From Virtual Reality to Regenerative Virtual Therapy: Some Insights from a Systematic Review Exploring Inner Body Perception in Anorexia and Bulimia Nervosa
Abstract
1. Introduction
Inner Body Perception in Eating Disorders
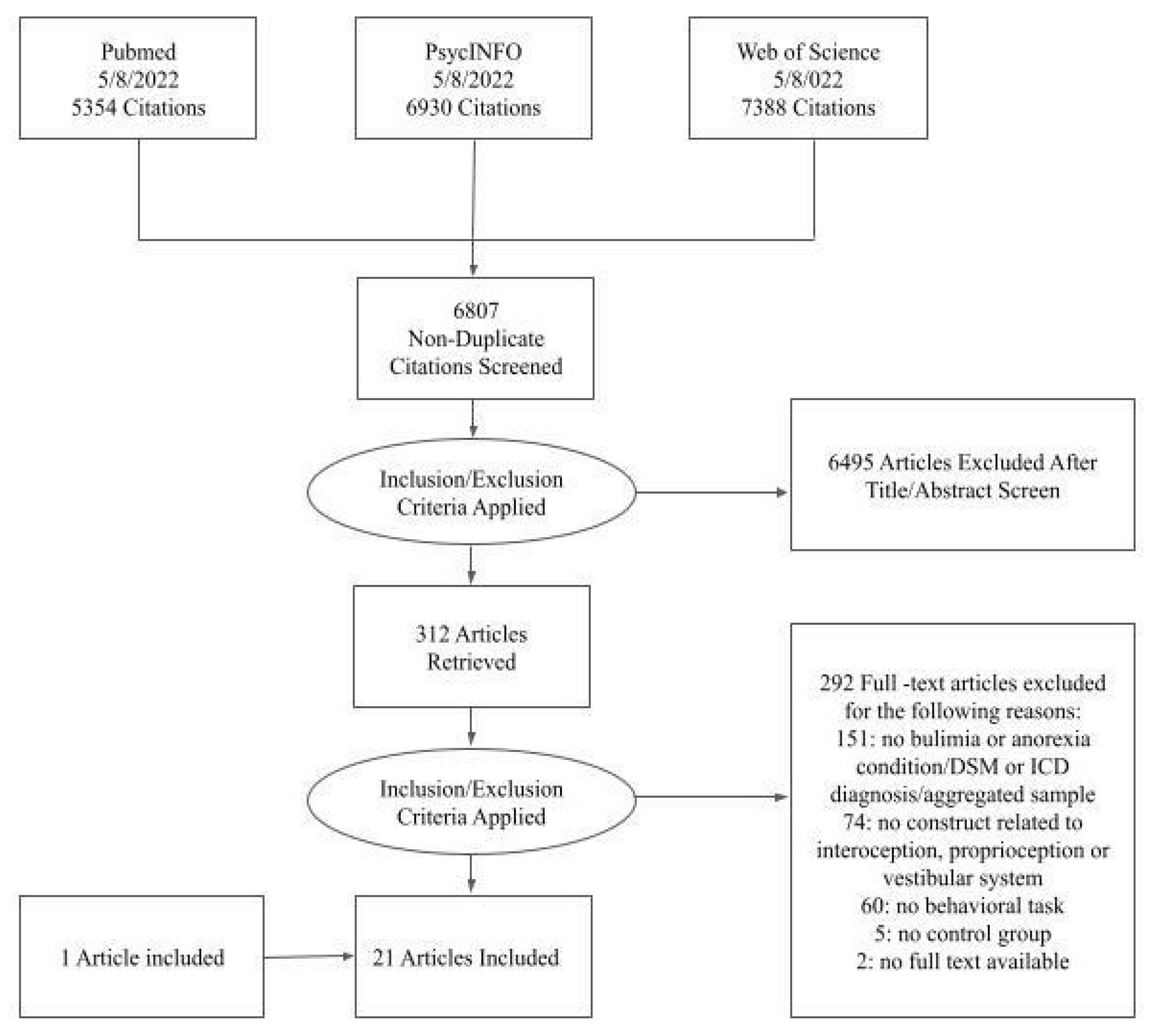
2. Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection and Inclusion Criteria
- (a)
- Investigate a sample of individuals that meet a current diagnosis of AN and BN, according to the Diagnostic and Statistical Manual of mental disorders (DSM) or International Classification of Diseases (ICD). In other words, studies in which the participants self-reported the diagnosis, used self-reported measures to identify participants’ diagnosis, or in which the diagnosis was not provided by a professional (e.g., a clinical psychologist) were not included in the systematic review. Both adults and adolescents with a current diagnosis of AN or BN were considered eligible. Studies that considered the participants’ sample as aggregated (e.g., reporting under the same category of EDs multiple diagnoses) were also not included in the systematic review;
- (b)
- Include a healthy control group (HC) or a population of normative values to compare the clinical group with;
- (c)
- Use tasks or instruments to evaluate interoception according to Craig’s definition [35,38]; or proprioception according to Blanke’s definition [40]; or vestibular perception according to Lopez’s definition [73]. Studies that employed self-reported questionnaires to assess such dimensions were excluded;
- (d)
- Use tasks that directly evaluate one or more sensory domains. Interoceptive input was considered present when the task tested sensitivity to visceral activity [35]. Proprioceptive input was considered present when the task was based on a sensory judgment about limb and body position [40]. Vestibular input was considered present when the task tested the sensation of any change in balance, position, direction, or movement of the eyes, head, or body [73].
