MCP1 Could Mediate FGF23 and Omega 6/Omega 3 Correlation Inversion in CKD
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Fatty Acid Analysis
2.3. Enzyme-Linked Immunosorbent (ELISA) of FGF23 Intact/C-Terminal and Monocyte Chemoattractant Protein 1 (MCP1)
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, J.; Alique, M.; Vida, C.; Bodega, G.; Ceprián, N.; Morales, E.; Praga, M.; de Sequera, P.; Ramírez, R. Mechanisms of Cardiovascular Disorders in Patients With Chronic Kidney Disease: A Process Related to Accelerated Senescence. Front. Cell Dev. Biol. 2020, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Fliser, D.; Kollerits, B.; Neyer, U.; Ankerst, D.P.; Lhotta, K.; Lingenhel, A.; Ritz, E.; Kronenberg, F. Fibroblast Growth Factor 23 (FGF23) Predicts Progression of Chronic Kidney Disease: The Mild to Moderate Kidney Disease (MMKD) Study. J. Am. Soc. Nephrol. 2007, 18, 2601–2608. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Quarles, L.D. How Fibroblast Growth Factor 23 Works. J. Am. Soc. Nephrol. 2007, 18, 1637–1647. [Google Scholar] [CrossRef]
- Mattinzoli, D.; Ikehata, M.; Tsugawa, K.; Alfieri, C.M.; Dongiovanni, P.; Trombetta, E.; Valenti, L.; Puliti, A.; Lazzari, L.; Messa, P. FGF23 and Fetuin-A Interaction in the Liver and in the Circulation. Int. J. Biol. Sci. 2018, 14, 586–598. [Google Scholar] [CrossRef]
- Richter, B.; Faul, C. FGF23 Actions on Target Tissues-With and Without Klotho. Front. Endocrinol. 2018, 9, 189. [Google Scholar] [CrossRef]
- Binnenmars, S.H.; Hoogslag, G.E.; Yeung SM, H.; Brouwers, F.P.; Bakker, S.J.L.; van Gilst, W.H.; Gansevoort, R.T.; Navis, G.; Voors, A.A.; de Borst, M.H. Fibroblast Growth Factor 23 and Risk of New Onset Heart Failure With Preserved or Reduced Ejection Fraction: The PREVEND Study. J. Am. Heart Assoc. 2022, 11, 24952. [Google Scholar] [CrossRef]
