Type 2 Diabetes and Mild Hyperglycemia. J. Clin. Endocrinol. Metab. 2008, 93, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Scherbaum, W.A.; Schweizer, A.; Mari, A.; Nilsson, P.M.; Lalanne, G.; Jauffret, S.; Foley, J.E. Efficacy and tolerability of vildagliptin in drug-naïve patients with type 2 diabetes and mild hyperglycaemia. Diabetes Obes. Metab. 2008, 10, 675–682. [Google Scholar] [CrossRef]
- Scherbaum, W.A.; Schweizer, A.; Mari, A.; Nilsson, P.M.; Lalanne, G.; Wang, Y.; Dunning, B.E.; Foley, J.E. Evidence that vildagliptin attenuates deterioration of glycaemic control during 2-year treatment of patients with type 2 diabetes and mild hyperglycaemia. Diabetes Obes. Metab. 2008, 10, 1114–1124. [Google Scholar] [CrossRef]
- Dejager, S.; Razac, S.; Foley, J.E.; Schweizer, A. Vildagliptin in drug-naïve patients with type 2 diabetes: A 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm. Metab. Res. 2007, 39, 218–223. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X.; Schweizer, A.; Mills, D.; Dejager, S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2007, 76, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Pratley, R.E.; Jauffret-Kamel, S.; Galbreath, E.; Holmes, D. Twelve-week Monotherapy with the DPP-4 Inhibitor Vildagliptin Improves Glycemic Control in Subjects with Type 2 Diabetes. Horm. Metab. Res. 2006, 38, 423–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ristić, S.; Byiers, S.; Foley, J.; Holmes, D. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: Vildagliptin (LAF237) dose response. Diabetes Obes. Metab. 2005, 7, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.C. Efficacy of vildagliptin on newly diagnosed type 2 diabetes complicated with nonalcoholic fatty liver disease. J. Clin. Intern. Med. 2016, 33, 709–710. [Google Scholar]
- Lin, N.; Liu, H.B. Effect of linagliptin on lipid metabolism and islet resistance in patients with type 2 diabetes. Clin. Res. 2017, 25, 77–78, 80. [Google Scholar]
- Chen, Y.; Ning, G.; Wang, C.; Gong, Y.; Patel, S.; Zhang, C.; Izumoto, T.; Woerle, H.; Wang, W. Efficacy and safety of linagliptin monotherapy in Asian patients with inadequately controlled type 2 diabetes mellitus: A multinational, 24-week, randomized, clinical trial. J. Diabetes Investig. 2015, 6, 692–698. [Google Scholar] [CrossRef]
- Wu, W.; Li, Y.; Chen, X.; Lin, D.; Xiang, S.; Shen, F.; Gu, X. Effect of Linagliptin on Glycemic Control in Chinese Patients with Newly-Diagnosed, Drug-Naïve Type 2 Diabetes Mellitus: A Randomized Controlled Trial. J. Pharmacol. Exp. Ther. 2015, 21, 2678–2684. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.J.; Chen, X.; Shen, F.X.; Xiang, S.Y.; Lin, D.N.; Gu, X.M. Effects of linagliptin treatment on hyperglycemia and islet function in newly-diagnosed type 2 diabetic patients. Chin. J. New Drugs Clin. Remedies 2014, 33, 263–266. [Google Scholar]
- Barnett, A.H.; Patel, S.; Harper, R.; Toorawa, R.; Thiemann, S.; von Eynatten, M.; Woerle, H.-J. Linagliptin monotherapy in type 2 diabetes patients for whom metformin is inappropriate: An 18-week randomized, double-blind, placebo-controlled phase III trial with a 34-week active-controlled extension. Diabetes Obes. Metab. 2012, 14, 1145–1154. [Google Scholar] [CrossRef]
- Kawamori, R.; Inagaki, N.; Araki, E.; Watada, H.; Hayashi, N.; Horie, Y.; Sarashina, A.; Gong, Y.; Von Eynatten, M.; Woerle, H.J.; et al. Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: A randomized, placebo and active comparator-controlled, double-blind study. Diabetes Obes. Metab. 2012, 14, 348–357. [Google Scholar] [CrossRef]
