Is Adynamic Bone Always a Disease? Lessons from Patients with Chronic Kidney Disease
Abstract
:1. Introduction
2. Epidemiology
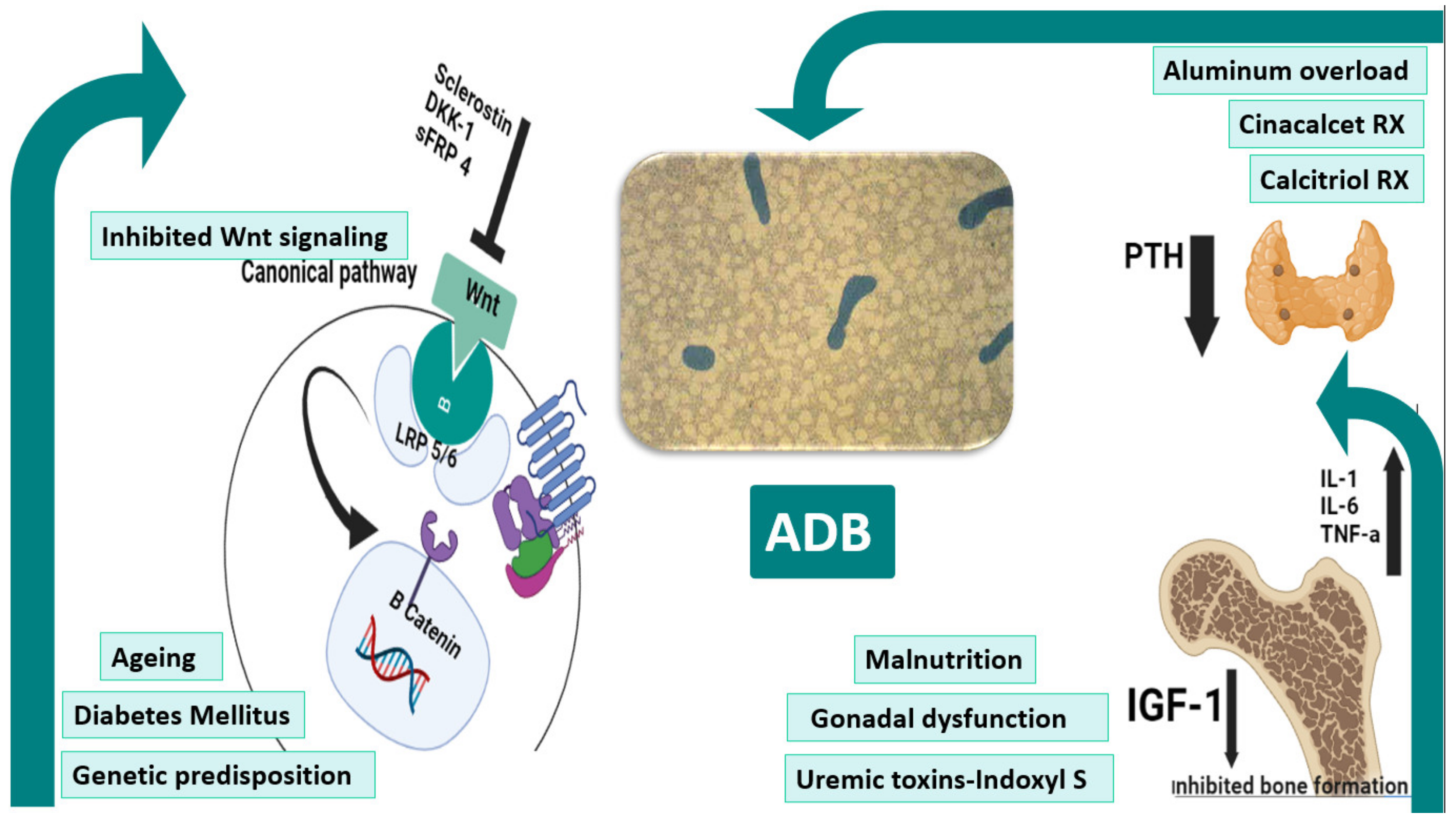
3. Pathogenesis
4. Impact of LBT on Bone Health/Fracture, Osteoporosis, and Mortality
5. ADB as a Risk Factor for Vascular Calcification
6. Variants of ADB
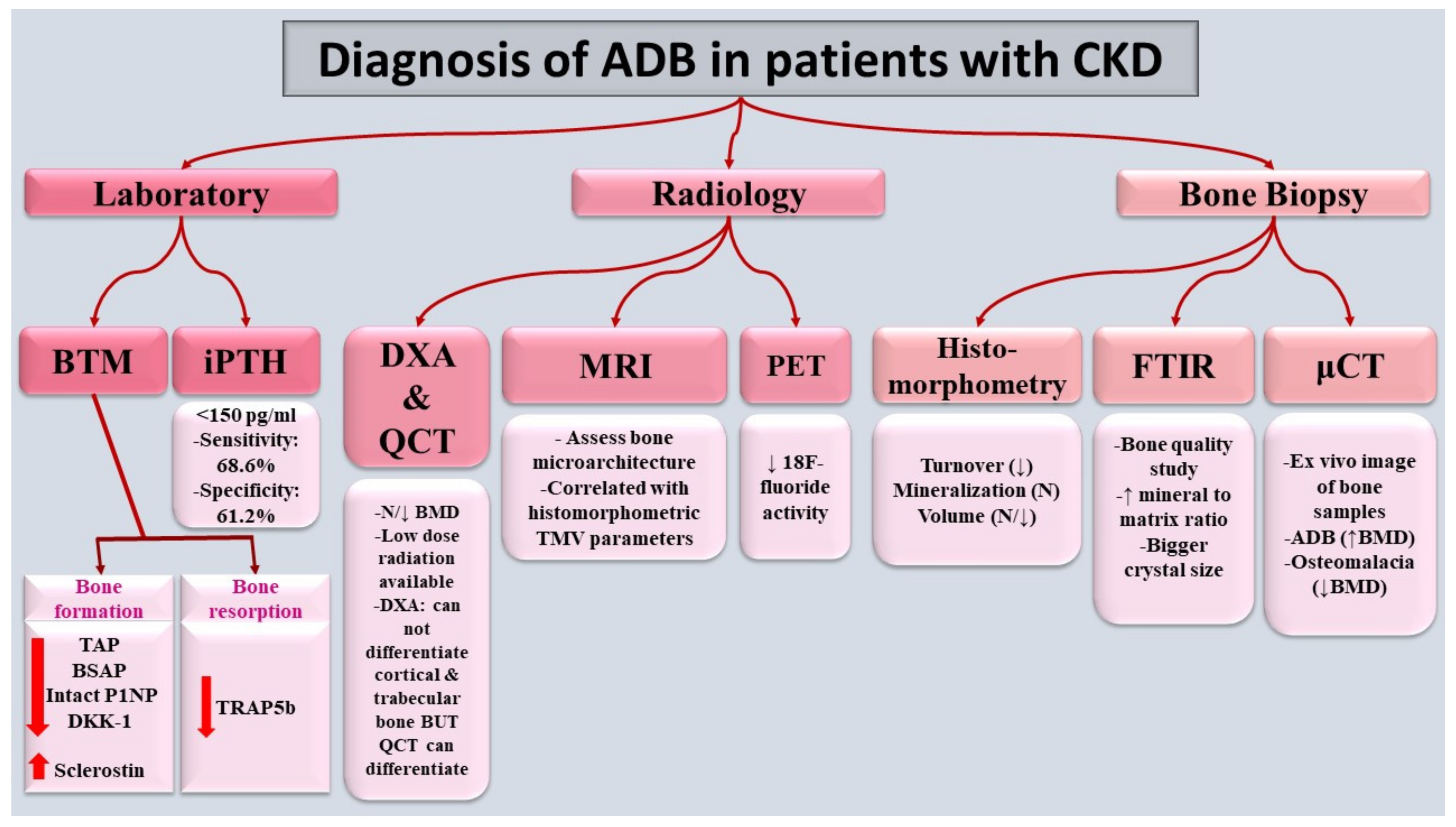
7. Diagnosis
7.1. Bone-Turnover Markers (BTMs)
7.1.1. PTH
7.1.2. Bone-Formation Biomarkers
7.1.3. Bone-Resorption Biomarkers
7.1.4. Other Biomarkers
7.2. Radiology
7.2.1. Dual-Energy X-ray Absorptiometry (DXA) Scan and Quantitative Computed Tomography (QCT)
7.2.2. High-Resolution Peripheral Quantitative Computed Tomography (HR-pQCT)
7.2.3. Positron Emission Tomography (PET) Scan
7.2.4. Magnetic-Resonance Imaging (MRI)
7.3. Bone Biopsy
8. Importance of Determining the Underlying Etiology of ADB
9. Prevention and Treatment of ADB in CKD
9.1. Over-Suppression of PTH Secretion
9.1.1. Vitamin D Analogs and Calcimimetics
9.1.2. Calcium Overload
Calcium Intake
Dialysate Calcium
9.2. Antiresorptives
9.3. Malnutrition–Inflammation Syndrome
9.4. Uremic Toxins
9.5. Drugs Used for Potential Treatment of ADB
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malluche, H.H.; Mawad, H.W.; Monier-Faugere, M.C. Renal osteodystrophy in the first decade of the new millennium: Analysis of 630 bone biopsies in black and white patients. J. Bone Miner. Res. 2011, 26, 1368–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D. Diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder: Synopsis of the kidney disease: Improving global outcomes 2017 clinical practice guideline update. Ann. Intern. Med. 2018, 168, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013, 28, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomiyama, C.; Carvalho, A.B.; Higa, A.; Jorgetti, V.; Draibe, S.A.; Canziani, M.E.F. Coronary calcification is associated with lower bone formation rate in CKD patients not yet in dialysis treatment. J. Bone Miner. Res. 2010, 25, 499–504. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Marty, C.; Marchais, S.J.; Guerin, A.P.; Metivier, F.; de Vernejoul, M.-C. Arterial calcifications and bone histomorphometry in end-stage renal disease. J. Am. Soc. Nephrol. 2004, 15, 1943–1951. [Google Scholar] [CrossRef]
- Hernandes, F.R.; Canziani, M.E.F.; Barreto, F.C.; Santos, R.O.; Moreira, V.d.M.; Rochitte, C.E.; Carvalho, A.B. The shift from high to low turnover bone disease after parathyroidectomy is associated with the progression of vascular calcification in hemodialysis patients: A 12-month follow-up study. PLoS ONE 2017, 12, e0174811. [Google Scholar] [CrossRef] [Green Version]
- Andreoli, S.P.; Bergstein, J.M.; Sherrard, D.J. Aluminum intoxication from aluminum-containing phosphate binders in children with azotemia not undergoing dialysis. N. Engl. J. Med. 1984, 310, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.; Ellis, H.; Feest, T.; Parkinson, I.; Kerr, D.; Herrington, J.; Goode, G. Osteomalacic dialysis osteodystrophy: Evidence for a water-borne aetiological agent, probably aluminium. Lancet 1978, 311, 841–845. [Google Scholar] [CrossRef]
- Coen, G.; Mazzaferro, S.; Ballanti, P.; Sardella, D.; Chicca, S.; Manni, M.; Bonucci, E.; Taggi, F. Renal bone disease in 76 patients with varying degrees of predialysis chronic renal failure: A cross-sectional study. Nephrol. Dial. Transplant. 1996, 11, 813–819. [Google Scholar] [CrossRef]
- Dukas, L.; Schacht, E.; Stähelin, H.B. In elderly men and women treated for osteoporosis a low creatinine clearance of< 65 ml/min is a risk factor for falls and fractures. Osteoporos. Int. 2005, 16, 1683–1690. [Google Scholar]
- Malluche, H.; Monier-Faugere, M.-C. Renal osteodystrophy: What’s in a name? Presentation of a clinically useful new model to interpret bone histologic findings. Clin. Nephrol. 2006, 65, 235–242. [Google Scholar] [CrossRef]
- Moe, S.; Drüeke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G. Kidney disease: Improving global outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006, 69, 1945–1953. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, A.J.; Whitehouse, R.W.; Boulton, H.F.; Adams, J.E.; Mawer, E.B.; Freemont, T.J.; Gokal, R. Correlation of bone histology with parathyroid hormone, vitamin D3, and radiology in end-stage renal disease. Kidney Int. 1993, 44, 1071–1077. [Google Scholar] [CrossRef] [Green Version]
- Sherrard, D.J.; Hercz, G.; Pei, Y.; Maloney, N.A.; Greenwood, C.; Manuel, A.; Saiphoo, C.; Fenton, S.S.; Segre, G.V. The spectrum of bone disease in end-stage renal failure—an evolving disorder. Kidney Int. 1993, 43, 436–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drüeke, T.B.; Massy, Z.A. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016, 89, 289–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprague, S.M.; Bellorin-Font, E.; Jorgetti, V.; Carvalho, A.B.; Malluche, H.H.; Ferreira, A.; D’Haese, P.C.; Drüeke, T.B.; Du, H.; Manley, T. Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am. J. Kidney Dis. 2016, 67, 559–566. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Moyses, R.M.A.; Neves, K.; Canziani, M.E.F.; Draibe, S.A.; Jorgetti, V.; Carvalho, A.B.D. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008, 73, 771–777. [Google Scholar] [CrossRef]
- Massy, Z.; Drueke, T. Adynamic bone disease is a predominant bone pattern in early stages of chronic kidney disease. J. Nephrol. 2017, 30, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.C.; Barreto, D.V.; Canziani, M.E.F.; Tomiyama, C.; Higa, A.; Mozar, A.; Glorieux, G.; Vanholder, R.; Massy, Z.; Carvalho, A.B.D. Association between indoxyl sulfate and bone histomorphometry in pre-dialysis chronic kidney disease patients. Braz. J. Nephrol. 2014, 36, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Husseini, A.; Abdalbary, M.; Lima, F.; Issa, M.; Ahmed, M.-T.; Winkler, M.; Srour, H.; Davenport, D.; Wang, G.; Faugere, M.-C. Low turnover renal osteodystrophy with abnormal bone quality and vascular calcification in patients with mild-to-moderate CKD. Kidney Int. Rep. 2022, 7, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Amr El-Husseini, M.M.A.; Issa, M.; Winkler, M.; Lima, F.; Faugere, M.; Srour, H.; Malluche, H.H. Progression of Renal Osteodystrophy and Vascular Calcifications in Patients with CKD Stage II-IV. In Kidney Week 2021; ASN: Orlando, FL, USA, 2021. [Google Scholar]
- Malluche, H.H.; Ritz, E.; Lange, H.P.; Kutschera, L.; Hodgson, M.; Seiffert, U.; Schoeppe, W. Bone histology in incipient and advanced renal failure. Kidney Int. 1976, 9, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.; Concepcion, M.T.; Lorenzo, V.; Martinez, M.E.; Rodriguez, A.; De Bonis, E.; Gonzalez-Posada, J.M.; Felsenfeld, A.J.; Rodriguez, M.; Torres, A. Adynamic bone disease with negative aluminium staining in predialysis patients: Prevalence and evolution after maintenance dialysis. Nephrol. Dial. Transpl. 1994, 9, 517–523. [Google Scholar] [CrossRef]