- Verbueken, D.; Moe, O.W. Strategies to Lower Fibroblast Growth Factor-23 Bioactivity. Nephrol. Dial. Transplant. 2021, 37, 1800–1807. [Google Scholar] [CrossRef]
- Turolo, S.; Edefonti, A.; Syren, M.L.; Marangoni, F.; Morello, W.; Agostoni, C.; Montini, G. Fatty Acids in Nephrotic Syndrome and Chronic Kidney Disease. J. Ren. Nutr. 2018, 28, 145–155. [Google Scholar] [CrossRef]
- Noels, H.; Lehrke, M.; Vanholder, R.; Jankowski, J. Lipoproteins and Fatty Acids in Chronic Kidney Disease: Molecular and Metabolic Alterations. Nat. Rev. Nephrol. 2021, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Ander, B.P.; Dupasquier CM, C.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated Fatty Acids and Their Effects on Cardiovascular Disease. Exp. Clin. Cardiol. 2003, 8, 164. [Google Scholar] [PubMed]
- Schmitz, G.; Ecker, J. The Opposing Effects of N-3 and N-6 Fatty Acids. Progress in Lipid Research. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Omega-3 Fatty Acids and Cardiovascular Disease: New Recommendations from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, E.; Blanchet, C.; Lemieux, S.; Sauvé, L.; Gingras, S.; Ayotte, P.; Holub, B.J. N-3 Fatty Acids and Cardiovascular Disease Risk Factors among the Inuit of Nunavik. Am. J. Clin. Nutr. 2001, 74, 464–473. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of Action of (N-3) Fatty Acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef]
- Hirafuji, M.; Machida, T.; Tsunoda, M.; Miyamoto, A.; Minami, M. Docosahexaenoic Acid Potentiates Interleukin-1beta Induction of Nitric Oxide Synthase through Mechanism Involving P44/42 MAPK Activation in Rat Vascular Smooth Muscle Cells. Br. J. Pharmacol. 2002, 136, 613–619. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hossain, S.; Al Mamun, A.; Matsuzaki, K.; Arai, H. Docosahexaenoic Acid: One Molecule Diverse Functions. Crit. Rev. Biotechnol. 2017, 37, 579–597. [Google Scholar] [CrossRef]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of Arachidonic Acid Metabolism: A Review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef]