- Del Prato, S.; Barnett, A.H.; Huisman, H.; Neubacher, D.; Woerle, H.J.; Dugi, K.A. Effect of linagliptin monotherapy on glycaemic control and markers of β-cell function in patients with inadequately controlled type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2011, 13, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Han, P.; Ji, Q.; Li, C.; Lu, J.; Yang, J.; Li, W.; Zeng, J.; Hsieh, A.-T.; Chan, J. Efficacy and safety of alogliptin in patients with type 2 diabetes mellitus: A multicentre randomized double-blind placebo-controlled Phase 3 study in mainland China, Taiwan, and Hong Kong. J. Diabetes 2017, 9, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, N.; Onouchi, H.; Maezawa, H.; Kuroda, S.; Kaku, K. Once-weekly trelagliptin versus daily alogliptin in Japanese patients with type 2 diabetes: A randomised, double-blind, phase 3, non-inferiority study. Lancet Diabetes Endocrinol. 2015, 3, 191–197. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Fleck, P.R.; Wilson, C.A.; Mekki, Q.; Group, A.S. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: A randomized, double-blind, placebo-controlled study. Diabetes Care 2008, 31, 2315–2317. [Google Scholar] [CrossRef] [PubMed]
- Kutoh, E.; Ukai, Y. Alogliptin as an initial therapy in patients with newly diagnosed, drug naïve type 2 diabetes: A randomized, control trial. Endocrine 2012, 41, 435–441. [Google Scholar] [CrossRef]
- Wang, W.Y. The clinical effect of dapagliflozin in the treatment of newly diagnosed type 2 diabetes. Electron. J. Clin. Med. Lit. 2020, 7, 159–160. [Google Scholar]
- Liao, X.; Wang, X.; Li, H.; Li, L.; Zhang, G.; Yang, M.; Yuan, L.; Liu, H.; Yang, G.; Gao, L. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitor Increases Circulating Zinc-A2-Glycoprotein Levels in Patients with Type 2 Diabetes. Sci. Rep. 2016, 6, 32887. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.; Ma, J.; Li, H.; Mansfield, T.A.; T’Joen, C.L.; Iqbal, N.; Ptaszynska, A.; List, J.F. Dapagliflozin as Monotherapy in Drug-Naive Asian Patients with Type 2 Diabetes Mellitus: A Randomized, Blinded, Prospective Phase III Study. Clin. Ther. 2014, 36, 84–100.e9. [Google Scholar] [CrossRef] [Green Version]
- Kaku, K.; Kiyosue, A.; Inoue, S.; Ueda, N.; Tokudome, T.; Yang, J.; Langkilde, A.M. Efficacy and safety of dapagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise. Diabetes Obes. Metab. 2014, 16, 1102–1110. [Google Scholar] [CrossRef]
- Liu, L.M.; Yang, Y.; Bao, M.J.; Li, P.Q.; Zhang, X.J.; Xian, Y.; Wu, J.C. Therapeutic effect of dapagliflozin on type 2 diabetes mellitus. J. Chin. Pract. Diagn. Ther. 2014, 28, 926–927, 929. [Google Scholar]
- Kaku, K.; Inoue, S.; Matsuoka, O.; Kiyosue, A.; Azuma, H.; Hayashi, N.; Tokudomé, T.; Langkilde, A.M.; Parikh, S. Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: A phase II multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 2013, 15, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Iqbal, N.; T’Joen, C.; List, J.F. Dapagliflozin monotherapy in drug-naïve patients with diabetes: A randomized-controlled trial of low-dose range. Diabetes Obes. Metab. 2012, 14, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Ramos, S.J.; Salsali, A.; Tang, W.; List, J.F. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: A randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2010, 33, 2217–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadowaki, T.; Haneda, M.; Inagaki, N.; Terauchi, Y.; Taniguchi, A.; Koiwai, K.; Rattunde, H.; Woerle, H.J.; Broedl, U.C. Empagliflozin Monotherapy in Japanese Patients with Type 2 Diabetes Mellitus: A Randomized, 12-Week, Double-Blind, Placebo-Controlled, Phase II Trial. Adv. Ther. 2014, 31, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, N.; Kondo, K.; Yoshinari, T.; Takahashi, N.; Susuta, Y.; Kuki, H. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: A 24-week, randomized, double-blind, placebo-controlled, Phase III study. Expert Opin. Pharmacother. 2014, 15, 1501–1515. [Google Scholar] [CrossRef]
- Inagaki, N.; Kondo, K.; Yoshinari, T.; Maruyama, N.; Susuta, Y.; Kuki, H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes. Metab. 2013, 15, 1136–1145. [Google Scholar] [CrossRef]