- (e)
- Use behavioral and cognitive tasks. Studies involving manipulation of the variable of interest (e.g., through medications or psychological interventions) were not included in the systematic review;
- (f)
- Be original articles: reviews, meeting abstracts, conference proceedings, notes, letters to the editor, research protocols, patents, editorials, books or chapters, and other editorial materials were not considered eligible for this systematic review;
- (g)
- Be quantitative studies: qualitative studies were not included;
- (h)
- Be in English, enroll humans (i.e., studies that use animals were excluded) and have an available full text.
2.3. Study Inclusion
2.4. Data Extraction
3. Results
3.1. Study Characteristics
3.2. Tasks Employed to Assess Interoception, Proprioception, and Vestibular Processes
3.2.1. Interoception
3.2.2. Proprioception
3.2.3. Vestibular System
3.3. Primary Outcomes in Anorexic and Bulimic Patients
3.3.1. Interoception Outcomes
3.3.2. Proprioception Outcomes
3.3.3. Vestibular Outcomes
4. Discussion
4.1. Interoceptive Deficits in Anorexia and Bulimia Nervosa
4.2. Proprioception in Anorexia and Bulimia Nervosa
4.3. Vestibular System in Anorexia and Bulimia Nervosa
5. Conclusions and Future Direction
Supplementary Materials
Funding
Conflicts of Interest
References
- Ciążyńska, J.; Maciaszek, J. Various Types of Virtual Reality-Based Therapy for Eating Disorders: A Systematic Review. J. Clin. Med. 2022, 11, 4956. [Google Scholar] [CrossRef]
- Clus, D.; Larsen, M.E.; Lemey, C.; Berrouiguet, S. The Use of Virtual Reality in Patients with Eating Disorders: Systematic Review. J. Med. Internet Res. 2018, 20, e157. [Google Scholar] [CrossRef]
- Ferrer-Garcia, M.; Gutiérrez-Maldonado, J.; Riva, G. Virtual Reality Based Treatments in Eating Disorders and Obesity: A Review. J. Contemp. Psychother. 2013, 43, 207–221. [Google Scholar] [CrossRef]
- Riva, G.; Bernardelli, L.; Castelnuovo, G.; Di Lernia, D.; Tuena, C.; Clementi, A.; Pedroli, E.; Malighetti, C.; Sforza, F.; Wiederhold, B.; et al. A Virtual Reality-Based Self-Help Intervention for Dealing with the Psychological Distress Associated with the COVID-19 Lockdown: An Effectiveness Study with a Two-Week Follow-Up. Int. J. Environ. Res. Public Health 2021, 18, 8188. [Google Scholar] [CrossRef]
- Riva, G.; Mantovani, F.; Capideville, C.S.; Preziosa, A.; Morganti, F.; Villani, D.; Gaggioli, A.; Botella, C.; Alcañiz, M. Affective Interactions Using Virtual Reality: The Link between Presence and Emotions. Cyberpsychol. Behav. 2007, 10, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Malighetti, C.; Serino, S. Virtual reality in the treatment of eating disorders. Clin. Psychol. Psychother. 2021, 28, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.; Vogel, E.N.; Adler, S.; Bohon, C.; Bullock, K.; Nameth, K.; Riva, G.; Safer, D.L.; Runfola, C.D. Bringing Virtual Reality from Clinical Trials to Clinical Practice for the Treatment of Eating Disorders: An Example Using Virtual Reality Cue Exposure Therapy. J. Med. Internet Res. 2020, 22, e16386. [Google Scholar] [CrossRef] [PubMed]
- Riva, G. Virtual Reality in Psychotherapy: Review. Cyberpsychol. Behav. 2005, 8, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Riva, G. The Key to Unlocking the Virtual Body: Virtual Reality in the Treatment of Obesity and Eating Disorders. J. Diabetes Sci. Technol. 2011, 5, 283–292. [Google Scholar] [CrossRef]
- Ferrer-García, M.; Gutiérrez-Maldonado, J. The use of virtual reality in the study, assessment, and treatment of body image in eating disorders and nonclinical samples: A review of the literature. Body Image 2012, 9, 1–11. [Google Scholar] [CrossRef]