- Hirafuji, M.; Machida, T.; Hamaue, N.; Minami, M. Cardiovascular Protective Effects of N-3 Polyunsaturated Fatty Acids with Special Emphasis on Docosahexaenoic Acid. J. Pharmacol. Sci. 2003, 92, 308–316. [Google Scholar] [CrossRef]
- Vas Dias, F.W.; Gibney, M.J.; Taylor, T.G. The Effect of Polyunsaturated Fatty Acids on the N-3 and N-6 Series on Platelet Aggregation and Platelet and Aortic Fatty Acid Composition in Rabbits. Atherosclerosis 1982, 43, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Baia, L.C.; Van den Berg, E.; Vervloet, M.G.; Heilberg, I.P.; Navis, G.; Bakker, S.J.L.; Geleijnse, J.M.; Kromhout, D.; Soedamah-Muthu, S.S.; De Borst, M.H. Fish and Omega-3 Fatty Acid Intake in Relation to Circulating Fibroblast Growth Factor 23 Levels in Renal Transplant Recipients. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Oberg, B.P.; McMenamin, E.; Lucas, F.L.; McMonagle, E.; Morrow, J.; Ikizler, T.A.; Himmelfarb, J. Increased Prevalence of Oxidant Stress and Inflammation in Patients with Moderate to Severe Chronic Kidney Disease. Kidney Int. 2004, 65, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Bertram, A.; Nadrowitz, F.; Menne, J. Monocyte Chemoattractant Protein-1 and the Kidney. Curr. Opin. Nephrol. Hypertens. 2016, 25, 42–49. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Van Der Laan, S.W.; Asare, Y.; Mekke, J.M.; Haitjema, S.; Schoneveld, A.H.; De Jager, S.C.A.; Nurmohamed, N.S.; Kroon, J.; Stroes, E.S.G.; et al. Monocyte-Chemoattractant Protein-1 Levels in Human Atherosclerotic Lesions Associate with Plaque Vulnerability. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Resende, A.L.; Elias, R.M.; Wolf, M.; dos Reis, L.M.; Graciolli, F.G.; Santos, G.D.; Dias, C.B.; Jorgetti, V.; Woronik, V.; Moysés, R.M.A. Serum Levels of Fibroblast Growth Factor 23 Are Elevated in Patients with Active Lupus Nephritis. Cytokine 2017, 91, 124–127. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Mannstadt, M.; Isakova, T.; Rauh-Hain, J.A.; Tamez, H.; Shah, A.; Smith, K.; Lee, H.; Thadhani, R.; Jüppner, H.; et al. Fibroblast Growth Factor 23 and Mortality among Patients Undergoing Hemodialysis. N. Engl. J. Med. 2008, 359, 584–592. [Google Scholar] [CrossRef]
- Caldiroli, L.; Vettoretti, S.; Armelloni, S.; Mattinzoli, D.; Ikehata, M.; Molinari, P.; Alfieri, C.; Messa, P.; Castellano, G. Possible Benefits of a Low Protein Diet in Older Patients With CKD at Risk of Malnutrition: A Pilot Randomized Controlled Trial. Front. Nutr. 2022, 8, 782499. [Google Scholar] [CrossRef]
- Tanisawa, K.; Taniguchi, H.; Sun, X.; Ito, T.; Kawakami, R.; Sakamoto, S.; Higuchi, M. Visceral Fat Area Is a Strong Predictor of Leukocyte Cell-Derived Chemotaxin 2, a Potential Biomarker of Dyslipidemia. PLoS ONE 2017, 12, e0173310. [Google Scholar] [CrossRef]
- Margiotta, E.; Caldiroli, L.; Callegari, M.L.; Miragoli, F.; Zanoni, F.; Armelloni, S.; Rizzo, V.; Messa, P.; Vettoretti, S. Association of Sarcopenia and Gut Microbiota Composition in Older Patients with Advanced Chronic Kidney Disease, Investigation of the Interactions with Uremic Toxins, Inflammation and Oxidative Stress. Toxins 2021, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Wijenayaka, A.R.; Prideaux, M.; Kogawa, M.; Ormsby, R.T.; Evdokiou, A.; Bonewald, L.F.; Findlay, D.M.; Atkins, G.J. Regulation of FGF23 Expression in IDG-SW3 Osteocytes and Human Bone by pro-Inflammatory Stimuli. Mol. Cell. Endocrinol. 2015, 399, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.; David, V. Inflammation Regulates Fibroblast Growth Factor 23 Production. Curr. Opin. Nephrol. Hypertens. 