- Stenlöf, K.; Cefalu, W.T.; Kim, K.; Alba, M.; Usiskin, K.; Tong, C.; Canovatchel, W.; Meininger, G. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes. Metab. 2013, 15, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.H. Efficacy and Safety of Exenatide Treatment in Type 2 Diabetes Mellitus; Zhejiang University: Hangzhou, China, 2016. [Google Scholar]
- Gastaldelli, A.; Brodows, R.G.; D’Alessio, D. The effect of chronic twice daily exenatide treatment on β-cell function in new onset type 2 diabetes. Clin. Endocrinol. 2014, 80, 545–553. [Google Scholar] [CrossRef]
- Moretto, T.J.; Milton, D.R.; Ridge, T.D.; MacConell, L.A.; Okerson, T.; Wolka, A.M.; Brodows, R.G. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, parallel-group study. Clin. Ther. 2008, 30, 1448–1460. [Google Scholar] [CrossRef]
- Zhu, H.Q. Effect of Exenatide on Vascular Endothelial Function and Cardiovascular Disease Risk Factors in Patients with Type 2 Diabetes; Guangxi Medical University: Nanning, China, 2017. [Google Scholar]
- Yamada, Y.; Katagiri, H.; Hamamoto, Y.; Deenadayalan, S.; Navarria, A.; Nishijima, K.; Seino, Y.; Fukushima, Y.; Hisatomi, A.; Ide, Y.; et al. Dose-response, efficacy, and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): A 52-week, phase 2/3a, randomised, controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 377–391. [Google Scholar] [CrossRef]
- Nino, A.; Okuda, I.; Wilson, T.H.; Yue, L.; Nakajima, H.; Tsuboi, M.; Carr, M.C.; Seino, Y. Weekly glucagon-like peptide-1 receptor agonist albiglutide as monotherapy improves glycemic parameters in Japanese patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. J. Diabetes Investig. 2018, 9, 558–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, S.; Kramer, C.K.; Zinman, B.; Choi, H.; Retnakaran, R. Effect of chronic liraglutide therapy and its withdrawal on time to postchallenge peak glucose in type 2 diabetes. Am. J. Physiol. Metab. 2018, 314, E287–E295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyagawa, J.; Odawara, M.; Takamura, T.; Iwamoto, N.; Takita, Y.; Imaoka, T. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide is non-inferior to once-daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: A 26-week randomized phase III study. Diabetes Obes Metab. 2015, 17, 974–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.R.; Liang, Z.R.; Li, S.B.; Tang, S.G.; Fang, C.; Tang, L.H.; Pi, Y.; Qin, F.; Wang, Y. Effects of GLP-1 receptor agonist on Zinc-α2-glycoprotein and its relationship with insulin resistance in patients with newly diagnosed type 2 diabetes. J. Chongqing Med. Univ. 2015, 40, 1395–1400. [Google Scholar]
- Retnakaran, R.; Kramer, C.K.; Choi, H.; Swaminathan, B.; Zinman, B. Liraglutide and the Preservation of Pancreatic Beta-Cell Function in Early Type 2 Diabetes: The LIBRA Trial. Can. J. Diabetes 2014, 38, S4–S5. [Google Scholar] [CrossRef]
- Seino, Y.; Rasmussen, M.; Zdravkovic, M.; Kaku, K. Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: A double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2008, 81, 161–168. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Brock, B.; Perrild, H.; Levin, K.; Lervang, H.-H.; Kølendorf, K.; Krarup, T.; Schmitz, O.; Zdravkovic, M.; Le-Thi, T.; et al. Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with Type 2 diabetes mellitus. Diabet. Med. 2008, 25, 152–156. [Google Scholar] [CrossRef]
- Fan, P. Efficacy of long-acting GLP-1 analogue combined with low-carbohydrate diet in the treatment of obese type 2 diabetes patients. J. Pract. Diabetol. 2020, 16, 43–44. [Google Scholar]
- Li, Q.Y.; Liu, X.; Li, H.X.; Yang, C.W.; Zhang, W.H.; Wang, T.T.; Guo, Z.H.; Zhou, Y.R. Clinical effect of liraglutide on type 2 diabetes mellitus complicated with polycystic ovary syndrome. China Med. 2018, 13, 549–552. [Google Scholar]
- Liu, M.R. The Clinical Efficacy and Safety Observation on a Low Carbohydrate Diet Combined with Liraglutide in Obese Patients with Diabetes; University of South China: Hengyang, China, 2017. [Google Scholar]