- Torres, A.; Lorenzo, V.; Hernández, D.; Rodríguez, J.C.; Concepción, M.T.; Rodríguez, A.P.; Hernández, A.; de Bonis, E.; Darias, E.; González-Posada, J.M.; et al. Bone disease in predialysis, hemodialysis, and CAPD patients: Evidence of a better bone response to PTH. Kidney Int. 1995, 47, 1434–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monier-Faugere, M.C.; Malluche, H.H. Trends in renal osteodystrophy: A survey from 1983 to 1995 in a total of 2248 patients. Nephrol. Dial. Transpl. 1996, 11 (Suppl. 3), 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, S.M.; Ambrosoni, P.; Lobão, R.R.; Caorsi, H.; Moysés, R.M.; Barreto, F.C.; Olaizola, I.; Cruz, E.A.; Petraglia, A.; Dos Reis, L.M.; et al. The renal osteodystrophy pattern in Brazil and Uruguay: An overview. Kidney Int. Suppl. 2003, S54–S56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreto, D.V.; Barreto Fde, C.; Carvalho, A.B.; Cuppari, L.; Draibe, S.A.; Dalboni, M.A.; Moyses, R.M.; Neves, K.R.; Jorgetti, V.; Miname, M.; et al. Association of changes in bone remodeling and coronary calcification in hemodialysis patients: A prospective study. Am. J. Kidney Dis. 2008, 52, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, C.E.M.; Reis, L.M.D.; Quadros, K.; Roza, N.A.V.; Sano, R.; Carvalho, A.B.; Jorgetti, V.; Oliveira, R.B. Renal osteodystrophy and clinical outcomes: Data from the Brazilian Registry of Bone Biopsies—REBRABO. J. Bras. Nefrol. 2020, 42, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Neto, R.; Pereira, L.; Magalhaes, J.; Quelhas-Santos, J.; Martins, S.; Carvalho, C.; Frazao, J.M. Sclerostin and DKK1 circulating levels associate with low bone turnover in patients with chronic kidney disease Stages 3 and 4. Clin. Kidney J. 2021, 14, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.S.; Behets, G.; Viaene, L.; Bammens, B.; Claes, K.; Meijers, B.; Naesens, M.; Sprangers, B.; Kuypers, D.; Cavalier, E.; et al. Diagnostic Accuracy of Noninvasive Bone Turnover Markers in Renal Osteodystrophy. Am. J. Kidney Dis. 2022, 79, 667–676.e1. [Google Scholar] [CrossRef] [PubMed]
- Hirata, J.; Hirai, K.; Asai, H.; Matsumoto, C.; Inada, M.; Miyaura, C.; Yamato, H.; Watanabe-Akanuma, M. Indoxyl sulfate exacerbates low bone turnover induced by parathyroidectomy in young adult rats. Bone 2015, 79, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Mozar, A.; Louvet, L.; Godin, C.; Mentaverri, R.; Brazier, M.; Kamel, S.; Massy, Z.A. Indoxyl sulphate inhibits osteoclast differentiation and function. Nephrol. Dial. Transpl. 2012, 27, 2176–2181. [Google Scholar] [CrossRef] [PubMed]
- Shyu, J.F.; Liu, W.C.; Zheng, C.M.; Fang, T.C.; Hou, Y.C.; Chang, C.T.; Liao, T.Y.; Chen, Y.C.; Lu, K.C. Toxic Effects of Indoxyl Sulfate on Osteoclastogenesis and Osteoblastogenesis. Int. J. Mol. Sci. 2021, 22, 11265. [Google Scholar] [CrossRef] [PubMed]
- Le Meur, Y.; Lorgeot, V.; Aldigier, J.C.; Wijdenes, J.; Leroux-Robert, C.; Praloran, V. Whole blood production of monocytic cytokines (IL-1beta, IL-6, TNF-alpha, sIL-6R, IL-1Ra) in haemodialysed patients. Nephrol. Dial. Transpl. 1999, 14, 2420–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monier-Faugere, M.C.; Malluche, H.H. Role of cytokines in renal osteodystrophy. Curr. Opin. Nephrol. Hypertens. 1997, 6, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Langub, M.C., Jr.; Koszewski, N.J.; Turner, H.V.; Monier-Faugere, M.C.; Geng, Z.; Malluche, H.H. Bone resorption and mRNA expression of IL-6 and IL-6 receptor in patients with renal osteodystrophy. Kidney Int. 1996, 50, 515–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haris, A.; Sherrard, D.J.; Hercz, G. Reversal of adynamic bone disease by lowering of dialysate calcium. Kidney Int. 2006, 70, 931–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akin, O.; Göl, K.; Aktürk, M.; Erkaya, S. Evaluation of bone turnover in postmenopausal patients with type 2 diabetes mellitus using biochemical markers and bone mineral density measurements. Gynecol. Endocrinol. 2003, 17, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, V.M.; Floege, J. Adynamic bone disease—bone and beyond. NDT Plus 2008, 1, 135–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nebeker, H.G.; Coburn, J.W. Aluminum and renal osteodystrophy. Annu. Rev. Med. 1986, 37, 79–95. [Google Scholar] [CrossRef]
- Cannata-Andía, J.B.; Cohen, J.J.; Harrington, J.T.; Madias, N.E.; Zusman, C.J. Hypokinetic azotemic osteodystrophy. Kidney Int. 1998, 54, 1000–1016. [Google Scholar] [CrossRef] [Green Version]
- Haarhaus, M.; Evenepoel, P.; European Renal Osteodystrophy (EUROD) Workgroup; Chronic Kidney Disease Mineral and Bone Disorder (CKD-MBD) Working Group of the European Renal Association–European Dialysis and Transplant Association. Differentiating the causes of adynamic bone in advanced chronic kidney disease informs osteoporosis treatment. Kidney Int. 2021, 100, 546–558. [Google Scholar] [CrossRef]
- Kakani, E.; Sloan, D.; Sawaya, B.P.; El-Husseini, A.; Malluche, H.H.; Rao, M. Long-term outcomes and management considerations after parathyroidectomy in the dialysis patient. In Seminars in Dialysis; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 541–552. [Google Scholar]
- Sánchez-González, M.C.; López-Barea, F.; Bajo, M.A.; Selgas, R. Serum albumin levels, an additional factor implicated in hyperparathyroidism outcome in peritoneal dialysis: A prospective study with paired bone biopsies. Adv. Perit. Dial. 2006, 22, 198–202. [Google Scholar]
- Gaipov, A.; Cseprekal, O.; Potukuchi, P.K.; Kabulbayev, K.; Remport, A.; Mathe, Z.; Talwar, M.; Balaraman, V.; Fulop, T.; Eason, J.D.; et al. Association between malnutrition-inflammation score and risk of subsequent self-reported bone fractures in prevalent kidney transplant recipients. Osteoporos. Int. 2019, 30, 611–620. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Yu, J.; Rho, J. Bone Loss Triggered by the Cytokine Network in Inflammatory Autoimmune Diseases. J. Immunol. Res. 2015, 2015, 832127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feroze, U.; Molnar, M.Z.; Dukkipati, R.; Kovesdy, C.P.; Kalantar-Zadeh, K. Insights into nutritional and inflammatory aspects of low parathyroid hormone in dialysis patients. J. Ren. Nutr. 2011, 21, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Carlstedt, E.; Ridefelt, P.; Lind, L.; Rastad, J. Interleukin-6 induced suppression of bovine parathyroid hormone secretion. Biosci. Rep. 1999, 19, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Akizawa, T.; Kinugasa, E.; Kurihara, R. Risk factors for the development of parathyroid hormone deficiency in dialysis patients. J. Am. Soc. Nephrol. 1998, 9, 561A. [Google Scholar]
- Riggs, B.L.; Khosla, S.; Melton, L.J., 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Brown, A.J. Mechanism of vitamin D action and its regulation. Am. J. Kidney Dis. 1998, 32 (Suppl. 2), S13–S24. [Google Scholar] [CrossRef] [PubMed]
- Andress, D.L. Adynamic bone in patients with chronic kidney disease. Kidney Int. 2008, 73, 1345–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vertino, A.M.; Bula, C.M.; Chen, J.R.; Almeida, M.; Han, L.; Bellido, T.; Kousteni, S.; Norman, A.W.; Manolagas, S.C. Nongenotropic, anti-apoptotic signaling of 1alpha,25(OH)2-vitamin D3 and analogs through the ligand binding domain of the vitamin D receptor in osteoblasts and osteocytes. Mediation by Src, phosphatidylinositol 3-, and JNK kinases. J. Biol. Chem. 2005, 280, 14130–14137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Ba, Y.; Xing, Q.; Du, J.L. Diabetes mellitus and the risk of fractures at specific sites: A meta-analysis. BMJ Open 2019, 9, e024067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krakauer, J.C.; McKenna, M.J.; Buderer, N.F.; Rao, D.S.; Whitehouse, F.W.; Parfitt, A.M. Bone loss and bone turnover in diabetes. Diabetes 1995, 44, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Hygum, K.; Starup-Linde, J.; Harsløf, T.; Vestergaard, P.; Langdahl, B.L. Mechanisms in Endocrinology: Diabetes mellitus, a state of low bone turnover—A systematic review and meta-analysis. Eur. J. Endocrinol. 2017, 176, R137–R157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Yamaguchi, T.; Kanazawa, I.; Sugimoto, T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem. Biophys. Res. Commun. 2015, 461, 193–199. [Google Scholar] [CrossRef]