2016, 25, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Graves, T.D.; Jiang, Y.; Valente, J.A. The Expression of Monocyte Chemoattractant Protein-1 and Other Chemokines by Osteoblasts. Front. Biosci. 1999, 4, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Grabner, A.; Yanucil, C.; Schramm, K.; Czaya, B.; Krick, S.; Czaja, M.J.; Bartz, R.; Abraham, R.; Di Marco, G.S.; et al. Fibroblast Growth Factor 23 Directly Targets Hepatocytes to Promote Inflammation in Chronic Kidney Disease. Kidney Int. 2016, 90, 985–996. [Google Scholar] [CrossRef]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-Derived Resolvins, Protectins, and Maresins in Airway Inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Husted, K.S.; Bouzinova, E.V. The Importance of n-6/n-3 Fatty Acids Ratio in the Major Depressive Disorder. Med. (Lith.) 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Frishberg, Y.; Ito, N.; Rinat, C.; Yamazaki, Y.; Feinstein, S.; Urakawa, I.; Navon-Elkan, P.; Becker-Cohen, R.; Yamashita, T.; Araya, K.; et al. Hyperostosis-Hyperphosphatemia Syndrome: A Congenital Disorder of O-Glycosylation Associated With Augmented Processing of Fibroblast Growth Factor 23. J. Bone Miner. Res. 2007, 22, 235–242. [Google Scholar] [CrossRef]
- David, V.; Martin, A.; Isakova, T.; Spaulding, C.; Qi, L.; Ramirez, V.; Zumbrennen-Bullough, K.B.; Sun, C.C.; Lin, H.Y.; Babitt, J.L.; et al. Inflammation and Functional Iron Deficiency Regulate Fibroblast Growth Factor 23 Production. Kidney Int. 2016, 89, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Gosling, J.; Slaymaker, S.; Gu, L.; Tseng, S.; Zlot, C.H.; Young, S.G.; Rollins, B.J.; Charo, I.F. MCP-1 Deficiency Reduces Susceptibility to Atherosclerosis in Mice That Overexpress Human Apolipoprotein B. J. Clin. Investig. 