- Fonseca, V.A.; Alvarado-Ruiz, R.; Raccah, D.; Boka, G.; Miossec, P.; Gerich, J.E. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: A randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care 2012, 35, 1225–1231. [Google Scholar] [CrossRef] [Green Version]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherifali, D.; Nerenberg, K.; Pullenayegum, E.; Cheng, J.E.; Gerstein, H.C. The effect of oral antidiabetic agents on A1C levels: A systematic review and meta-analysis. Diabetes Care 2010, 33, 1859–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention (CDC). Prevalence of overweight and obesity among adults with diagnosed diabetes—United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep. 2004, 53, 1066–1068. [Google Scholar]
- Nichols, G.A.; Bell, K.; Kimes, T.M.; O’Keeffe-Rosetti, M. Medical Care Costs Associated with Long-term Weight Maintenance Versus Weight Gain among Patients with Type 2 Diabetes. Diabetes Care 2016, 39, 1981–1986. [Google Scholar] [CrossRef] [Green Version]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L.; et al. Benefits of Modest Weight Loss in Improving Cardiovascular Risk Factors in Overweight and Obese Individuals with Type 2 Diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [Green Version]
- Williamson, D.F.; Thompson, T.J.; Thun, M.; Flanders, D.; Pamuk, E.; Byers, T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000, 23, 1499–1504. [Google Scholar] [CrossRef] [Green Version]
- Redmon, J.B.; Bertoni, A.G.; Connelly, S.; Feeney, P.A.; Glasser, S.P.; Glick, H.; Greenway, F.; Hesson, L.A.; Lawlor, M.S.; Montez, M.; et al. Effect of the Look AHEAD Study Intervention on Medication Use and Related Cost to Treat Cardiovascular Disease Risk Factors in Individuals with Type 2 Diabetes. Diabetes Care 2010, 33, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef]
- Bolen, S.; Feldman, L.; Vassy, J.; Wilson, L.; Yeh, H.-C.; Marinopoulos, S.; Wiley, C.; Selvin, E.; Wilson, R.; Bass, E.; et al. Systematic Review: Comparative Effectiveness and Safety of Oral Medications for Type 2 Diabetes Mellitus. Ann. Intern. Med. 2007, 147, 386–399. [Google Scholar] [CrossRef]
- Emdin, C.A.; Rahimi, K.; Neal, B.; Callender, T.; Perkovic, V.; Patel, A. Blood pressure lowering in type 2 diabetes: A systematic review and meta-analysis. JAMA 2015, 313, 603–615. [Google Scholar] [CrossRef]
- Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Lancet 2000, 355, 253–259. [Google Scholar] [CrossRef]
- Tsapas, A.; Karagiannis, T.; Kakotrichi, P.; Avgerinos, I.; Mantsiou, C.; Tousinas, G.; Manolopoulos, A.; Liakos, A.; Malandris, K.; Matthews, D.R.; et al. Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetes Obes. Metab. 2021, 23, 2116–2124. [Google Scholar] [CrossRef] [PubMed]
- Frier, B.M. Hypoglycaemia in diabetes mellitus: Epidemiology and clinical implications. Nat. Rev. Endocrinol. 2014, 10, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fonseca, V.; Childs, B. Economic burden of diabetes-related hypoglycemia on patients, payors, and employers. J. Diabetes Its Complicat. 2021, 35, 107916. [Google Scholar] [CrossRef] [PubMed]
| Glucose-Lowering Drug | HbA1c, % | FPG, mmol/L | BMI, kg/m2 | TC, mmol/L | HDL-C, mmol/L | SBP, mmHg | Hypoglycemia |
| Metformin | −0.96 (−1.16, −0.76) * | −1.65 (−2.02, −1.27) * | −1.28 (−2.26, −0.31) * | −0.30 (−0.47, −0.14) * | 0.05 (0.02, 0.09) * | −1.50 (−3.78, 0.79) | 1.53 (0.98, 2.40) |
| SUs | −1.39 (−1.63, −1.16) * | −2.70 (−3.18, −2.23) * | 1.22 (0.13, 2.31) * | −0.40 (−1.20, 0.40) | −0.00 (−0.18, 0.18) | 1.84 (−4.57, 8.25) | 5.44 (2.11, 14.02) * |
| Glyburide | −1.50 (−2.69, −0.30) * | −2.35 (−3.59, −1.12) * | 0.27 (−1.48, 2.03) | −0.80 (−1.94, 0.34) | −0.20 (−0.63, 0.23) | — | — |
| Glimepiride | −1.36 (−1.57, −1.16) * | −2.41 (−3.09, −1.73) * | 1.79 (0.46, 3.12) * | −0.22 (−1.60, 1.17) | 0.12 (−0.04, 0.29) | 1.84 (−4.57, 8.25) | 2.88 (0.45, 18.58) |