- Kang, J.; Boonanantanasarn, K.; Baek, K.; Woo, K.M.; Ryoo, H.M.; Baek, J.H.; Kim, G.S. Hyperglycemia increases the expression levels of sclerostin in a reactive oxygen species- and tumor necrosis factor-alpha-dependent manner. J. Periodontal Implant Sci. 2015, 45, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Daniele, G.; Winnier, D.; Mari, A.; Bruder, J.; Fourcaudot, M.; Pengou, Z.; Tripathy, D.; Jenkinson, C.; Folli, F. Sclerostin and Insulin Resistance in Prediabetes: Evidence of a Cross Talk Between Bone and Glucose Metabolism. Diabetes Care 2015, 38, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.H.; Chiang, T.I.; Chang, I.C.; Lin, F.H.; Wei, C.C.; Cheng, Y.W. Increased levels of circulating advanced glycation end-products in menopausal women with osteoporosis. Int. J. Med. Sci. 2014, 11, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Valcourt, U.; Merle, B.; Gineyts, E.; Viguet-Carrin, S.; Delmas, P.D.; Garnero, P. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J. Biol. Chem. 2007, 282, 5691–5703. [Google Scholar] [CrossRef] [Green Version]
- Napoli, N.; Chandran, M.; Pierroz, D.D.; Abrahamsen, B.; Schwartz, A.V.; Ferrari, S.L. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017, 13, 208–219. [Google Scholar] [PubMed]
- Clowes, J.A.; Allen, H.C.; Prentis, D.M.; Eastell, R.; Blumsohn, A. Octreotide abolishes the acute decrease in bone turnover in response to oral glucose. J. Clin. Endocrinol. Metab. 2003, 88, 4867–4873. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Pantoja, E.L.; Ranganath, L.R.; Gallagher, J.A.; Wilson, P.J.; Fraser, W.D. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011, 11, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eastell, R.; Boonen, S.; Cosman, F.; Reid, I.R.; Palermo, L.; Cummings, S.R.; Black, D.M. Relationship between pretreatment rate of bone loss and bone density response to once-yearly ZOL: HORIZON-PFT extension study. J. Bone Miner. Res. 2015, 30, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.H.; Omelon, S.; Variola, F.; Allo, B.; Willett, T.L.; Alman, B.A.; Grynpas, M.D. Adynamic bone decreases bone toughness during aging by affecting mineral and matrix. J. Bone Miner. Res. 2016, 31, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, B.; Endo, I.; Ohnishi, Y.; Mitsui, Y.; Kurahashi, K.; Kanai, M.; Hiasa, M.; Teramachi, J.; Tenshin, H.; Fukumoto, S. Persistent Activation of Calcium-Sensing Receptor Suppresses Bone Turnover, Increases Microcracks, and Decreases Bone Strength. JBMR Plus 2019, 3, e10182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Husseini, A.; Chakraborty, A.; Yuan, Q.; Inayatullah, S.; Bush, H.; Sawaya, B.P. Urinary calcium excretion and bone turnover in osteoporotic patients. Clin. Nephrol. 2017, 88, 239. [Google Scholar] [CrossRef]
- Barreto, F.d.C.; Barreto, D.V.; Moyses, R.M.A.; Neves, C.; Jorgetti, V.; Draibe, S.A.; Canziani, M.E.; Carvalho, A. Osteoporosis in hemodialysis patients revisited by bone histomorphometry: A new insight into an old problem. Kidney Int. 2006, 69, 1852–1857. [Google Scholar] [CrossRef] [Green Version]
- Hughes-Austin, J.M.; Katz, R.; Semba, R.D.; Kritchevsky, S.B.; Bauer, D.C.; Sarnak, M.J.; Ginsberg, C.; Shlipak, M.G.; Lima, F.; Malluche, H.H. Biomarkers of bone turnover identify subsets of chronic kidney disease patients at higher risk for fracture. J. Clin. Endocrinol. Metab. 2020, 105, e2903–e2911. [Google Scholar] [CrossRef]
- Al Salmi, I.; Bieber, B.; Al Rukhaimi, M.; AlSahow, A.; Shaheen, F.; Al-Ghamdi, S.M.G.; Al Wakeel, J.; Al Ali, F.; Al-Aradi, A.; Hejaili, F.A.; et al. Parathyroid Hormone Serum Levels and Mortality among Hemodialysis Patients in the Gulf Cooperation Council Countries: Results from the DOPPS (2012–2018). Kidney360 2020, 1, 1083–1090. [Google Scholar] [CrossRef]
- Ganesh, S.K.; Stack, A.G.; Levin, N.W.; Hulbert-Shearon, T.; Port, F.K. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J. Am. Soc. Nephrol. 2001, 12, 2131–2138. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, H.; Zhang, Y.; Huang, H.; Liu, W.; Diao, Z. Low Parathyroid Hormone Versus Secondary Hyperparathyroidism and Survival in Patients Undergoing Hemodialysis: A Propensity-Matched Analysis. Front. Endocrinol. 2022, 13, 869330. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.A.; Kim, J.H.; Kim, Y.K.; Chang, Y.K.; Park, C.W.; Kim, S.Y.; Kim, Y.S.; Kang, S.W.; Kim, N.H.; Kim, Y.L.; et al. Low parathyroid hormone level predicts infection-related mortality in incident dialysis patients: A prospective cohort study. Korean J. Intern. Med. 2020, 35, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Jean, G.; Lataillade, D.; Genet, L.; Legrand, E.; Kuentz, F.; Moreau-Gaudry, X.; Fouque, D.; ARNOS Study Investigators. Association between very low PTH levels and poor survival rates in haemodialysis patients: Results from the French ARNOS cohort. Nephron Clin. Pract. 2011, 118, c211–c216. [Google Scholar] [CrossRef] [PubMed]
- Naves-Diaz, M.; Passlick-Deetjen, J.; Guinsburg, A.; Marelli, C.; Fernandez-Martin, J.L.; Rodriguez-Puyol, D.; Cannata-Andia, J.B. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol. Dial. Transpl. 2011, 26, 1938–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tentori, F.; Wang, M.; Bieber, B.A.; Karaboyas, A.; Li, Y.; Jacobson, S.H.; Andreucci, V.E.; Fukagawa, M.; Frimat, L.; Mendelssohn, D.C.; et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: The DOPPS study. Clin. J. Am. Soc. Nephrol. 2015, 10, 98–109. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Kuwae, N.; Regidor, D.; Kovesdy, C.; Kilpatrick, R.; Shinaberger, C.; McAllister, C.; Budoff, M.; Salusky, I.; Kopple, J. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006, 70, 771–780. [Google Scholar] [CrossRef] [Green Version]
- Bover, J.; Ureña, P.; Brandenburg, V.; Goldsmith, D.; Ruiz, C.; DaSilva, I.; Bosch, R.J. Adynamic bone disease: From bone to vessels in chronic kidney disease. In Seminars in Nephrology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 626–640. [Google Scholar]
- Jankowski, J.; Floege, J.; Fliser, D.; Bohm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Wu, M.; Rementer, C.; Giachelli, C.M. Vascular calcification: An update on mechanisms and challenges in treatment. Calcif. Tissue Int. 2013, 93, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Kurz, P.; Monier-Faugere, M.C.; Bognar, B.; Werner, E.; Roth, P.; Vlachojannis, J.; Malluche, H.H. Evidence for abnormal calcium homeostasis in patients with adynamic bone disease. Kidney Int. 1994, 46, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Lund, R.J.; Strebeck, F.; Tustison, K.S.; Geurs, T.; Hruska, K.A. Reversal of the adynamic bone disorder and decreased vascular calcification in chronic kidney disease by sevelamer carbonate therapy. J. Am. Soc. Nephrol. 2007, 18, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Alexander, A.J.; Jahangir, D.; Lazarus, M.; Sprague, S.M. Imaging in Chronic Kidney Disease-Metabolic Bone Disease. Semin. Dial. 2017, 30, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Asci, G.; Ok, E.; Savas, R.; Ozkahya, M.; Duman, S.; Toz, H.; Kayikcioglu, M.; Branscum, A.J.; Monier-Faugere, M.C.; Herberth, J.; et al. The link between bone and coronary calcifications in CKD-5 patients on haemodialysis. Nephrol. Dial. Transpl. 2011, 26, 1010–1015. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, R.A.; Barreto, F.C.; Mendes, M.; dos Reis, L.M.; Castro, J.H.; Britto, Z.M.; Marques, I.D.; Carvalho, A.B.; Moyses, R.M.; Jorgetti, V. Peritoneal dialysis per se is a risk factor for sclerostin-associated adynamic bone disease. Kidney Int. 2015, 87, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Neto, R.; Pereira, L.; Magalhaes, J.; Quelhas-Santos, J.; Frazao, J. Low bone turnover is associated with plain X-ray vascular calcification in predialysis patients. PLoS ONE 2021, 16, e0258284. [Google Scholar] [CrossRef] [PubMed]