- Sansoni, M.; Malighetti, C.; Riva, G. Psychological and Educational Interventions Among Cancer Patients: A Systematic Review to Analyze the Role of Immersive Virtual Reality for Improving Patients’ Well-Being. In Extended Reality. XR Salento; De Paolis, L.T., Arpaia, P., Sacco, M., Eds.; Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2022; Volume 13446. [Google Scholar] [CrossRef]
- Sansoni, M.; Scarzello, G.; Serino, S.; Groff, E.; Riva, G. Mitigating negative emotions through virtual reality and embodiment. Front. Hum. Neurosci. 2022, 16, 916227. [Google Scholar] [CrossRef]
- Sansoni, M.; Riva, G. 360-VIRTOncology: Virtual Reality to Improve Cancer Patients’ Well-Being. Cyberpsychol. Behav. Soc. Netw. 2022, 25, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Gorini, A.; Capideville, C.S.; De Leo, G.; Mantovani, F.; Riva, G. The Role of Immersion and Narrative in Mediated Presence: The Virtual Hospital Experience. Cyberpsychol. Behav. Soc. Netw. 2011, 14, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Garcia, M.; Pla-Sanjuanelo, J.; Dakanalis, A.; Vilalta-Abella, F.; Riva, G.; Fernandez-Aranda, F.; Forcano, L.; Riesco, N.; Sánchez, I.; Clerici, M.; et al. A Randomized Trial of Virtual Reality-Based Cue Exposure Second-Level Therapy and Cognitive Behavior Second-Level Therapy for Bulimia Nervosa and Binge-Eating Disorder: Outcome at Six-Month Followup. Cyberpsychol. Behav. Soc. Netw. 2019, 22, 60–68. [Google Scholar] [CrossRef]
- Ferrer-García, M.; Gutiérrez-Maldonado, J.; Pla-Sanjuanelo, J.; Vilalta-Abella, F.; Riva, G.; Clerici, M.; Ribas-Sabaté, J.; Andreu-Gracia, A.; Fernandez-Aranda, F.; Forcano, L.; et al. A Randomised Controlled Comparison of Second-Level Treatment Approaches for Treatment-Resistant Adults with Bulimia Nervosa and Binge Eating Disorder: Assessing the Benefits of Virtual Reality Cue Exposure Therapy. Eur. Eat. Disord. Rev. 2017, 25, 479–490. [Google Scholar] [CrossRef]
- Marco, J.H.; Perpiñá, C.; Botella, C. Effectiveness of cognitive behavioral therapy supported by virtual reality in the treatment of body image in eating disorders: One year follow-up. Psychiatry Res. 2013, 209, 619–625. [Google Scholar] [CrossRef]
- Chirico, A.; Malighetti, C.; Serino, S.; Cipresso, P.; Pedroli, E.; Tuena, C.; Riva, G. Towards an advancement of multisensory integration deficits in anorexia nervosa: Exploring temporal discrimination processing of visuo-auditory stimuli. Annu. Rev. Cybertherapy Telemed. 2020, 17, 53–58. [Google Scholar]
- Dakanalis, A.; Gaudio, S.; Serino, S.; Clerici, M.; Carrà, G.; Riva, G. Body-image distortion in anorexia nervosa. Nat. Rev. Dis. Prim. 2016, 2, 16026. [Google Scholar] [CrossRef]
- Riva, G.; Dakanalis, A. Altered Processing and Integration of Multisensory Bodily Representations and Signals in Eating Disorders: A Possible Path toward the Understanding of Their Underlying Causes. Front. Hum. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Teaford, M.; McMurray, M.S.; Billock, V.; Filipkowski, M.; Smart, L.J. The somatosensory system in anorexia nervosa: A scoping review. J. Exp. Psychopathol. 2021, 12, 2043808720987346. [Google Scholar] [CrossRef]
- Riva, G. Neuroscience and eating disorders: The allocentric lock hypothesis. Med. Hypotheses 2012, 78, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Gaudio, S. Allocentric lock in anorexia nervosa: New evidences from neuroimaging studies. Med. Hypotheses 2012, 79, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Gaudio, S.; Dakanalis, A. I’m in a virtual body: A locked allocentric memory may impair the experience of the body in both obesity and anorexia nervosa. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2013, 19, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Riva, G. Out of my real body: Cognitive neuroscience meets eating disorders. Front. Hum. Neurosci. 2014, 8, 234. [Google Scholar] [CrossRef]