| Gliclazide | −1.40 (−2.70, −0.10) * | −2.22 (−3.47, −0.97) * | — | −0.75 (−1.84, 0.34) | 0.07 (−0.08, 0.22) | — | 5.00 (0.25, 99.95) |
| Glipizide | −1.47 (−1.87, −1.06) * | −3.02 (−3.85, −2.20) * | — | 0.20 (−3.19, 3.59) | 0.41 (−1.64, 2.47) | — | 7.11 (2.18, 23.24) * |
| TZDs | −0.89 (−1.04, −0.73) * | −1.91 (−2.23, −1.60) * | 0.63 (0.26, 0.99) * | 0.01 (−0.19, 0.22) | 0.12 (0.07, 0.17) * | 0.78 (−2.37, 3.93) | 0.49 (0.23, 1.03) |
| Rosiglitazone | −0.68 (−0.98, −0.38) * | −1.73 (−2.32, −1.14) * | 0.91 (0.48, 1.35) * | 0.25 (0.03, 0.46) * | 0.06 (0.03, 0.08) * | 2.43 (−1.55, 6.42) | 0.43 (0.15, 1.26) |
| Pioglitazone | −1.00 (−1.17, −0.82) * | −2.01 (−2.34, −1.67) * | 0.38 (−0.07, 0.82) | −0.09 (−0.32, 0.14) | 0.18 (0.09, 0.26) * | −1.79 (−6.33, 2.74) | 0.55 (0.20, 1.53) |
| NIDEs | −0.44 (−0.69, −0.20) * | −0.75 (−1.04, −0.45) * | 0.08 (−1.29, 1.44) | 0.21 (−0.39, 0.81) | 0.08 (−0.21, 0.37) | −5.98 (−12.33, 0.37) | 1.37 (0.34, 5.59) |
| Repaglinide | −0.45 (−0.81, −0.09) * | −0.64 (−1.27, −0.01) * | — | — | — | — | 0.97 (0.14, 6.77) |
| Nateglinide | −0.45 (−0.79, −0.10) * | −0.70 (−1.04, −0.36) * | 0.08 (−1.29, 1.44) | 0.21 (−0.39, 0.81) | 0.08 (−0.21, 0.37) | −5.98 (−12.33, 0.37) | 2.00 (0.26, 15.33) |
| AGIs | −0.62 (−0.79, −0.45) * | −1.19 (−1.73, −0.64) * | −0.49 (−1.26, 0.28) | −0.29 (−0.58, −0.00) * | 0.03 (−0.12, 0.17) | −1.40 (−4.71, 1.90) | 0.86 (0.51, 1.45) |
| Acarbose | −0.74 (−0.96, −0.52) * | −1.17 (−1.83, −0.50) * | −0.60 (−1.66, 0.46) | −0.34 (−0.65, −0.03) * | 0.07 (−0.11, 0.25) | −1.40 (−4.71, 1.90) | 1.19 (0.34, 4.23) |
| Voglibose | −0.20 (−0.33, −0.07) * | −1.78 (−3.58, 0.02) | 0.10 (−0.13, 0.33) | −0.15 (−0.92, 0.62) | −0.17 (−0.48, 0.14) | — | 0.93 (0.10, 8.79) |
| Miglitol | −0.53 (−0.85, −0.21) * | −0.01 (−0.88, 0.86) | — | — | — | — | 0.79 (0.44, 1.44) |
| DPP−4is | −0.63 (−0.68, −0.58) * | −0.94 (−1.03, −0.85) * | 0.47 (−0.01, 0.95) | −0.04 (−0.11, 0.02) | 0.03 (−0.01, 0.06) | 0.02 (−1.10, 1.14) | 0.89 (0.67, 1.18) |
| Sitagliptin | −0.73 (−0.82, −0.65) * | −1.07 (−1.20, −0.95) * | 0.10 (−1.24, 1.44) | 0.06 (0.01, 0.11) * | 0.06 (−0.02, 0.14) | 0.18 (−1.27, 1.64) | 0.79 (0.52, 1.20) |
| Saxagliptin | −0.52 (−0.61, −0.44) * | −0.83 (−1.00, −0.67) * | −0.46 (−2.04, 1.12) | — | — | — | 1.21 (0.52, 2.81) |
| Vildagliptin | −0.48 (−0.57, −0.38) * | −0.56 (−0.82, −0.30) * | −0.58 (−2.09, 0.93) | −0.12 (−0.83, 0.59) | — | — | 1.07 (0.54, 2.13) |
| Linagliptin | −0.68 (−0.79, −0.58) * | −0.94 (−1.16, −0.73) * | — | −0.06 (−0.21, 0.09) | 0.03 (−0.06, 0.12) | −1.74 (−4.75, 1.26) | 0.52 (0.26, 1.01) |
| Alogliptin | −0.68 (−0.76, −0.61) * | −1.07 (−1.27, −0.86) * | 0.81 (0.27, 1.35) * | −0.19 (−0.31, −0.07) * | −0.00 (−0.04, 0.03) | 0.59 (−1.59, 2.76) | 2.97 (1.00, 8.77) * |
| SGLT2is | −0.80 (−0.87, −0.72) * | −1.58 (−1.81, −1.36) * | −0.60 (−1.89, 0.69) | 0.22 (0.13, 0.31) * | 0.09 (0.07, 0.11) * | −4.18 (−4.84, −3.53) * | 0.86 (0.55, 1.37) |
| Dapagliflozin | −0.68 (−0.77, −0.59) * | −1.27 (−1.49, −1.06) * | −0.60 (−1.89, 0.69) | −0.02 (−0.24, 0.20) | 0.03 (−0.09, 0.15) | −3.89 (−5.02, −2.75) * | 1.29 (0.70, 2.36) |
| Empagliflozin | −0.79 (−0.86, −0.72) * | −1.84 (−1.97, −1.72) * | — | 0.20 (0.06, 0.34) * | 0.08 (0.07, 0.10) * | −3.29 (−4.34, −2.25) * | 0.31 (0.14, 0.71) * |
| Canagliflozin | −0.99 (−1.06, −0.92) * | −2.09 (−2.31, −1.87) * | — | 0.30 (0.19, 0.40) * | 0.10 (0.07, 0.12) * | −5.36 (−6.59, −4.14) * | 1.57 (0.79, 3.10) |
| GLP−1RAs | −0.99 (−1.20, −0.78) * | −1.64 (−2.00, −1.28) * | −1.05 (−1.81, −0.29) * | −0.42 (−0.61, −0.22) * | 0.03 (−0.01, 0.06) | −2.98 (−4.67, −1.30) * | 1.57 (0.82, 3.02) |
| Exenatide twice-daily | −0.64 (−0.82, −0.47) * | −1.13 (−1.49, −0.77) * | −1.65 (−2.26, −1.04) * | −0.28 (−0.50, −0.06) * | 0.01 (−0.01, 0.03) | −3.19 (−5.47, −0.90) * | 3.36 (0.84, 13.52) |
| Liraglutide | −1.17 (−1.47, −0.87) * | −1.97 (−2.47, −1.47) * | −0.80 (−1.66, 0.07) | −0.56 (−0.78, −0.34) * | 0.13 (0.05, 0.21) * | −2.74 (−5.24, −0.25) * | 1.37 (0.58, 3.25) |