- Nigwekar, S.U.; Kroshinsky, D.; Nazarian, R.M.; Goverman, J.; Malhotra, R.; Jackson, V.A.; Kamdar, M.M.; Steele, D.J.; Thadhani, R.I. Calciphylaxis: Risk factors, diagnosis, and treatment. Am. J. Kidney Dis. 2015, 66, 133–146. [Google Scholar] [CrossRef] [Green Version]
- Mawad, H.W.; Sawaya, B.P.; Sarin, R.; Malluche, H.H. Calcific uremic arteriolopathy in association with low turnover uremic bone disease. Clin. Nephrol. 1999, 52, 160–166. [Google Scholar] [PubMed]
- Patecki, M.; Lehmann, G.; Brasen, J.H.; Schmitz, J.; Bertram, A.; Berthold, L.D.; Haller, H.; Gwinner, W. A case report of severe calciphylaxis—suggested approach for diagnosis and treatment. BMC Nephrol. 2017, 18, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elder, G.; Kumar, K.S. Calciphylaxis associated with chronic kidney disease and low bone turnover: Management with recombinant human PTH-(1-34). NDT Plus 2008, 1, 97–99. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, V.M.; Kramann, R.; Rothe, H.; Kaesler, N.; Korbiel, J.; Specht, P.; Schmitz, S.; Kruger, T.; Floege, J.; Ketteler, M. Calcific uraemic arteriolopathy (calciphylaxis): Data from a large nationwide registry. Nephrol. Dial. Transpl. 2017, 32, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Cejka, D.; Herberth, J.; Branscum, A.J.; Fardo, D.W.; Monier-Faugere, M.C.; Diarra, D.; Haas, M.; Malluche, H.H. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin. J. Am. Soc. Nephrol. 2011, 6, 877–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graciolli, F.G.; Neves, K.R.; Barreto, F.; Barreto, D.V.; Dos Reis, L.M.; Canziani, M.E.; Sabbagh, Y.; Carvalho, A.B.; Jorgetti, V.; Elias, R.M.; et al. The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease. Kidney Int. 2017, 91, 1436–1446. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Kramann, R.; Koos, R.; Kruger, T.; Schurgers, L.; Muhlenbruch, G.; Hubner, S.; Gladziwa, U.; Drechsler, C.; Ketteler, M. Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: A cross-sectional study. BMC Nephrol. 2013, 14, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morena, M.; Jaussent, I.; Dupuy, A.M.; Bargnoux, A.S.; Kuster, N.; Chenine, L.; Leray-Moragues, H.; Klouche, K.; Vernhet, H.; Canaud, B.; et al. Osteoprotegerin and sclerostin in chronic kidney disease prior to dialysis: Potential partners in vascular calcifications. Nephrol. Dial. Transpl. 2015, 30, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Mace, M.L.; Gravesen, E.; Nordholm, A.; Egstrand, S.; Morevati, M.; Nielsen, C.; Kjaer, A.; Behets, G.; D’Haese, P.; Olgaard, K.; et al. Chronic Kidney Disease-Induced Vascular Calcification Impairs Bone Metabolism. J. Bone Min. Res. 2021, 36, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Mace, M.L.; Egstrand, S.; Morevati, M.; Olgaard, K.; Lewin, E. New Insights to the Crosstalk between Vascular and Bone Tissue in Chronic Kidney Disease-Mineral and Bone Disorder. Metabolites 2021, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, F.L.; Elias, R.M.; dos Reis, L.M.; Graciolli, F.G.; Zampieri, F.G.; Oliveira, R.B.; Jorgetti, V.; Moyses, R.M. Serum sclerostin is an independent predictor of mortality in hemodialysis patients. BMC Nephrol. 2014, 15, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean, G.; Chazot, C.; Bresson, E.; Zaoui, E.; Cavalier, E. High Serum Sclerostin Levels Are Associated with a Better Outcome in Haemodialysis Patients. Nephron 2016, 132, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Kalousova, M.; Dusilova-Sulkova, S.; Kubena, A.A.; Zakiyanov, O.; Tesar, V.; Zima, T. Sclerostin levels predict cardiovascular mortality in long-term hemodialysis patients: A prospective observational cohort study. Physiol. Res. 2019, 68, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Evenepoel, P.; Vervloet, M.G.; Wanner, C.; Ketteler, M.; Marx, N.; Floege, J.; Dekker, F.W.; Brandenburg, V.M.; Group, N.S. High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: Results from the NECOSAD study. Nephrol. Dial. Transpl. 2015, 30, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Yang, M.; Wang, J.; Cui, L.; Jiang, Z.; Ding, J.; Li, M.; Zhou, H. Association of sclerostin with cardiovascular events and mortality in dialysis patients. Ren. Fail. 2020, 42, 282–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, S.; Takahashi, H.E.; Ito, A.; Saito, N.; Nawata, M.; Horiuchi, H.; Ohta, H.; Ito, A.; Iorio, R.; Yamamoto, N.; et al. Trabecular minimodeling in human iliac bone. Bone 2003, 32, 163–169. [Google Scholar] [CrossRef]
- Ubara, Y.; Tagami, T.; Nakanishi, S.; Sawa, N.; Hoshino, J.; Suwabe, T.; Katori, H.; Takemoto, F.; Hara, S.; Takaichi, K. Significance of minimodeling in dialysis patients with adynamic bone disease. Kidney Int. 2005, 68, 833–839. [Google Scholar] [CrossRef] [Green Version]
- Ubara, Y.; Fushimi, T.; Tagami, T.; Sawa, N.; Hoshino, J.; Yokota, M.; Katori, H.; Takemoto, F.; Hara, S. Histomorphometric features of bone in patients with primary and secondary hypoparathyroidism. Kidney Int. 2003, 63, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Gal-Moscovici, A.; Popovtzer, M.M. Parathyroid hormone-independent osteoclastic resorptive bone disease: A new variant of adynamic bone disease in haemodialysis patients. Nephrol. Dial. Transpl. 2002, 17, 620–624. [Google Scholar] [CrossRef] [Green Version]
- Rocha, L.A.; Higa, A.; Barreto, F.C.; dos Reis, L.M.; Jorgetti, V.; Draibe, S.A.; Carvalho, A.B. Variant of adynamic bone disease in hemodialysis patients: Fact or fiction? Am. J. Kidney Dis. 2006, 48, 430–436. [Google Scholar] [CrossRef]
- Damasiewicz, M.J.; Nickolas, T.L. Rethinking Bone Disease in Kidney Disease. JBMR Plus 2018, 2, 309–322. [Google Scholar]
- Ureña, P.; Hruby, M.; Ferreira, A.; Ang, K.S.; de Vernejoul, M.C. Plasma total versus bone alkaline phosphatase as markers of bone turnover in hemodialysis patients. J. Am. Soc. Nephrol. 1996, 7, 506–512. [Google Scholar] [CrossRef]
- Moore, C.; Yee, J.; Malluche, H.; Rao, D.S.; Monier-Faugere, M.C.; Adams, E.; Daramola-Ogunwuyi, O.; Fehmi, H.; Bhat, S.; Osman-Malik, Y. Relationship between bone histology and markers of bone and mineral metabolism in African-American hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1484–1493. [Google Scholar] [CrossRef] [Green Version]
- Vervloet, M.G.; Brandenburg, V.M. Circulating markers of bone turnover. J. Nephrol. 2017, 30, 663–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eastell, R.; Szulc, P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017, 5, 908–923. [Google Scholar]
- Mazzaferro, S.; Tartaglione, L.; Rotondi, S.; Bover, J.; Goldsmith, D.; Pasquali, M. News on biomarkers in CKD-MBD. Semin. Nephrol. 2014, 34, 598–611. [Google Scholar] [CrossRef]
- Alvarez, L.; Torregrosa, J.V.; Peris, P.; Monegal, A.; Bedini, J.L.; Martinez De Osaba, M.J.; Filella, X.; Martin, G.; Ricos, C.; Oppenheimer, F.; et al. Effect of hemodialysis and renal failure on serum biochemical markers of bone turnover. J. Bone Min. Metab. 2004, 22, 254–259. [Google Scholar] [CrossRef]
- Ureña, P.; De Vernejoul, M.C. Circulating biochemical markers of bone remodeling in uremic patients. Kidney Int. 1999, 55, 2141–2156. [Google Scholar]
- Ho, L.T.; Sprague, S.M. Renal osteodystrophy in chronic renal failure. Semin. Nephrol. 2002, 22, 488–493. [Google Scholar] [CrossRef]