1999, 103, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Okada, Y.; Clinton, S.K.; Gerard, C.; Sukhova, G.K.; Libby, P.; Rollins, B.J. Absence of Monocyte Chemoattractant Protein-1 Reduces Atherosclerosis in Low Density Lipoprotein Receptor-Deficient Mice. Mol. Cell 1998, 2, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
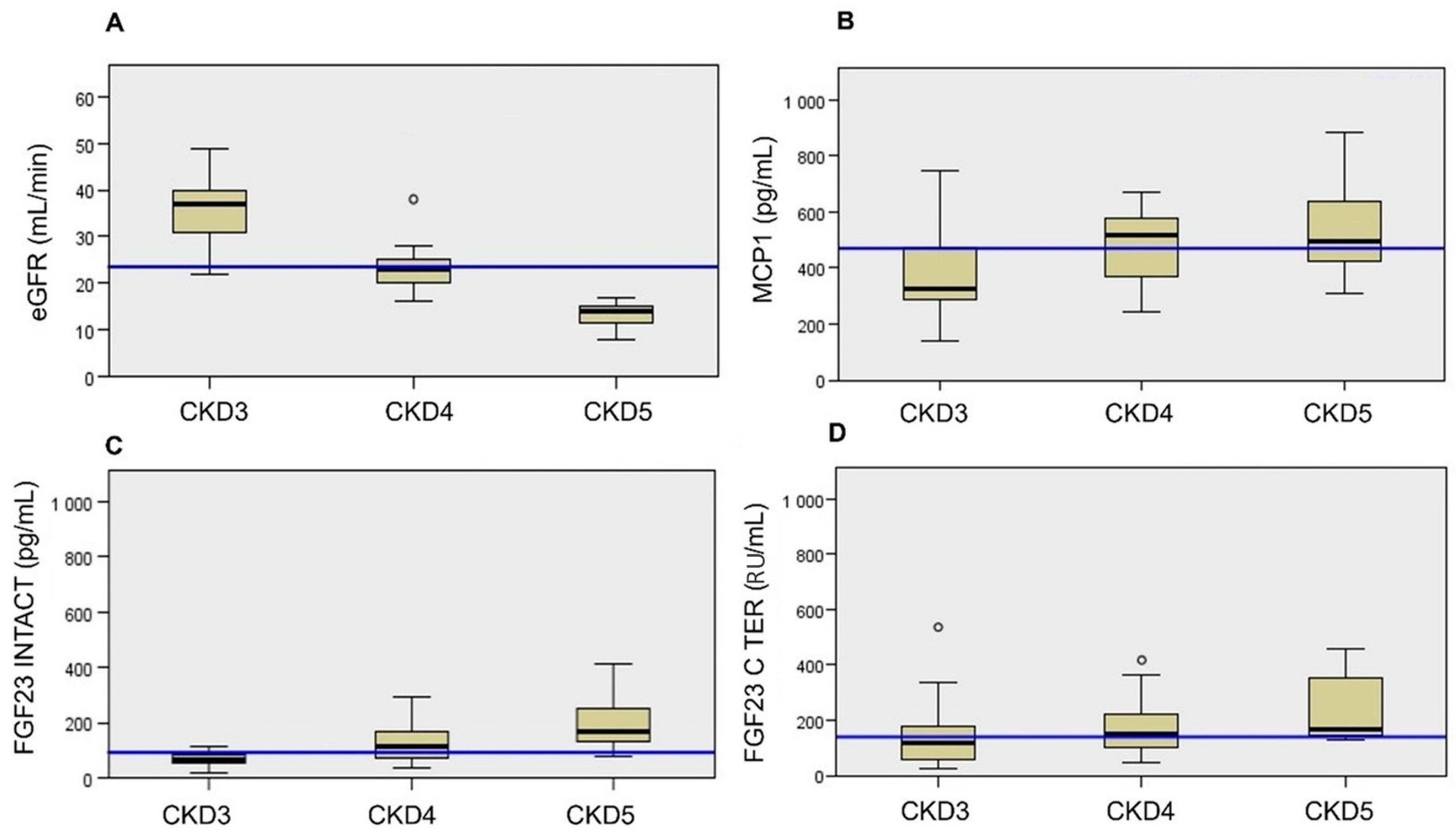
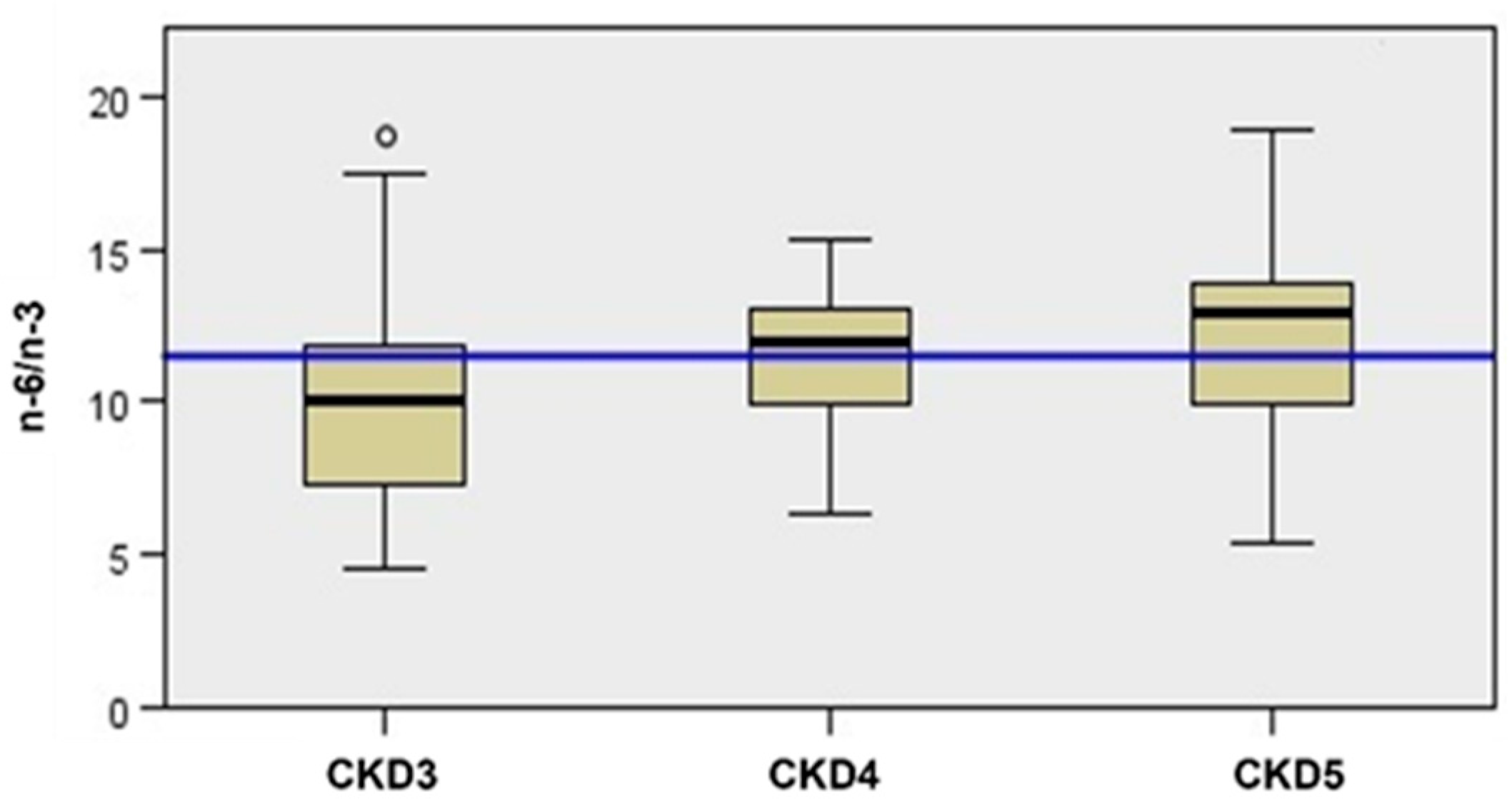
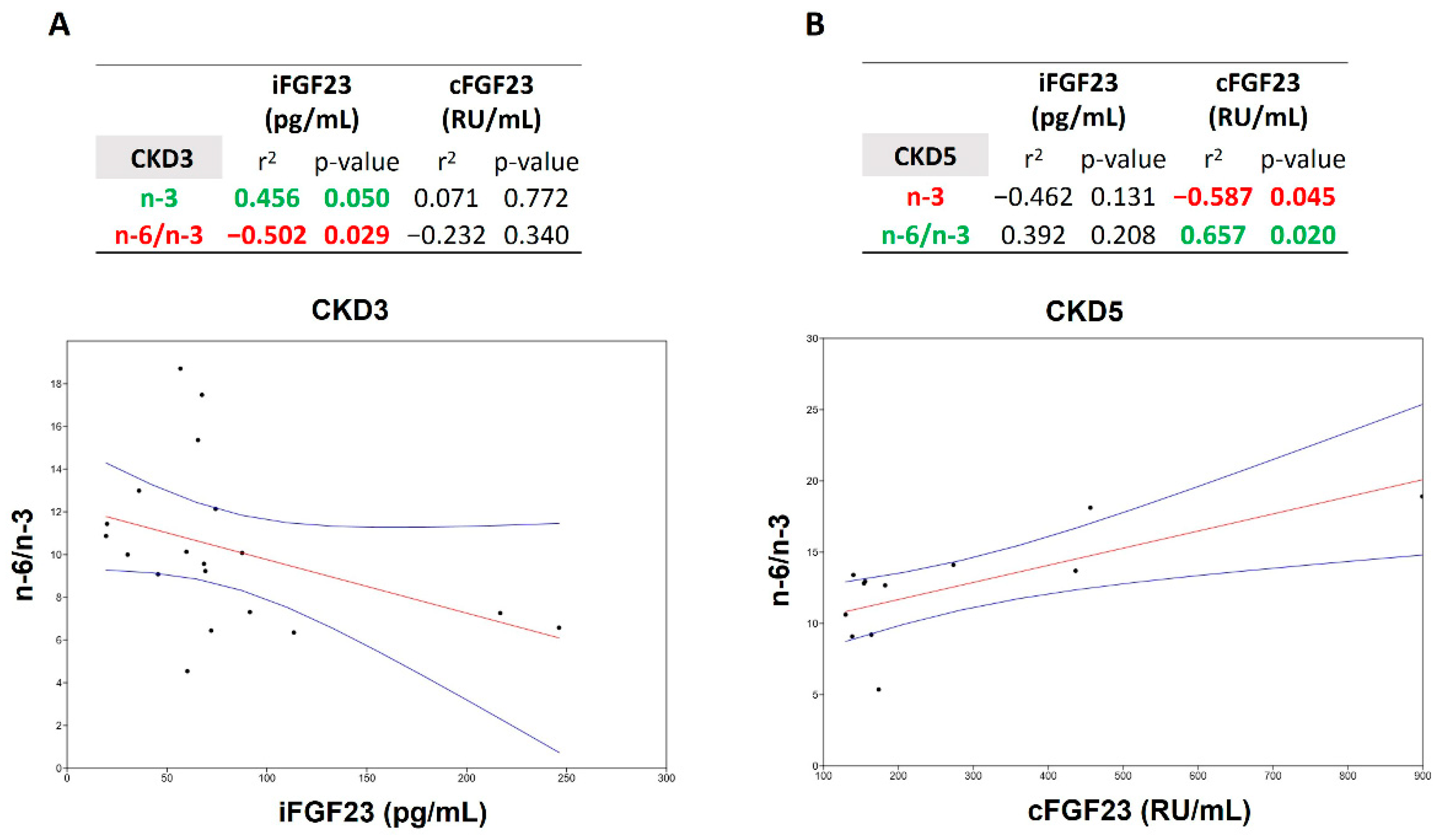
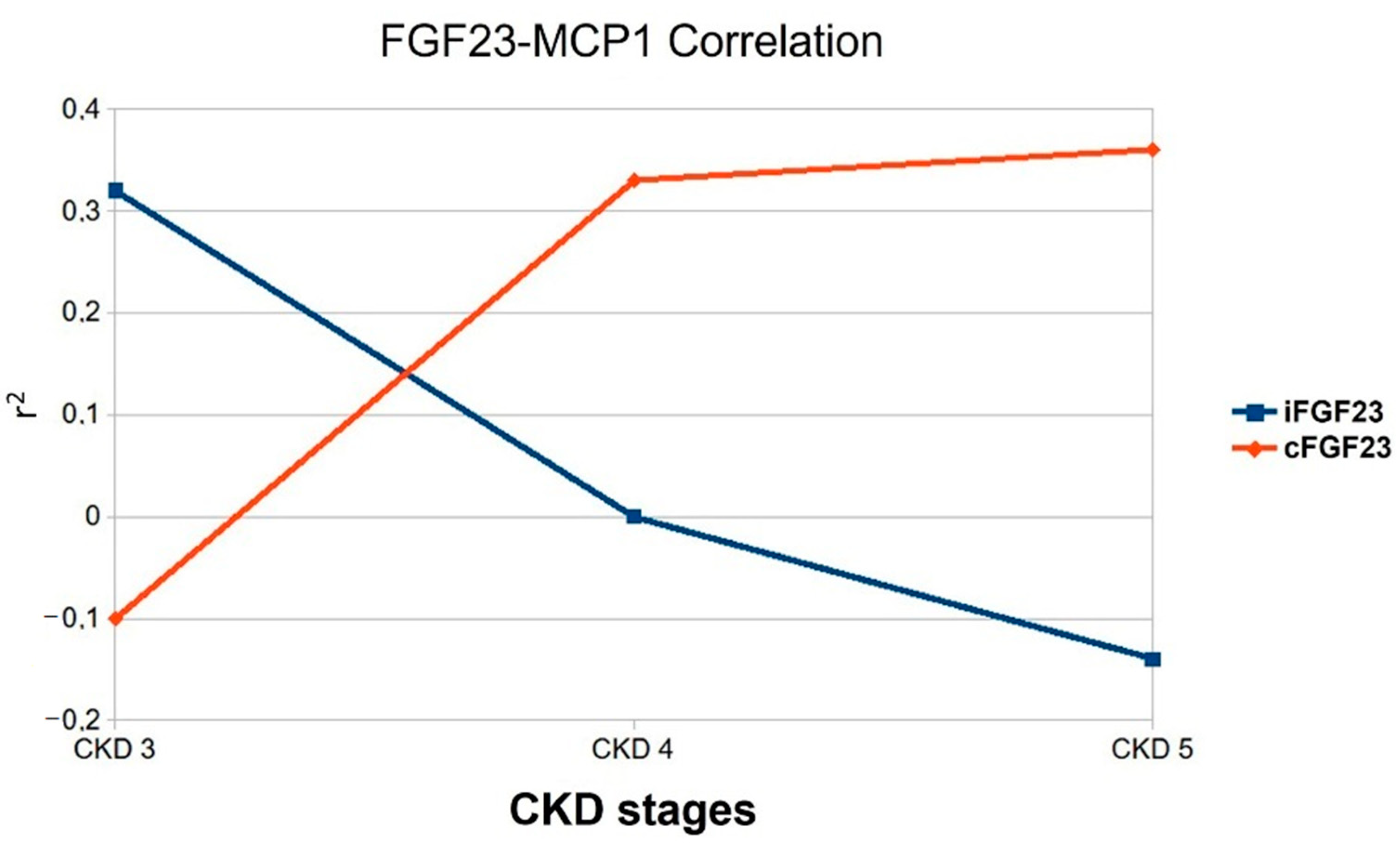
| TOTAL (56 Patients) | CKD 3 (19 Patients) | CKD 4 (25 Patients) | CKD 5 ND (12 Patients) | p-Value 3 vs. 4 | p-Value 3 vs. 5 | p-Value 4 vs. 5 | |
|---|---|---|---|---|---|---|---|
| Demographic data | |||||||
| Age (years) | 78 ± 8 | 74 ± 20 | 81 ± 6 | 78 ± 8 | 0.08 | 0.49 | 0.12 |
| Gender (m/f) | 37/19 | 15/4 | 12/13 | 10/2 | 0.03 | 0.76 | 0.04 |
| Clinical data | |||||||
| Diabetic subjects n (%) | 33 (58) | 11 (57) | 16 (64) | 6 (50) | 0.68 | 0.66 | 0.41 |
| CV events n (%) | 31 (55) | 8 (42) | 17 (68) | 6 (50) | 0.08 | 0.66 | 0.29 |
| Tot. cholesterol (mg/dL) | 166 ± 37 | 169 ± 43 | 160 ± 27 | 171 ± 47 | 0.40 | 0.84 | 0.31 |
| HDL (mg/dL) | 52 ±15 | 52 ± 13 | 52 ± 15 | 55 ± 17 | 0.98 | 0.59 | 0.60 |
| LDL (mg/dL) | 88 ± 33 | 90 ± 38 | 84 ± 21 | 92 ± 45 | 0.51 | 0.80 | 0.37 |
| Na (mmol/24 h) | 141 ± 2 | 141 ± 2 | 141 ± 2.5 | 141 ± 1 | 0.67 | 0.7 | 0.97 |
| K (mmol/L) | 4.6 ± 0.4 | 4.4 ± 0.4 | 4.6 ± 0.4 | 4.9 ± 0.5 | 0.19 | 0.07 | 0.22 |
| Biomarker data | |||||||
| Ca (mmol/L) | 9.2 ± 0.4 | 9.3 ± 0.4 | 9.3 ± 0.4 | 9.0 ± 0.6 | 0.82 | 0.12 | 0.15 |
| Vit. D (ng/mL) | 30 ± 15 | 35 ± 15 | 29 ± 16 | 23 ± 13 | 0.24 | 0.04 | 0.32 |
| Phosphoremia (mg/dL) | 3.4 ± 0.5 | 3.1 ± 0.3 | 3.4 ± 0.5 | 3.8 ± 0.7 | 0.12 | 0.05 | 0.08 |
| Phosphaturia (mg/24 h) | 501 ± 195 | 571 ± 194 | 487 ± 199 | 436 ± 176 | 0.18 | 0.04 | 0.4 |