- Serino, S.; Pedroli, E.; Keizer, A.; Triberti, S.; Dakanalis, A.; Pallavicini, F.; Chirico, A.; Riva, G. Virtual Reality Body Swapping: A Tool for Modifying the Allocentric Memory of the Body. Cyberpsychol. Behav. Soc. Netw. 2016, 19, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Serino, S.; Chirico, A.; Pedroli, E.; Polli, N.; Cacciatore, C.; Riva, G. Two-phases innovative treatment for anorexia nervosa: The potential of virtual reality body-swap. Ann. Rev. Cyber Ther. Telemed. 2017, 15, 111–115. [Google Scholar]
- Serino, S.; Dakanalis, A. Bodily illusions and weight-related disorders: Clinical insights from experimental research. Ann. Phys. Rehabil. Med. 2017, 60, 217–219. [Google Scholar] [CrossRef]
- Serino, S.; Polli, N.; Riva, G. From avatars to body swapping: The use of virtual reality for assessing and treating body-size distortion in individuals with anorexia. J. Clin. Psychol. 2018, 75, 313–322. [Google Scholar] [CrossRef]
- Keizer, A.; Van Elburg, A.; Helms, R.; Dijkerman, H.C. A Virtual Reality Full Body Illusion Improves Body Image Disturbance in Anorexia Nervosa. PLoS ONE 2016, 11, e0163921. [Google Scholar] [CrossRef] [PubMed]
- Petkova, V.I.; Ehrsson, H.H. If I Were You: Perceptual Illusion of Body Swapping. PLoS ONE 2008, 3, e3832. [Google Scholar] [CrossRef]
- Matamala-Gomez, M.; Maselli, A.; Malighetti, C.; Realdon, O.; Mantovani, F.; Riva, G. Virtual Body Ownership Illusions for Mental Health: A Narrative Review. J. Clin. Med. 2021, 10, 139. [Google Scholar] [CrossRef]
- Riva, G. Letter to the Editor: Virtual reality in the treatment of eating and weight disorders. Psychol. Med. 2017, 47, 2567–2568. [Google Scholar] [CrossRef] [PubMed]
- Riva, G. The neuroscience of body memory: From the self through the space to the others. Cortex 2018, 104, 241–260. [Google Scholar] [PubMed]
- Craig, A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, M.; Jiménez, A.T.; Costantini, M. Just a heartbeat away from one’s body: Interoceptive sensitivity predicts malleability of body-representations. Proc. R. Soc. B Biol. Sci. 2011, 278, 2470–2476. [Google Scholar] [CrossRef] [PubMed]
- Babo-Rebelo, M.; Wolpert, N.; Adam, C.; Hasboun, D.; Tallon-Baudry, C. Is the cardiac monitoring function related to the self in both the default network and right anterior insula? Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160004. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, S.N.; Seth, A.K.; Barrett, A.B.; Suzuki, K.; Critchley, H.D. Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 2015, 104, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, S.N.; Critchley, H.D. Interoception, emotion and brain: New insights link internal physiology to social behaviour. Commentary on: “Anterior insular cortex mediates bodily sensibility and social anxiety” by Terasawa et al. (2012). Soc. Cogn. Affect. Neurosci. 2013, 8, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Blanke, O. Multisensory brain mechanisms of bodily self-consciousness. Nat. Rev. Neurosci. 2012, 13, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Proske, U. The role of muscle proprioceptors in human limb position sense: A hypothesis. J. Anat. 2015, 227, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Azañón, E.; Tamè, L.; Maravita, A.; Linkenauger, S.A.; Ferrè, E.R.; Tajadura-Jiménez, A.; Longo, M.R. Multimodal contributions to body representation. Multisens. Res. 2016, 29, 635–661. [Google Scholar] [CrossRef]