- Coen, G.; Mazzaferro, S.; Ballanti, P.; Bonucci, E.; Bondatti, F.; Manni, M.; Pasquali, M.; Perruzza, I.; Sardella, D.; Spurio, A. Procollagen type I C-terminal extension peptide in predialysis chronic renal failure. Am. J. Nephrol. 1992, 12, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Nickolas, T.L.; Stein, E.M.; Dworakowski, E.; Nishiyama, K.K.; Komandah-Kosseh, M.; Zhang, C.A.; McMahon, D.J.; Liu, X.S.; Boutroy, S.; Cremers, S.; et al. Rapid cortical bone loss in patients with chronic kidney disease. J. Bone Min. Res. 2013, 28, 1811–1820. [Google Scholar] [CrossRef]
- Kuo, T.H.; Lin, W.H.; Chao, J.Y.; Wu, A.B.; Tseng, C.C.; Chang, Y.T.; Liou, H.H.; Wang, M.C. Serum sclerostin levels are positively related to bone mineral density in peritoneal dialysis patients: A cross-sectional study. BMC Nephrol. 2019, 20, 266. [Google Scholar] [CrossRef] [Green Version]
- Ishimura, E.; Okuno, S.; Ichii, M.; Norimine, K.; Yamakawa, T.; Shoji, S.; Nishizawa, Y.; Inaba, M. Relationship between serum sclerostin, bone metabolism markers, and bone mineral density in maintenance hemodialysis patients. J. Clin. Endocrinol. Metab. 2014, 99, 4315–4320. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.; Magalhães, J.; Mendonça, L.; Neto, R.; Santos, J.; Carvalho, C.G.; Oliveira, A.; Beco, A.; Frazão, J. Evaluation of Renal Osteodystrophy and Serum Bone-Related Biomarkers in a Peritoneal Dialysis Population. J. Bone Min. Res. 2022, 37, 1689–1699. [Google Scholar] [CrossRef]
- Evenepoel, P.; Cavalier, E.; D’Haese, P.C. Biomarkers Predicting Bone Turnover in the Setting of CKD. Curr. Osteoporos. Rep. 2017, 15, 178–186. [Google Scholar] [CrossRef]
- Shidara, K.; Inaba, M.; Okuno, S.; Yamada, S.; Kumeda, Y.; Imanishi, Y.; Yamakawa, T.; Ishimura, E.; Nishizawa, Y. Serum levels of TRAP5b, a new bone resorption marker unaffected by renal dysfunction, as a useful marker of cortical bone loss in hemodialysis patients. Calcif. Tissue Int. 2008, 82, 278–287. [Google Scholar] [CrossRef]
- Henriksen, K.; Tanko, L.B.; Qvist, P.; Delmas, P.D.; Christiansen, C.; Karsdal, M.A. Assessment of osteoclast number and function: Application in the development of new and improved treatment modalities for bone diseases. Osteoporos. Int. 2007, 18, 681–685. [Google Scholar] [CrossRef]
- Staines, K.A.; MacRae, V.E.; Farquharson, C. The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling. J. Endocrinol. 2012, 214, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Wu, H.; Zhou, W.; Luo, M.; Tan, Y.; Miao, L.; Cai, L. Sirtuin 1: A Target for Kidney Diseases. Mol. Med. 2015, 21, 87–97. [Google Scholar]
- Asadipooya, K.; Abdalbary, M.; Ahmad, Y.; Kakani, E.; Monier-Faugere, M.C.; El-Husseini, A. Bone Quality in CKD Patients: Current Concepts and Future Directions—Part I. Kidney Dis. 2021, 7, 268–277. [Google Scholar] [CrossRef]
- Aaltonen, L.; Koivuviita, N.; Seppänen, M.; Burton, I.S.; Kröger, H.; Löyttyniemi, E.; Metsärinne, K. Bone Histomorphometry and (18)F-Sodium Fluoride Positron Emission Tomography Imaging: Comparison Between only Bone Turnover-based and Unified TMV-based Classification of Renal Osteodystrophy. Calcif. Tissue Int. 2021, 109, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Boutroy, S.; Bouxsein, M.L.; Munoz, F.; Delmas, P.D. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J. Clin. Endocrinol. Metab. 2005, 90, 6508–6515. [Google Scholar] [CrossRef]
- Tsuji, K.; Kitamura, M.; Chiba, K.; Muta, K.; Yokota, K.; Okazaki, N.; Osaki, M.; Mukae, H.; Nishino, T. Comparison of bone microstructures via high-resolution peripheral quantitative computed tomography in patients with different stages of chronic kidney disease before and after starting hemodialysis. Ren. Fail. 2022, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Cejka, D.; Patsch, J.M.; Weber, M.; Diarra, D.; Riegersperger, M.; Kikic, Z.; Krestan, C.; Schueller-Weidekamm, C.; Kainberger, F.; Haas, M. Bone microarchitecture in hemodialysis patients assessed by HR-pQCT. Clin. J. Am. Soc. Nephrol. 2011, 6, 2264–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blake, G.M.; Park-Holohan, S.J.; Cook, G.J.; Fogelman, I. Quantitative studies of bone with the use of 18F-fluoride and 99mTc-methylene diphosphonate. Semin. Nucl. Med. 2001, 31, 28–49. [Google Scholar] [PubMed]
- Aaltonen, L.; Koivuviita, N.; Seppänen, M.; Tong, X.; Kröger, H.; Löyttyniemi, E.; Metsärinne, K. Correlation between (18)F-Sodium Fluoride positron emission tomography and bone histomorphometry in dialysis patients. Bone 2020, 134, 115267. [Google Scholar] [CrossRef]
- Sharma, A.K.; Toussaint, N.D.; Elder, G.J.; Masterson, R.; Holt, S.G.; Robertson, P.L.; Ebeling, P.R.; Baldock, P.; Miller, R.C.; Rajapakse, C.S. Magnetic resonance imaging based assessment of bone microstructure as a non-invasive alternative to histomorphometry in patients with chronic kidney disease. Bone 2018, 114, 14–21. [Google Scholar] [CrossRef]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D.; et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s changed and why it matters. Kidney Int. 2017, 92, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Min. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef]
- El-Husseini, A.; Sawaya, B.P. What is the role of bone biopsy in the management of adult dialysis patients? Semin. Dial. 2014, 27, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Misof, B.M.; Blouin, S.; Roschger, P.; Werzowa, J.; Klaushofer, K.; Lehmann, G. Bone matrix mineralization and osteocyte lacunae characteristics in patients with chronic kidney disease—mineral bone disorder (CKD-MBD). J. Musculoskelet. Neuronal Interact. 2019, 19, 196–206. [Google Scholar]
- Evenepoel, P.; D’Haese, P.; Bacchetta, J.; Cannata-Andia, J.; Ferreira, A.; Haarhaus, M.; Mazzaferro, S.; Lafage Proust, M.H.; Salam, S.; Spasovski, G.; et al. Bone biopsy practice patterns across Europe: The European renal osteodystrophy initiative-a position paper. Nephrol. Dial. Transpl. 2017, 32, 1608–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørgensen, H.S.; Ferreira, A.C.; D’Haese, P.; Haarhaus, M.; Vervloet, M.; Lafage-Proust, M.H.; Ferreira, A.; Evenepoel, P. Bone histomorphometry for the diagnosis of renal osteodystrophy: A call for harmonization of reference ranges. Kidney Int. 2022, 102, 431–434. [Google Scholar] [CrossRef]
- Malluche, H.H.; Porter, D.S.; Pienkowski, D. Evaluating bone quality in patients with chronic kidney disease. Nat. Rev. Nephrol. 2013, 9, 671–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.C.; Bischoff, D.S.; Yamaguchi, D.; Salusky, I.B.; Wesseling-Perry, K. Micro-CT in the Assessment of Pediatric Renal Osteodystrophy by Bone Histomorphometry. Clin. J. Am. Soc. Nephrol. 2016, 11, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amr El-Husseini, F.L.; Meulendyke, K.; Abdalbary, M.; Nagy, E.; Srour, H.; Faugere, M.; Malluche, H. Changes in Bone Quality Over Two Years in Patients with CKD II-IV. In Kidney Week 2022; ASN: Orlando, FL, USA, 2022. [Google Scholar]
- Iseri, K.; Watanabe, M.; Yoshikawa, H.; Mitsui, H.; Endo, T.; Yamamoto, Y.; Iyoda, M.; Ryu, K.; Inaba, T.; Shibata, T. Effects of Denosumab and Alendronate on Bone Health and Vascular Function in Hemodialysis Patients: A Randomized, Controlled Trial. J. Bone Min. Res. 2019, 34, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.J.; Burkart, J.; Piazza, M.; Russell, G.; Rohr, M.; Carr, J.J. Changes in cardiovascular calcification after parathyroidectomy in patients with ESRD. Am. J. Kidney Dis. 2005, 46, 464–469. [Google Scholar] [CrossRef]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar]