| Lixisenatide | −0.60 (−0.83, −0.37) * | −0.97 (−1.47, −0.48) * | — | — | — | — | 1.04 (0.25, 4.29) |
| Glucose-Lowering Drug | Death | Total Vascular Events | Myocardial Infarction | Heart Failure | Stroke | Diabetic Nephropathy | AE-Induced Discontinuations |
| Metformin | 0.88 (0.46, 1.69) | 0.91 (0.22, 3.73) | 0.98 (0.10, 9.30) | 0.98 (0.10, 9.30) | 0.98 (0.10, 9.30) | 1.59 (0.20, 12.85) | 1.03 (0.74, 1.43) |
| SUs | 1.10 (0.27, 4.52) | 0.46 (0.03, 7.00) | 1.41 (0.06, 33.26) | — | 0.16 (0.01, 3.70) | — | 2.25 (0.74, 6.81) |
| Glyburide | 1.09 (0.07, 16.30) | — | — | — | — | — | 2.24 (0.31, 16.50) |
| Glimepiride | 1.16 (0.15, 9.23) | 0.46 (0.03, 7.00) | 1.41 (0.06, 33.26) | — | 0.16 (0.01, 3.70) | — | 0.99 (0.10, 9.40) |
| Gliclazide | 1.00 (0.02, 48.82) | — | — | — | — | — | 1.00 (0.02, 48.82) |
| Glipizide | 1.02 (0.02, 50.81) | — | — | — | — | — | 4.64 (0.74, 28.95) |
| TZDs | 0.95 (0.48, 1.90) | 1.44 (0.38, 5.39) | 0.82 (0.13, 5.06) | 0.97 (0.19, 4.92) | 0.96 (0.13, 7.21) | 0.97 (0.02, 48.35) | 1.25 (0.81, 1.95) |
| Rosiglitazone | 0.94 (0.29, 3.00) | 1.00 (0.02, 46.40) | 1.00 (0.02, 46.40) | 1.00 (0.02, 46.40) | — | — | 0.97 (0.43, 2.23) |
| Pioglitazone | 0.96 (0.41, 2.26) | 1.51 (0.37, 6.17) | 0.78 (0.10, 6.12) | 0.96 (0.16, 5.78) | 0.96 (0.13, 7.21) | 0.97 (0.02, 48.35) | 1.38 (0.82, 2.33) |
| NIDEs | 0.96 (0.20, 4.68) | — | — | — | — | — | 0.97 (0.24, 3.81) |
| Repaglinide | 0.97 (0.14, 6.77) | — | — | — | — | — | 0.97 (0.14, 6.77) |
| Nateglinide | 0.94 (0.06, 14.46) | — | — | — | — | — | 0.96 (0.14, 6.67) |
| AGIs | 1.07 (0.41, 2.78) | 1.55 (0.19, 12.51) | 1.92 (0.16, 22.74) | — | — | — | 2.57 (1.64, 4.03) * |
| Acarbose | 0.97 (0.28, 3.31) | — | — | — | — | — | 2.15 (1.23, 3.75) * |
| Voglibose | 0.93 (0.10, 8.78) | 0.90 (0.02, 45.04) | — | — | — | — | 0.92 (0.19, 4.46) |
| Miglitol | 1.60 (0.20, 12.92) | 1.92 (0.16, 22.74) | 1.92 (0.16, 22.74) | — | — | — | 5.37 (2.11, 13.69) * |
| DPP-4is | 0.89 (0.51, 1.58) | 0.82 (0.47, 1.41) | 0.47 (0.19, 1.16) | 1.00 (0.14, 7.05) | 0.99 (0.14, 7.00) | 0.98 (0.14, 6.94) | 0.92 (0.74, 1.14) |
| Sitagliptin | 0.82 (0.30, 2.20) | 0.68 (0.28, 1.66) | 0.56 (0.09, 3.53) | 1.03 (0.06, 16.28) | 0.34 (0.01, 8.36) | — | 0.89 (0.62, 1.28) |
| Saxagliptin | 1.44 (0.49, 4.22) | 0.71 (0.29, 1.73) | 0.33 (0.08, 1.30) | — | — | 0.33 (0.01, 8.15) | 1.28 (0.68, 2.42) |
| Vildagliptin | 0.48 (0.10, 2.36) | — | — | — | — | — | 1.08 (0.73, 1.60) |
| Linagliptin | 0.85 (0.16, 4.50) | 0.95 (0.21, 4.25) | 0.31 (0.04, 2.52) | — | — | — | 0.55 (0.30, 0.99) * |
| Alogliptin | 0.79 (0.18, 3.45) | 2.19 (0.39, 12.23) | 1.88 (0.16, 22.23) | 0.97 (0.06, 15.40) | 1.87 (0.16, 22.19) | 1.87 (0.16, 22.19) | 0.82 (0.42, 1.60) |
| SGLT2is | 0.81 (0.41, 1.60) | 1.00 (0.45, 2.21) | 0.79 (0.17, 3.64) | 1.00 (0.10, 9.50) | 0.58 (0.12, 2.88) | 1.53 (0.56, 4.18) | 0.89 (0.63, 1.24) |
| Dapagliflozin | 1.07 (0.41, 2.80) | 1.19 (0.38, 3.68) | — | — | — | 1.19 (0.38, 3.68) | 1.66 (0.84, 3.27) |
| Empagliflozin | 0.53 (0.15, 1.81) | 0.81 (0.21, 3.09) | 0.64 (0.08, 5.18) | — | 0.34 (0.04, 3.27) | 3.95 (0.44, 35.38) | 0.56 (0.36, 0.87) * |
| Canagliflozin | 0.77 (0.17, 3.55) | 1.00 (0.10, 9.50) | 1.00 (0.10, 9.50) | 1.00 (0.10, 9.50) | 1.00 (0.10, 9.50) | — | 1.78 (0.78, 4.07) |
| GLP-1RAs | 0.89 (0.33, 2.43) | 0.39 (0.10, 1.57) | 0.67 (0.04, 10.13) | 0.67 (0.04, 10.13) | 0.67 (0.04, 10.13) | 0.80 (0.05, 12.41) | 1.23 (0.60, 2.54) |
| Exenatide twice-daily | 0.96 (0.14, 6.73) | 0.86 (0.02, 40.01) | 0.86 (0.02, 40.01) | 0.86 (0.02, 40.01) | 0.86 (0.02, 40.01) | 0.86 (0.02, 40.01) | 2.63 (0.49, 14.04) |
| Liraglutide | 0.86 (0.27, 2.79) | 0.35 (0.08, 1.55) | 0.51 (0.01, 25.66) | 0.51 (0.01, 25.66) | 0.51 (0.01, 25.66) | 0.75 (0.02, 37.38) | 0.63 (0.25, 1.61) |
| Lixisenatide | — | — | — | — | — | — | 4.01 (0.85, 18.83) |
| HbA1c, % (Left Lower Half) | FPG, mmol/L (Right Upper Half) | ||||||
| Metformin | 1.05 (0.45, 1.66) * | 0.26 (−0.23, 0.75) | −0.90 (−1.38, −0.42) * | −0.46 (−1.12, 0.20) | −0.71 (−1.10, −0.32) * | −0.07 (−0.51, 0.37) | −0.01 (−0.53, 0.51) |