| PTH (pg/mL) | 69 ± 40 | 54 ± 31 | 60 ± 30 | 112 ± 45 | 0.53 | 0.0002 | 0.0002 |
| eGFR | 25.5 ± 10,3 | 37.4 ± 6.5 | 22.36 ± 3.0 | 13.1 ± 2.7 | <0.0001 | <0.0001 | 0.0003 |
| MCP1 (pg/mL) | 465.1± 159.4 | 384.8 ± 161 | 478.4 ± 124 | 552.1 ± 177 | 0.04 | 0.15 | 0.01 |
| FGF23 Cter (RU/mL) | 187.7 ± 151.0 | 151.6 ± 132.0 | 172.5 ± 99.7 | 275.2 ± 226.9 | 0.55 | 0.06 | 0.06 |
| FGF23 Intact (pg/mL) | 135.0 ± 124.8 | 78.9 ± 58.9 | 127.6 ± 70.9 | 238.7 ± 207.5 | 0.02 | 0.02 | 0.003 |
| CKD | p-Value | |||||
|---|---|---|---|---|---|---|
| Stage 3 | Stage 4 | Stage 5 | 3 vs. 4 | 3 vs. 5 | 4 vs. 5 | |
| PUFA | 36.75 ± 3.83 | 35.63 ± 3.71 | 39.44 ± 4.27 | 0.33 | 0.07 | 0.009 |
| PUFA n-3 | 3.59 ± 1.32 | 2.91 ± 0.65 | 3.14 ± 1.1 | 0.03 | 0.33 | 0.43 |
| α-Linolenic acid | 0.36 ± 0.17 | 0.3 ± 0.08 | 0.36 ± 0.15 | 0.15 | 0.93 | 0.174 |
| EPA | 0.88 ± 0.62 | 0.6 ± 0.24 | 0.58 ± 0.39 | 0.09 | 0.13 | 0.85 |
| DPA | 0.42 ± 0.14 | 0.36 ± 0.1 | 0.37 ± 0.09 | 0.04 | 0.34 | 0.62 |
| DHA | 1.93 ± 0.67 | 1.65 ± 0.39 | 1.83 ± 0.66 | 0.09 | 0.69 | 0.30 |
| PUFA n-6 | 32.93 ± 3.64 | 32.42 ± 3.62 | 36.11 ± 4.27 | 0.64 | 0.03 | 0.01 |
| Linoleic acid | 22.96 ± 3.77 | 22.28 ± 3.72 | 26.13 ± 3.89 | 0.55 | 0.03 | 0.006 |
| Υ-Linolenic acid | 0.36 ± 0.17 | 0.30 ± 0.18 | 0.36 ± 0.15 | 0.94 | 0.16 | 0.13 |
| DGLA | 1.7 ± 0.31 | 1.84 ± 0.49 | 1.81 ± 0.36 | 0.28 | 0.38 | 0.84 |
| Arachidonic acid | 7.41 ± 1.76 | 7.37 ± 1.55 | 7.39 ± 2.16 | 0.93 | 0.98 | 0.96 |
| Osbond acid | 0.17 ± 0.07 | 0.20 ± 0.08 | 0.17 ± 0.05 | 0.20 | 0.89 | 0.19 |
| MCP1 (pg/mL) | CKD3 | CKD4 | CKD5 | |||
|---|---|---|---|---|---|---|
| r2 | p-value | r2 | p-value | r2 | p-value | |
| PUFA | 0.422 | 0.092 | 0.147 | 0.493 | −0.231 | 0.471 |
| n-3 | 0.162 | 0.535 | −0.032 | 0.881 | −0.28 | 0.379 |
| 18:3n3 | −0.209 | 0.421 | −0.256 | 0.228 | 0.21 | 0.513 |
| 20:5n3 | 0.13 | 0.619 | −0.012 | 0.955 | 0.119 | 0.713 |