- Kawata, T.; Nagano, N.; Obi, M.; Miyata, S.; Koyama, C.; Kobayashi, N.; Wakita, S.; Wada, M. Cinacalcet suppresses calcification of the aorta and heart in uremic rats. Kidney Int. 2008, 74, 1270–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Tocados, J.M.; Rodríguez-Ortiz, M.E.; Almadén, Y.; Pineda, C.; Martínez-Moreno, J.M.; Herencia, C.; Vergara, N.; Pendón-Ruiz de Mier, M.V.; Santamaría, R.; Rodelo-Haad, C.; et al. Calcimimetics maintain bone turnover in uremic rats despite the concomitant decrease in parathyroid hormone concentration. Kidney Int. 2019, 95, 1064–1078. [Google Scholar] [CrossRef]
- Yajima, A.; Inaba, M.; Tominaga, Y.; Ito, A. Bone formation by minimodeling is more active than remodeling after parathyroidectomy. Kidney Int. 2008, 74, 775–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.L.; Shyu, J.F.; Wu, C.C.; Hung, C.F.; Liao, M.T.; Liu, W.C.; Zheng, C.M.; Hou, Y.C.; Lin, Y.F.; Lu, K.C. Association of Anabolic Effect of Calcitriol with Osteoclast-Derived Wnt 10b Secretion. Nutrients 2018, 10, 1164. [Google Scholar] [CrossRef] [Green Version]
- Pataki, A.; Müller, K.; Green, J.R.; Ma, Y.F.; Li, Q.N.; Jee, W.S. Effects of short-term treatment with the bisphosphonates zoledronate and pamidronate on rat bone: A comparative histomorphometric study on the cancellous bone formed before, during, and after treatment. Anat. Rec. 1997, 249, 458–468. [Google Scholar] [CrossRef]
- Kalaitzoglou, E.; Popescu, I.; Bunn, R.C.; Fowlkes, J.L.; Thrailkill, K.M. Effects of Type 1 Diabetes on Osteoblasts, Osteocytes, and Osteoclasts. Curr. Osteoporos. Rep. 2016, 14, 310–319. [Google Scholar] [CrossRef]
- Sanches, C.P.; Vianna, A.G.D.; Barreto, F.C. The impact of type 2 diabetes on bone metabolism. Diabetol. Metab. Synd. 2017, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- McGee, M.E.; Maki, A.J.; Johnson, S.E.; Nelson, O.L.; Robbins, C.T.; Donahue, S.W. Decreased bone turnover with balanced resorption and formation prevent cortical bone loss during disuse (hibernation) in grizzly bears (Ursus arctos horribilis). Bone 2008, 42, 396–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGee-Lawrence, M.E.; Wojda, S.J.; Barlow, L.N.; Drummer, T.D.; Castillo, A.B.; Kennedy, O.; Condon, K.W.; Auger, J.; Black, H.L.; Nelson, O.L.; et al. Grizzly bears (Ursus arctos horribilis) and black bears (Ursus americanus) prevent trabecular bone loss during disuse (hibernation). Bone 2009, 45, 1186–1191. [Google Scholar] [CrossRef]
- McGee-Lawrence, M.; Buckendahl, P.; Carpenter, C.; Henriksen, K.; Vaughan, M.; Donahue, S. Suppressed bone remodeling in black bears conserves energy and bone mass during hibernation. J. Exp. Biol. 2015, 218 Pt 13, 2067–2074. [Google Scholar] [CrossRef] [Green Version]
- Evenepoel, P.; Cunningham, J.; Ferrari, S.; Haarhaus, M.; Javaid, M.K.; Lafage-Proust, M.H.; Prieto-Alhambra, D.; Torres, P.U.; Cannata-Andia, J. European Consensus Statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4-G5D. Nephrol. Dial. Transpl. 2021, 36, 42–59. [Google Scholar] [CrossRef] [PubMed]
- El-Husseini, A.; Sobh, M.; Elshabrawy, N.; Abdalbary, M. Antiresorptives in patients with chronic kidney disease with adynamic bone: Is absence of evidence of harm equal to no harm? Kidney Int. 2021, 100, 1341–1342. [Google Scholar] [CrossRef]
- Sista, S.K.; Arum, S.M. Management of adynamic bone disease in chronic kidney disease: A brief review. J. Clin. Transl. Endocrinol. 2016, 5, 32–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, W.G.; Ramirez, J.A.; Belin, T.R.; Chon, Y.; Gales, B.; Segre, G.V.; Salusky, I.B. Development of adynamic bone in patients with secondary hyperparathyroidism after intermittent calcitriol therapy. Kidney Int. 1994, 46, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Malluche, H.H.; Mawad, H.; Koszewski, N.J. Update on vitamin D and its newer analogues: Actions and rationale for treatment in chronic renal failure. Kidney Int. 2002, 62, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Pahl, M.; Jara, A.; Bover, J.; Felsenfeld, A.J. Studies in a hemodialysis patient indicating that calcitriol may have a direct suppressive effect on bone. Nephron 1995, 71, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Monier-Faugere, M.C.; Mawad, H.; Malluche, H.H. Opposite effects of calcitriol and paricalcitol on the parathyroid hormone-(1-84)/large carboxy-terminal-parathyroid hormone fragments ratio in patients with stage 5 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Hansen, D.; Rasmussen, K.; Danielsen, H.; Meyer-Hofmann, H.; Bacevicius, E.; Lauridsen, T.G.; Madsen, J.K.; Tougaard, B.G.; Marckmann, P.; Thye-Roenn, P.; et al. No difference between alfacalcidol and paricalcitol in the treatment of secondary hyperparathyroidism in hemodialysis patients: A randomized crossover trial. Kidney Int. 2011, 80, 841–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. (2011) 2017, 7, 1–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evenepoel, P.; Bover, J.; Urena Torres, P. Parathyroid hormone metabolism and signaling in health and chronic kidney disease. Kidney Int. 2016, 90, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. 1), S1–S107. [Google Scholar]
- Ferreira, A.; Frazao, J.M.; Monier-Faugere, M.C.; Gil, C.; Galvao, J.; Oliveira, C.; Baldaia, J.; Rodrigues, I.; Santos, C.; Ribeiro, S.; et al. Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J. Am. Soc. Nephrol. 2008, 19, 405–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Wang, Y.; Chen, H.; Zhu, X.; Zhou, L.; Yang, Y. The effects of non-calcium-based phosphate binders versus calcium-based phosphate binders on cardiovascular calcification and bone remodeling among dialysis patients: A meta-analysis of randomized trials. Ren. Fail. 2014, 36, 1244–1252. [Google Scholar] [CrossRef]
- Wang, A.Y. Calcium balance and negative impact of calcium load in peritoneal dialysis patients. Perit. Dial. Int. 2014, 34, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Fujimori, A.; Yorifuji, M.; Sakai, M.; Oyama, M.; Nakao, N.; Tokuyama, M.; Fukagawa, M. Low-calcium dialysate improves mineral metabolism in hemodialysis patients. Clin. Nephrol. 2007, 67, 20–24. [Google Scholar] [CrossRef]
- Lezaic, V.; Pejanovic, S.; Kostic, S.; Pljesa, S.; Dimkovic, N.; Komadina, L.; Jovanovic, D.; Marinkovic, J.; Djukanovic, L. Effects of lowering dialysate calcium concentration on mineral metabolism and parathyroid hormone secretion: A multicentric study. Apher. Dial. 2007, 11, 121–130. [Google Scholar] [CrossRef]
- Spasovski, G.; Gelev, S.; Masin-Spasovska, J.; Selim, G.; Sikole, A.; Vanholder, R. Improvement of bone and mineral parameters related to adynamic bone disease by diminishing dialysate calcium. Bone 2007, 41, 698–703. [Google Scholar] [CrossRef]
- Burr, D.B. Fifty years of bisphosphonates: What are their mechanical effects on bone? Bone 2020, 138, 115518. [Google Scholar] [CrossRef]
- Bone, H.G.; Wagman, R.B.; Brandi, M.L.; Brown, J.P.; Chapurlat, R.; Cummings, S.R.; Czerwinski, E.; Fahrleitner-Pammer, A.; Kendler, D.L.; Lippuner, K.; et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017, 5, 513–523. [Google Scholar] [CrossRef]
- Dempster, D.W.; Brown, J.P.; Fahrleitner-Pammer, A.; Kendler, D.; Rizzo, S.; Valter, I.; Wagman, R.B.; Yin, X.; Yue, S.V.; Boivin, G. Effects of Long-Term Denosumab on Bone Histomorphometry and Mineralization in Women With Postmenopausal Osteoporosis. J. Clin. Endocrinol. Metab. 2018, 103, 2498–2509. [Google Scholar] [CrossRef]
- Jamal, S.A.; Bauer, D.C.; Ensrud, K.E.; Cauley, J.A.; Hochberg, M.; Ishani, A.; Cummings, S.R. Alendronate treatment in women with normal to severely impaired renal function: An analysis of the fracture intervention trial. J. Bone Min. Res. 2007, 22, 503–508. [Google Scholar] [CrossRef]