| 0.43 (0.12, 0.74) * | SUs | −0.79 (−1.36, −0.22) * | −1.95 (−2.51, −1.39) * | −1.51 (−2.23, −0.79) * | −1.76 (−2.24, −1.28) * | −1.12 (−1.65, −0.59) * | −1.06 (−1.66, −0.46) * |
| −0.07 (−0.32, 0.18) | −0.50 (−0.78, −0.22) * | TZDs | −1.16 (−1.59, −0.73) * | −0.72 (−1.35, −0.09) * | −0.97 (−1.30, −0.64) * | −0.33 (−0.72, 0.06) | −0.27 (−0.75, 0.21) |
| −0.52 (−0.84, −0.20) * | −0.95 (−1.29, −0.61) * | −0.45 (−0.74, −0.16) * | NIDEs | 0.44 (−0.18, 1.06) | 0.19 (−0.12, 0.50) | 0.83 (0.46, 1.20) * | 0.89 (0.43, 1.36) * |
| −0.34 (−0.60, −0.08) * | −0.77 (−1.06, −0.48) * | −0.27 (−0.50, −0.04) * | 0.18 (−0.12, 0.48) | AGIs | −0.25 (−0.80, 0.30) | 0.39 (−0.20, 0.98) | 0.45 (−0.20, 1.10) |
| −0.33 (−0.54, −0.12) * | −0.76 (−1.00, −0.52) * | −0.26 (−0.42, −0.10) * | 0.19 (−0.06, 0.44) | 0.01 (−0.17, 0.19) | DPP−4is | 0.64 (0.40, 0.88) * | 0.70 (0.33, 1.07) * |
| −0.16 (−0.37, 0.05) | −0.59 (−0.84, −0.34) * | −0.09 (−0.26, 0.08) | 0.36 (0.10, 0.62) * | 0.18 (−0.01, 0.37) | 0.17 (0.08, 0.26) * | SGLT2is | 0.06 (−0.37, 0.49) |
| 0.03 (−0.26, 0.32) | −0.40 (−0.72, −0.09) * | 0.10 (−0.16, 0.36) | 0.55 (0.23, 0.87) * | 0.37 (0.10, 0.64) * | 0.36 (0.14, 0.58) * | 0.19 (−0.03, 0.41) | GLP−1RAs |
| BMI, kg/m2 (Left Lower Half) | TC, mmol/L (Right Upper Half) | ||||||
| Metformin | 0.10 (−0.72, 0.92) | −0.31 (−0.57, −0.05) * | −0.51 (−1.13, 0.11) | −0.01 (−0.34, 0.32) | −0.26 (−0.44, −0.08) * | −0.52 (−0.71, −0.33) * | 0.12 (−0.14, 0.38) |
| −2.50 (−3.96, −1.04) * | SUs | −0.41 (−1.24, 0.42) | −0.61 (−1.61, 0.39) | −0.11 (−0.96, 0.74) | −0.36 (−1.16, 0.44) | −0.62 (−1.43, 0.19) | 0.02 (−0.80, 0.84) |
| −1.91 (−2.95, −0.87) * | 0.59 (−0.56, 1.74) | TZDs | −0.20 (−0.83, 0.43) | 0.30 (−0.06, 0.66) | 0.05 (−0.17, 0.27) | −0.21 (−0.43, 0.01) | 0.43 (0.15, 0.71) * |
| −1.36 (−3.04, 0.32) | 1.14 (−0.61, 2.89) | 0.55 (−0.86, 1.96) | NIDEs | 0.50 (−0.17, 1.17) | 0.25 (−0.35, 0.85) | −0.01 (−0.62, 0.60) | 0.63 (−0.00, 1.26) |
| −0.79 (−2.03, 0.45) | 1.71 (0.38, 3.05) * | 1.12 (0.27, 1.97) * | 0.57 (−1.00, 2.14) | AGIs | −0.25 (−0.55, 0.05) | −0.51 (−0.81, −0.21) * | 0.13 (−0.22, 0.48) |
| −1.75 (−2.84, −0.66) * | 0.75 (−0.44, 1.94) | 0.16 (−0.44, 0.76) | −0.39 (−1.84, 1.06) | −0.96 (−1.87, −0.05) * | DPP−4is | −0.26 (−0.37, −0.15) * | 0.38 (0.17, 0.59) * |
| −0.68 (−2.30, 0.94) | 1.82 (0.13, 3.51) * | 1.23 (−0.11, 2.57) | 0.68 (−1.20, 2.56) | 0.11 (−1.39, 1.61) | 1.07 (−0.31, 2.45) | SGLT2is | 0.64 (0.43, 0.86) * |
| −0.23 (−1.47, 1.01) | 2.27 (0.94, 3.60) * | 1.68 (0.84, 2.52) * | 1.13 (−0.43, 2.69) | 0.56 (−0.52, 1.64) | 1.52 (0.62, 2.42) * | 0.45 (−1.05, 1.95) | GLP−1RAs |
| HDL-C, mmol/L (Left Lower Half) | SBP, mmHg (Right Upper Half) | ||||||
| Metformin | −3.34 (−10.15, 3.47) | −2.28 (−6.17, 1.61) | 4.48 (−2.27, 11.23) | −0.10 (−4.12, 3.92) | −1.52 (−4.07, 1.03) | 2.68 (0.30, 5.06) * | 1.48 (−1.36, 4.32) |
| 0.05 (−0.13, 0.23) | SUs | 1.06 (−6.08, 8.20) | 7.82 (−1.20, 16.84) | 3.24 (−3.97, 10.45) | 1.82 (−4.69, 8.33) | 6.02 (−0.42, 12.46) | 4.82 (−1.81, 11.45) |
| −0.07 (−0.13, −0.01) * | −0.12 (−0.31, 0.07) | TZDs | 6.76 (−0.33, 13.85) | 2.18 (−2.39, 6.75) | 0.76 (−2.58, 4.10) | 4.96 (1.74, 8.18) * | 3.76 (0.19, 7.33) * |
| −0.03 (−0.32, 0.26) | −0.08 (−0.42, 0.26) | 0.04 (−0.25, 0.33) | NIDEs | −4.58 (−11.74, 2.58) | −6.00 (−12.45, 0.45) | −1.80 (−8.18, 4.58) | −3.00 (−9.57, 3.57) |
| 0.02 (−0.13, 0.17) | −0.03 (−0.26, 0.20) | 0.09 (−0.06, 0.24) | 0.05 (−0.27, 0.37) | AGIs | −1.42 (−4.91, 2.07) | 2.78 (−0.59, 6.15) | 1.58 (−2.13, 5.29) |
| 0.02 (−0.03, 0.07) | −0.03 (−0.21, 0.15) | 0.09 (0.03, 0.15) * | 0.05 (−0.24, 0.34) | 0.00 (−0.15, 0.15) | DPP−4is | 4.20 (2.90, 5.50) * | 3.00 (0.98, 5.02) * |
| −0.04 (−0.08, 0.00) | −0.09 (−0.27, 0.09) | 0.03 (−0.02, 0.08) | −0.01 (−0.30, 0.28) | −0.06 (−0.21, 0.09) | −0.06 (−0.10, −0.02) * | SGLT2is | −1.20 (−3.01, 0.61) |