- Berthoz, A. How does the cerebral cortex process and utilize vestibular signals. In Disorders of the Vestibular System; Oxford University Press: New York, NY, USA, 1996. [Google Scholar]
- Ferrè, E.R.; Haggard, P. The vestibular body: Vestibular contributions to bodily representations. Cogn. Neuropsychol. 2016, 33, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Cabanac, M.; Duclaux, R. Obesity: Absence of Satiety Aversion to Sucrose. Science 1970, 168, 496–497. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, S.; Iavarone, A.; La Marra, M.; Messina, G.; Villano, I.; Ranucci, S.; Messina, A.; Piombino, L.; Dalia, C.; Monda, M. Memory for proprioceptive targets in bulimia nervosa. J. Psychiatry 2015, 18, 2. [Google Scholar]
- Gaudio, S.; Brooks, S.; Riva, G. Nonvisual Multisensory Impairment of Body Perception in Anorexia Nervosa: A Systematic Review of Neuropsychological Studies. PLoS ONE 2014, 9, e110087. [Google Scholar] [CrossRef] [PubMed]
- Natenshon, A.H. Eating Disorders: A Treatment Apart. The Unique Use of the Therapist’s Self in the Treatment of Eating Disorders. In Eating Disorders: A Paradigm of the Biopsychosocial Model of Illness; Intech: Vienna, Austria, 2017; pp. 159–192. [Google Scholar]
- Kerr, K.L.; Moseman, S.E.; Avery, J.A.; Bodurka, J.; Zucker, N.L.; Simmons, W.K. Altered Insula Activity during Visceral Interoception in Weight-Restored Patients with Anorexia Nervosa. Neuropsychopharmacology 2015, 41, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Nunn, K.; Frampton, I.; Fuglset, T.S.; Törzsök-Sonnevend, M.; Lask, B. Anorexia nervosa and the insula. Med. Hypotheses 2011, 76, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Pollatos, O.; Kurz, A.-L.; Albrecht, J.; Schreder, T.; Kleemann, A.M.; Schöpf, V.; Kopietz, R.; Wiesmann, M.; Schandry, R. Reduced perception of bodily signals in anorexia nervosa. Eat. Behav. 2008, 9, 381–388. [Google Scholar] [CrossRef]
- Zucker, N.L.; Merwin, R.M.; Bulik, C.M.; Moskovich, A.; Wildes, J.E.; Groh, J. Subjective experience of sensation in anorexia nervosa. Behav. Res. Ther. 2013, 51, 256–265. [Google Scholar] [CrossRef]
- Oberndorfer, T.A.; Frank, G.; Simmons, A.N.; Wagner, A.; McCurdy, D.; Fudge, J.L.; Yang, T.T.; Paulus, M.P.; Kaye, W.H. Altered Insula Response to Sweet Taste Processing After Recovery from Anorexia and Bulimia Nervosa. Am. J. Psychiatry 2013, 170, 1143–1151. [Google Scholar] [CrossRef]
- Blechert, J.; Ansorge, U.; Tuschen-Caffier, B. A body-related dot-probe task reveals distinct attentional patterns for bulimia nervosa and anorexia nervosa. J. Abnorm. Psychol. 2010, 119, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Guardia, D.; Lafargue, G.; Thomas, P.; Dodin, V.; Cottencin, O.; Luyat, M. Anticipation of body-scaled action is modified in anorexia nervosa. Neuropsychologia 2010, 48, 3961–3966. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Nagamitsu, S.; Ozono, S.; Yamashita, Y.; Ishibashi, M.; Matsuishi, T. Regional cerebral blood flow changes in early-onset anorexia nervosa before and after weight gain. Brain Dev. 2010, 32, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Nico, D.; Daprati, E.; Nighoghossian, N.; Carrier, E.; Duhamel, J.-R.; Sirigu, A. The role of the right parietal lobe in anorexia nervosa. Psychol. Med. 2009, 40, 1531–1539. [Google Scholar] [CrossRef]
- Sachdev, P.; Mondraty, N.; Wen, W.; Gulliford, K. Brains of anorexia nervosa patients process self-images differently from non-self-images: An fMRI study. Neuropsychologia 2008, 46, 2161–2168. [Google Scholar] [CrossRef]