- Dupont, J.; Laurent, M.R.; Dedeyne, L.; Luyten, F.P.; Gielen, E.; Dejaeger, M. Rebound-associated vertebral fractures after stopping denosumab: Report of four cases. Jt. Bone Spine 2020, 87, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Maugars, Y.; Guillot, P.; Glémarec, J.; Berthelot, J.M.; Le Goff, B.; Darrieutort-Laffite, C. Long-term follow up after denosumab treatment for osteoporosis—rebound associated with hypercalcemia, parathyroid hyperplasia, severe bone mineral density loss, and multiple fractures: A case report. J. Med. Case Rep. 2020, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, P.; Faouzi, M.; Buclin, T.; Lamy, O. Fractures After Denosumab Discontinuation: A Retrospective Study of 797 Cases. J. Bone Min. Res. 2021, 36, 1717–1728. [Google Scholar] [CrossRef]
- Niimi, R.; Kono, T.; Nishihara, A.; Hasegawa, M.; Kono, T.; Sudo, A. Second rebound-associated vertebral fractures after denosumab discontinuation. Arch. Osteoporos. 2020, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Makras, P.; Yavropoulou, M.P.; Tabacco, G.; Naciu, A.M.; Palermo, A. Denosumab Discontinuation and the Rebound Phenomenon: A Narrative Review. J. Clin. Med. 2021, 10, 152. [Google Scholar] [CrossRef]
- Cummings, S.R.; Ferrari, S.; Eastell, R.; Gilchrist, N.; Jensen, J.B.; McClung, M.; Roux, C.; Törring, O.; Valter, I.; Wang, A.T.; et al. Vertebral Fractures After Discontinuation of Denosumab: A Post Hoc Analysis of the Randomized Placebo-Controlled FREEDOM Trial and Its Extension. J. Bone Min. Res. 2018, 33, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Mazurenko, S.; Feofanova, S. Severe rebound effect and multiple fractures after denosumab discontinuation in patient with chronic kidney disease stage 5. Bone Rep. 2020, 13, 100562. [Google Scholar] [CrossRef]
- Pimentel, A.; Urena-Torres, P.; Zillikens, M.C.; Bover, J.; Cohen-Solal, M. Fractures in patients with CKD-diagnosis, treatment, and prevention: A review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int. 2017, 92, 1343–1355. [Google Scholar] [CrossRef] [Green Version]
- Saab, G.; Whaley-Connell, A.; McFarlane, S.I.; Li, S.; Chen, S.C.; Sowers, J.R.; McCullough, P.A.; Bakris, G.L.; Kidney Early Evaluation Program, Investigators. Obesity is associated with increased parathyroid hormone levels independent of glomerular filtration rate in chronic kidney disease. Metabolism 2010, 59, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Yamato, H.; Nii-Kono, T.; Fujieda, A.; Uchida, M.; Hosokawa, A.; Motojima, M.; Fukagawa, M. Administration of oral charcoal adsorbent (AST-120) suppresses low-turnover bone progression in uraemic rats. Nephrol. Dial. Transpl. 2006, 21, 2768–2774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheshwari, V.; Tao, X.; Thijssen, S.; Kotanko, P. Removal of Protein-Bound Uremic Toxins Using Binding Competitors in Hemodialysis: A Narrative Review. Toxins 2021, 13, 622. [Google Scholar] [CrossRef]
- Khairallah, P.; Nickolas, T.L. Management of Osteoporosis in CKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 962–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.H.; Lo, W.C.; Hu, P.J.; Chan, H.C.; Shen, W.C.; Wu, M.S.; Wu, M.Y. Efficacy of Osteoporosis Medications for Patients With Chronic Kidney Disease: An Updated Systematic Review and Network Meta-Analysis. Front. Pharm. 2022, 13, 822178. [Google Scholar] [CrossRef]
- Ayodele, O.; Rejnmark, L.; Mu, F.; Lax, A.; Berman, R.; Swallow, E.; Gosmanova, E.O. Five-Year Estimated Glomerular Filtration Rate in Adults with Chronic Hypoparathyroidism Treated with rhPTH(1-84): A Retrospective Cohort Study. Adv. Ther. 2022, 39, 5013–5024. [Google Scholar] [CrossRef] [PubMed]
- Krege, J.H.; Wan, X. Teriparatide and the risk of nonvertebral fractures in women with postmenopausal osteoporosis. Bone 2012, 50, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Hattersley, G.; Mitlak, B.H.; Hu, M.Y.; Fitzpatrick, L.A.; Dabrowski, C.; Miller, P.D.; Papapoulos, S.E. Abaloparatide in patients with mild or moderate renal impairment: Results from the ACTIVE phase 3 trial. Curr. Med. Res. Opin. 2019, 35, 2097–2102. [Google Scholar] [CrossRef]
- Miller, P.D.; Hattersley, G.; Riis, B.J.; Williams, G.C.; Lau, E.; Russo, L.A.; Alexandersen, P.; Zerbini, C.A.; Hu, M.Y.; Harris, A.G.; et al. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. JAMA 2016, 316, 722–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef] [Green Version]
- Miller, P.D.; Adachi, J.D.; Albergaria, B.H.; Cheung, A.M.; Chines, A.A.; Gielen, E.; Langdahl, B.L.; Miyauchi, A.; Oates, M.; Reid, I.R.; et al. Efficacy and Safety of Romosozumab Among Postmenopausal Women With Osteoporosis and Mild-to-Moderate Chronic Kidney Disease. J. Bone Min. Res. 2022, 37, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Mizobuchi, M.; Kato, T.; Suzuki, T.; Fujiwara, Y.; Kanamori, N.; Makuuchi, M.; Honda, H. One-Year Romosozumab Treatment Followed by One-Year Denosumab Treatment for Osteoporosis in Patients on Hemodialysis: An Observational Study. Calcif. Tissue Int. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Sato, M.; Inaba, M.; Yamada, S.; Emoto, M.; Ohno, Y.; Tsujimoto, Y. Efficacy of romosozumab in patients with osteoporosis on maintenance hemodialysis in Japan; an observational study. J. Bone Min. Metab. 2021, 39, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Mitsopoulos, E.; Ginikopoulou, E.; Economidou, D.; Zanos, S.; Pateinakis, P.; Minasidis, E.; Memmos, D.; Thodis, E.; Vargemezis, V.; Tsakiris, D. Impact of long-term cinacalcet, ibandronate or teriparatide therapy on bone mineral density of hemodialysis patients: A pilot study. Am. J. Nephrol. 2012, 36, 238–244. [Google Scholar] [CrossRef]
- Cejka, D.; Kodras, K.; Bader, T.; Haas, M. Treatment of Hemodialysis-Associated Adynamic Bone Disease with Teriparatide (PTH1-34): A Pilot Study. Kidney Blood Press Res. 2010, 33, 221–226. [Google Scholar] [CrossRef]
- Sumida, K.; Ubara, Y.; Hoshino, J.; Mise, K.; Hayami, N.; Suwabe, T.; Kawada, M.; Imafuku, A.; Hiramatsu, R.; Hasegawa, E.; et al. Once-weekly teriparatide in hemodialysis patients with hypoparathyroidism and low bone mass: A prospective study. Osteoporos. Int. 2016, 27, 1441–1450. [Google Scholar] [CrossRef]
- Fitzpatrick, L.A.; Dabrowski, C.E.; Cicconetti, G.; Gordon, D.N.; Fuerst, T.; Engelke, K.; Genant, H.K. Ronacaleret, a calcium-sensing receptor antagonist, increases trabecular but not cortical bone in postmenopausal women. J. Bone Min. Res. 2012, 27, 255–262. [Google Scholar] [CrossRef]
| Study | Patient Population | Finding |
|---|---|---|
| 1. Malluche et al. 1976 [22] | 50 patients in different stages of CKD (GFR 6–80 mL/min/1.73 m2) | Prevalence of woven osteoid, as expression of osteitis fibrosa, was increasing with decreasing GFR. Osteoclastic surface resorption was abnormally high with GFR < 50. Mineralization defect was present only in patients with GFR < 40 mL/min/1.73 m2. |
| 2. Hutchison et al. 1993 [13] | 30 ESKD on CAPD | Osteitis fibrosa was the most common histological diagnosis (50%). ADB was found in 27%, mixed ROD in 13%, and osteomalacia in 7% of patients. |
| 3. Sherrard et al. 1993 [14] | 259 HD and PD patients | In PD patients 66% had LBT, whereas in HD patients 62% had HBT.Osteomalacia was the least common pattern (4% of all patients). |
| 4. Hernandez et al. 1994 [23] | 92 pre-dialysis patients with GFR < 10 mL/min/1.73 m2 | ADB reported in 32%. Stainable bone aluminum surface was < 3% in all patients with ADB. |