| 0.02 (−0.03, 0.07) | −0.03 (−0.21, 0.15) | 0.09 (0.03, 0.15) * | 0.05 (−0.24, 0.34) | 0.00 (−0.15, 0.15) | 0.00 (−0.05, 0.05) | 0.06 (0.02, 0.10) * | GLP-1RAs |
| Hypoglycemia (Left Lower Half) | Death (Right Upper Half) | ||||||
| Metformin | 0.80 (0.17, 3.80) | 0.93 (0.36, 2.39) | 0.92 (0.17, 5.09) | 0.83 (0.26, 2.63) | 0.99 (0.42, 2.35) | 1.09 (0.42, 2.79) | 0.99 (0.30, 3.29) |
| 0.28 (0.10, 0.81) * | SUs | 1.15 (0.24, 5.55) | 1.15 (0.14, 9.57) | 1.03 (0.19, 5.67) | 1.23 (0.27, 5.65) | 1.36 (0.28, 6.51) | 1.24 (0.22, 7.01) |
| 3.13 (1.32, 7.42) * | 11.08 (3.33, 36.87) * | TZDs | 0.99 (0.18, 5.58) | 0.89 (0.28, 2.90) | 1.07 (0.44, 2.61) | 1.18 (0.45, 3.09) | 1.07 (0.32, 3.62) |
| 1.12 (0.26, 4.90) | 3.97 (0.73, 21.66) | 0.36 (0.07, 1.76) | NIDEs | 0.90 (0.14, 5.71) | 1.08 (0.20, 5.78) | 1.19 (0.21, 6.64) | 1.08 (0.17, 7.05) |
| 1.79 (0.89, 3.57) | 6.33 (2.14, 18.73) * | 0.57 (0.23, 1.42) | 1.60 (0.36, 7.16) | AGIs | 1.20 (0.39, 3.64) | 1.32 (0.41, 4.27) | 1.20 (0.30, 4.82) |
| 1.73 (1.02, 2.95) * | 6.14 (2.28, 16.51) * | 0.55 (0.25, 1.22) | 1.55 (0.37, 6.49) | 0.97 (0.53, 1.76) | DPP-4is | 1.10 (0.46, 2.67) | 1.01 (0.32, 3.19) |
| 1.78 (0.93, 3.38) | 6.30 (2.20, 18.05) * | 0.57 (0.24, 1.36) | 1.59 (0.36, 6.96) | 0.99 (0.49, 2.00) | 1.03 (0.60, 1.76) | SGLT2is | 0.91 (0.27, 3.07) |
| 0.97 (0.44, 2.15) | 3.45 (1.09, 10.91) * | 0.31 (0.12, 0.84) * | 0.87 (0.19, 4.10) | 0.55 (0.24, 1.26) | 0.56 (0.28, 1.15) | 0.55 (0.25, 1.22) | GLP-1RAs |
| Total Vascular Events (Left Lower Half) | AE-Induced Discontinuations (Right Upper Half) | ||||||
| Metformin | 0.46 (0.14, 1.46) | 0.82 (0.48, 1.42) | 1.07 (0.26, 4.37) | 0.40 (0.23, 0.70) * | 1.12 (0.76, 1.66) | 1.16 (0.73, 1.85) | 0.84 (0.38, 1.85) |
| 1.99 (0.09, 42.75) | SUs | 1.79 (0.54, 5.91) | 2.33 (0.40, 13.59) | 0.88 (0.27, 2.90) | 2.45 (0.79, 7.58) | 2.53 (0.80, 8.06) | 1.82 (0.49, 6.85) |
| 0.63 (0.09, 4.37) | 0.32 (0.02, 6.59) | TZDs | 1.30 (0.31, 5.48) | 0.49 (0.26, 0.92) * | 1.37 (0.84, 2.23) | 1.41 (0.81, 2.46) | 1.02 (0.44, 2.37) |
| — | — | — | NIDEs | 0.38 (0.09, 1.59) | 1.05 (0.26, 4.22) | 1.09 (0.27, 4.46) | 0.78 (0.17, 3.69) |
| 0.59 (0.05, 7.30) | 0.30 (0.01, 9.16) | 0.93 (0.08, 10.98) | — | AGIs | 2.80 (1.70, 4.61) * | 2.89 (1.65, 5.07) * | 2.08 (0.89, 4.88) |
| 1.12 (0.25, 5.08) | 0.56 (0.04, 9.07) | 1.76 (0.42, 7.38) | — | 1.90 (0.22, 16.48) | DPP-4is | 1.03 (0.69, 1.54) | 0.75 (0.35, 1.58) |
| 0.91 (0.18, 4.61) | 0.46 (0.03, 7.86) | 1.44 (0.31, 6.74) | — | 1.56 (0.17, 14.53) | 0.82 (0.31, 2.15) | SGLT2is | 0.72 (0.33, 1.60) |
| 2.33 (0.32, 16.87) | 1.17 (0.06, 24.97) | 3.67 (0.54, 25.02) | — | 3.96 (0.32, 48.69) | 2.08 (0.47, 9.30) | 2.55 (0.51, 12.66) | GLP-1RAs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, S.; Hu, X.; Shi, L.; Zhen, X.; Sun, X.; Huang, M.; Gu, Y.; Dong, H. Choice of Glucose-Lowering Drugs as Initial Monotherapy for Type 2 Diabetes Patients with Contraindications or Intolerance to Metformin: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7094. https://doi.org/10.3390/jcm11237094
Gu S, Hu X, Shi L, Zhen X, Sun X, Huang M, Gu Y, Dong H. Choice of Glucose-Lowering Drugs as Initial Monotherapy for Type 2 Diabetes Patients with Contraindications or Intolerance to Metformin: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(23):7094. https://doi.org/10.3390/jcm11237094
Chicago/Turabian StyleGu, Shuyan, Xiaoqian Hu, Lizheng Shi, Xuemei Zhen, Xueshan Sun, Minzhuo Huang, Yuxuan Gu, and Hengjin Dong. 2022. "Choice of Glucose-Lowering Drugs as Initial Monotherapy for Type 2 Diabetes Patients with Contraindications or Intolerance to Metformin: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 23: 7094. https://doi.org/10.3390/jcm11237094
APA StyleGu, S., Hu, X., Shi, L., Zhen, X., Sun, X., Huang, M., Gu, Y., & Dong, H. (2022). Choice of Glucose-Lowering Drugs as Initial Monotherapy for Type 2 Diabetes Patients with Contraindications or Intolerance to Metformin: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(23), 7094. https://doi.org/10.3390/jcm11237094