| 22:5n3 | −0.02 | 0.94 | 0.325 | 0.122 | 0.056 | 0.863 |
| 22:6n3 | 0.223 | 0.39 | −0.056 | 0.796 | −0.326 | 0.301 |
| n-6 | 0.377 | 0.135 | 0.12 | 0.576 | −0.182 | 0.572 |
| 18:2n6 | 0.27 | 0.295 | −0.134 | 0.533 | −0.077 | 0.812 |
| 18:3n6 | −0.267 | 0.299 | 0.187 | 0.381 | 0.677 | 0.016 |
| 20:3n6 | −0.304 | 0.236 | 0.594 | 0.002 | 0.371 | 0.236 |
| 20:4n6 | 0.377 | 0.135 | 0.424 | 0.039 | 0.007 | 0.983 |
| 22.4n6 | −0.347 | 0.173 | 0.344 | 0.099 | 0.092 | 0.776 |
| 22:5n6 | 0.062 | 0.814 | 0.521 | 0.009 | 0.158 | 0.624 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattinzoli, D.; Turolo, S.; Alfieri, C.M.; Ikehata, M.; Caldiroli, L.; Armelloni, S.; Montini, G.; Agostoni, C.; Messa, P.; Vettoretti, S.; et al. MCP1 Could Mediate FGF23 and Omega 6/Omega 3 Correlation Inversion in CKD. J. Clin. Med. 2022, 11, 7099. https://doi.org/10.3390/jcm11237099
Mattinzoli D, Turolo S, Alfieri CM, Ikehata M, Caldiroli L, Armelloni S, Montini G, Agostoni C, Messa P, Vettoretti S, et al. MCP1 Could Mediate FGF23 and Omega 6/Omega 3 Correlation Inversion in CKD. Journal of Clinical Medicine. 2022; 11(23):7099. https://doi.org/10.3390/jcm11237099
Chicago/Turabian StyleMattinzoli, Deborah, Stefano Turolo, Carlo Maria Alfieri, Masami Ikehata, Lara Caldiroli, Silvia Armelloni, Giovanni Montini, Carlo Agostoni, Piergiorgio Messa, Simone Vettoretti, and et al. 2022. "MCP1 Could Mediate FGF23 and Omega 6/Omega 3 Correlation Inversion in CKD" Journal of Clinical Medicine 11, no. 23: 7099. https://doi.org/10.3390/jcm11237099
APA StyleMattinzoli, D., Turolo, S., Alfieri, C. M., Ikehata, M., Caldiroli, L., Armelloni, S., Montini, G., Agostoni, C., Messa, P., Vettoretti, S., & Castellano, G. (2022). MCP1 Could Mediate FGF23 and Omega 6/Omega 3 Correlation Inversion in CKD. Journal of Clinical Medicine, 11(23), 7099. https://doi.org/10.3390/jcm11237099