| 5. Torres et al. 1995 [24] | 119 unselected CKD5: 38 were pre-dialysis, 49 on HD, and 32 on CAPD | ADB was a common finding (48%, 32%, and 48% in pre-dialysis, HD, and CAPD, respectively). |
| 6. Monier-Faugere and Malluche 1996 [25] | 2248 bone biopsies from ESKD patients on dialysis | HBT in 24.5%, mixed ROD in 52.9%, osteomalacia in 6.2%, and ADB in 16.4%. Patients on CAPD exhibited more histological signs of ADB. |
| 7. Coen et al. 1996 [9] | 76 pre-dialysis CKD patients, mean GFR 20 mL/min per 1.73 m2 | Normal bone histology in 13%, ADB in 12% (all negative for aluminum staining), mixed ROD in 63%, predominant osteomalacia in 9%, and predominant HBT in 3%. Patients with ADB had a less severe degree of CKD. |
| 8. Araújo et al. 2003 [26] | 2507 dialysis patients who underwent bone biopsies (1985–2001) for diagnostic purposes. | Comparing the 1980s to the 1990s, the prevalence of patients with HBT increased from 32.3% to 44.0%, whereas aluminum overload decreased from 61.3% to 42.4%. Osteomalacia decreased whereas the prevalence of ADB increased from 15.7% to 20.4%. |
| 9. Barreto et al. 2008 [27] | 38 bone biopsies from HD patients | Baseline bone biopsies showed HBT in 37% and LBT in 63%; after 1 year of sevelamer or calcium acetate HBT was reported in 39% and LBT in 61% of patients. |
| 10. Malluche et al. 2011 [1] | 630 dialysis patients | LBT was diagnosed in 58%, normal turnover in 18%, and HBT in 24% of patients. With 3.5 mEq/L of dialyzed calcium, there were more LBT patients than with 2.5 mEq/L. White patients exhibited predominantly LBT, whereas HBT was the prominent feature in black patients. |
| 11. Barreto et al. 2014 [19] | 49 pre-dialysis CKD stages 2–5 patients | Patients at CKD stages 2–3 presented remarkable LBT. In comparison to patients with CKD stages 2 and 3, patients with CKD stages 4–5 showed higher osteoid volume, osteoblast and osteoclast surface, bone fibrosis, and BFR, as well as a lower MLT. |
| 12. Sprague et al. 2016 [16] | 492 dialysis patients | LBT in 59% of patients, HBT in 17% of patients, and 24% had normal turnover. |
| 13. Carbonara et al. 2020 [28] | 260 CKD–MBD stage 3–5D patients | HBT, mixed ROD, ADB, osteomalacia, osteoporosis, and aluminum accumulation were detected in 33%, 17%, 10%, 4%, 30%, and 25% of patients, respectively. |
| 14. Neto et al. 2021 [29] | 56 patients with CKD stages 3–4. | ADB in 38%, HBT in 21%, and mixed ROD in 2% of patients, whereas 41% had normal bone histology. |
| 15. Jørgensen et al. 2022 [30] | 199 kidney-transplant candidates and recipients | Bone turnover was low in 17%, normal in 55%, and high in 29% of patients. |
| 16. El-Husseini et al. 2022 [20] | 32 CKD stage 2–4 patients with no specific bone-biopsy indication | 84% had LBT, 6% had HBT, and 10% had normal turnover. LBT with abnormal bone quality was predominant in early CKD stages. |
| Drugs | Mechanism of Action | Main Studies | Results |
|---|---|---|---|
| Teriparatide (PTH 1–34) | –A recombinant form of PTH, consisting of amino acids 1–34 that bind to PTH type 1 receptor stimulating osteoblast activity | Mitsopoulos et al.: 9 hemodialysis patients; 48 weeks of therapy [200]. | With teriparatide: –Improvement in BMD (femoral neck: 2.7%; lumbar: 4.9%). |
| Cejka et al.: 7 patients with ADB, 6-month therapy [201]. | With teriparatide: –Improvement in BMD at lumbar spine. –No changes in BMD at femoral neck, bone-turnover markers, or CAC. | ||
| Sumida et al.: 22 patients on dialysis; 56.5 μg dose once-weekly for 48 weeks [202]. | With teriparatide: –High rate of discontinuation (50%) due to transient hypotension. –Improvement of BMD at lumbar spine by 3.3% and 3.0% at weeks 24 and 48, respectively. –No change in the BMD at femoral neck and distal radius. | ||
| Abaloparatide | –Fragment of parathyroid hormone-related peptide | Miller et al.: ACTIVE was phase 3, double blinded, RCT included 1645 postmenopausal women who received SC 80 μg abaloparatide or placebo daily [195]. | With abaloparatide: Improvement in BMD at 1.5 years: –At total hip by 4.18%. –At femoral neck by 3.6%. –At lumber spine by 11.2%. |
| Bilezikian et al.: post hoc analysis of ACTIVE to evaluate safety and efficacy of abaloparatide in patients with different kidney functions [194]. | With abaloparatide: Improvement in BMD at 1.5 years: –At lumbar spine by 9.91% in patients with eGFR < 60 mL/min. –At femoral neck by 3.06% in patients with eGFR < 60 mL/min. | ||
| Romosozumab | –Sclerostin-humanized monoclonal antibody –Has anabolic properties | Miller et al.: post hoc analysis of FRAME and ARCH trials. FRAME included 7147 osteoporotic postmenopausal women who received 210 mg SC romosozumab or monthly placebo and ARCH (received 210 mg SC romosozumab monthly or 70 mg oral alendronate weekly) enrolled 4077 postmenopausal females with osteoporosis and fragility fractures [197]. | With romosozumab In FRAME: Improvement in BMD at 1 year: –At lumbar spine by 13% and 10.9% in patients with mild and moderate CKD, respectively. –At total hip by 5.9% and 5.2% in patients with mild and moderate CKD, respectively. –At femoral neck by 5.3% and 4.6% in patients with mild and moderate CKD, respectively. In ARCH: Improvement in BMD at 1 year: –At lumbar spine by 8.8% and 8.1% in patients with mild and moderate CKD, respectively. –At total hip by 3.2% and 3% in patients with mild and moderate CKD, respectively. –At femoral neck by 3.2% and 2.7% in patients with mild and moderate CKD, respectively. |
| Sato et al.: included 76 HD patients with high risk of fractures who received 210 mg SC romosozumab monthly and 55 HD untreated patients [199]. | With romosozumab: –Improvement in BMD at lumber spine at 1 year by 15.3%. –Improvement in BMD at femoral neck at 1 year by 7.2%. | ||
| Ronacaleret | –Calcium-sensing receptor antagonist –Calcilytic –Increases endogenous production of PTH | Fitzpatrick et al.: placebo-controlled, dose-ranging trial. 569 women with post-menopausal osteoporosis, teriparatide 20 µg SC once daily or 100 mg, 200 mg, 300 mg, or 400 mg ronacaleret once daily; 70 mg alendronate once weekly; or matching placebos in a double-blind fashion [203]. | –Improvement in spine integral vBMD (0.49% to 3.9%). –Improvement in trabecular vBMD (1.8% to 13.3%). –Non-dose-dependent decrease (1.79%) in integral vBMD at proximal femur. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, E.; Sobh, M.M.; Abdalbary, M.; Elnagar, S.; Elrefaey, R.; Shabaka, S.; Elshabrawy, N.; Shemies, R.; Tawfik, M.; Santos, C.G.S.; et al. Is Adynamic Bone Always a Disease? Lessons from Patients with Chronic Kidney Disease. J. Clin. Med. 2022, 11, 7130. https://doi.org/10.3390/jcm11237130
Nagy E, Sobh MM, Abdalbary M, Elnagar S, Elrefaey R, Shabaka S, Elshabrawy N, Shemies R, Tawfik M, Santos CGS, et al. Is Adynamic Bone Always a Disease? Lessons from Patients with Chronic Kidney Disease. Journal of Clinical Medicine. 2022; 11(23):7130. https://doi.org/10.3390/jcm11237130
Chicago/Turabian StyleNagy, Eman, Mahmoud M. Sobh, Mohamed Abdalbary, Sherouk Elnagar, Rabab Elrefaey, Shimaa Shabaka, Nehal Elshabrawy, Rasha Shemies, Mona Tawfik, Cássia Gomes S. Santos, and et al. 2022. "Is Adynamic Bone Always a Disease? Lessons from Patients with Chronic Kidney Disease" Journal of Clinical Medicine 11, no. 23: 7130. https://doi.org/10.3390/jcm11237130
APA StyleNagy, E., Sobh, M. M., Abdalbary, M., Elnagar, S., Elrefaey, R., Shabaka, S., Elshabrawy, N., Shemies, R., Tawfik, M., Santos, C. G. S., Barreto, F. C., & El-Husseini, A. (2022). Is Adynamic Bone Always a Disease? Lessons from Patients with Chronic Kidney Disease. Journal of Clinical Medicine, 11(23), 7130. https://doi.org/10.3390/jcm11237130