- Grunwald, M.; Ettrich, C.; Assmann, B.; Dähne, A.; Krause, W.; Busse, F.; Gertz, H.-J. Deficits in haptic perception and right parietal theta power changes in patients with anorexia nervosa before and after weight gain. Int. J. Eat. Disord. 2001, 29, 417–428. [Google Scholar] [CrossRef]
- Mohr, H.M.; Zimmermann, J.; Röder, C.; Lenz, C.; Overbeck, G.; Grabhorn, R. Separating two components of body image in anorexia nervosa using fMRI. Psychol. Med. 2009, 40, 1519–1529. [Google Scholar] [CrossRef]
- Cipolletta, S.; Malighetti, C.; Serino, S.; Riva, G.; Winter, D. Intrapersonal, interpersonal, and physical space in anorexia nervosa: A virtual reality and repertory grid investigation. Psychiatry Res. 2017, 252, 87–93. [Google Scholar] [CrossRef]
- Malighetti, C.; Serino, S.; Riva, G.; Cipolletta, S. Inside and outside the self. Virtual reality and repertory grids in the spatial analysis of anorexic patients’ meanings. Annu. Rev. Cybertherapy Telemed. 2016, 14, 78–81. [Google Scholar]
- Bartholdy, S.; Musiat, P.; Campbell, I.C.; Schmidt, U. The Potential of Neurofeedback in the Treatment of Eating Disorders: A Review of the Literature. Eur. Eat. Disord. Rev. 2013, 21, 456–463. [Google Scholar] [CrossRef]
- Duriez, P.; Khalil, R.B.; Chamoun, Y.; Maatoug, R.; Strumila, R.; Seneque, M.; Gorwood, P.; Courtet, P.; Guillaume, S. Brain Stimulation in Eating Disorders: State of the Art and Future Perspectives. J. Clin. Med. 2020, 9, 2358. [Google Scholar] [CrossRef]
- Dunlop, K.A.; Ewoodside, B.; Edownar, J. Targeting Neural Endophenotypes of Eating Disorders with Non-invasive Brain Stimulation. Front. Neurosci. 2016, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Schonherr, A.; Albrecht May, C. Influence of Caloric Vestibular Stimulation on Body Experience of Anorectic Patients. Case Stud. J. 2015, 4, 2886236. [Google Scholar]
- Miller, S.M. Vestibular neuromodulation: Stimulating the neural crossroads of psychiatric illness. Bipolar Disord. 2016, 18, 539–543. [Google Scholar] [CrossRef]
- Been, G.; Ngo, T.T.; Miller, S.M.; Fitzgerald, P.B. The use of tDCS and CVS as methods of non-invasive brain stimulation. Brain Res. Rev. 2007, 56, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Elmagarmid, A.; Fedorowicz, Z.; Hammady, H.; Ilyas, I.; Khabsa, M.; Ouzzani, M. Rayyan: A systematic reviews web app for exploring and filtering searches for eligible studies for Cochrane Reviews. In Evidence-Informed Public Health: Opportunities and Challenges; Abstracts of the 22nd Cochrane Colloquium; John Wiley & Sons: Hyderabad, India, 2014; pp. 21–26. [Google Scholar]
- Goldzak-Kunik, G.; Friedman, R.; Spitz, M.; Sandler, L.; Leshem, M. Intact sensory function in anorexia nervosa. Am. J. Clin. Nutr. 2012, 95, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Berlucchi, G.; Aglioti, S.M. The body in the brain revisited. Exp. Brain Res. 2010, 200, 25. [Google Scholar] [CrossRef]
- Lopez, C. Making Sense of the Body: The Role of Vestibular Signals. Multisens. Res. 2015, 28, 525–557. [Google Scholar] [CrossRef]
- Ambrosecchia, M.; Ardizzi, M.; Russo, E.; Ditaranto, F.; Speciale, M.; Vinai, P.; Todisco, P.; Maestro, S.; Gallese, V. Interoception and Autonomic Correlates during Social Interactions. Implications for Anorexia. Front. Hum. Neurosci. 2017, 11, 219. [Google Scholar] [CrossRef]
- Aschenbrenner, K.; Scholze, N.; Joraschky, P.; Hummel, T. Gustatory and olfactory sensitivity in patients with anorexia and bulimia in the course of treatment. J. Psychiatr. Res. 2008, 43, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Bär, K.J.; Boettger, S.; Wagner, G.; Wilsdorf, C.; Gerhard, U.J.; Boettger, M.K.; Blanz, B.; Sauer, H. Changes of pain perception, autonomic function, and endocrine parameters during treatment of anorectic adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Bellard, A.; Trotter, P.; McGlone, F.; Cazzato, V. Vicarious ratings of self vs. other-directed social touch in women with and recovered from Anorexia Nervosa. Sci. Rep. 2022, 12, 13429. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A.; Perry, T.R.; Kaye, W.H.; Wierenga, C.E. Pilot study of a water load test as a measure of gastric interoception in anorexia nervosa. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2022, 27, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Case, L.K.; Wilson, R.C.; Ramachandran, V.S. Diminished size–weight illusion in anorexia nervosa: Evidence for visuo-proprioceptive integration deficit. Exp. Brain Res. 2011, 217, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Crucianelli, L.; Cardi, V.; Treasure, J.; Jenkinson, P.M.; Fotopoulou, A. The perception of affective touch in anorexia nervosa. Psychiatry Res. 2016, 239, 72–78. [Google Scholar] [CrossRef]
- Crucianelli, L.; Demartini, B.; Goeta, D.; Nisticò, V.; Saramandi, A.; Bertelli, S.; Todisco, P.; Gambini, O.; Fotopoulou, A. The Anticipation and Perception of Affective Touch in Women with and Recovered from Anorexia Nervosa. Neuroscience 2020, 464, 143–155. [Google Scholar] [CrossRef]
- Demartini, B.; Goeta, D.; Romito, L.; Anselmetti, S.; Bertelli, S.; D’Agostino, A.; Gambini, O. Anorexia nervosa and functional motor symptoms: Two faces of the same coin? J. Neuropsychiatry Clin. Neurosci. 2017, 29, 383–390. [Google Scholar] [CrossRef]
- Di Lernia, D.; Serino, S.; Polli, N.; Cacciatore, C.; Persani, L.; Riva, G. Interoceptive Axes Dissociation in Anorexia Nervosa: A Single Case Study with Follow Up Post-recovery Assessment. Front. Psychol. 2019, 9, 2488. [Google Scholar] [CrossRef]
- Epstein, J.; Wiseman, C.V.; Sunday, S.R.; Klapper, F.; Alkalay, L.; Halmi, K.A. Neurocognitive evidence favors “top down” over “bottom up” mechanisms in the pathogenesis of body size distortions in anorexia nervosa. Eat. Weight. Disord.-Stud. Anorex. Bulim. Obes. 2001, 6, 140–147. [Google Scholar] [CrossRef]
- Fontana, M.P.; Menegoni, F.; Vismara, L.; Galli, M.; Romei, M.; Bergamini, E.; Petroni, M.L.; Capodaglio, P. Balance in patients with anorexia and bulimia nervosa. Eur. J. Phys. Rehabil. Med. 2009, 45, 335–340. [Google Scholar] [PubMed]
- Kinnaird, E.; Stewart, C.; Tchanturia, K. Interoception in Anorexia Nervosa: Exploring Associations with Alexithymia and Autistic Traits. Front. Psychiatry 2020, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.P.; Schulz, A.; Voderholzer, U.; Koch, S.; van Dyck, Z.; Vögele, C.V. Enhanced cortical processing of cardio-afferent signals in anorexia nervosa. Clin. Neurophysiol. 2019, 130, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Mergen, J.; Keizer, A.; Koelkebeck, K.; Heuvel, M.R.V.D.; Wagner, H. Women with Anorexia Nervosa do not show altered tactile localization compared to healthy controls. Psychiatry Res. 2018, 267, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Pollatos, O.; Herbert, B.M.; Berberich, G.; Zaudig, M.; Krauseneck, T.; Tsakiris, M. Atypical self-focus effect on interoceptive accuracy in anorexia nervosa. Front. Hum. Neurosci. 2016, 10, 484. [Google Scholar] [CrossRef]
- Richard, A.; Meule, A.; Georgii, C.; Voderholzer, U.; Cuntz, U.; Wilhelm, F.H.; Blechert, J. Associations between interoceptive sensitivity, intuitive eating, and body mass index in patients with anorexia nervosa and normal-weight controls. Eur. Eat. Disord. Rev. 2019, 27, 571–577. [Google Scholar] [CrossRef]
- Wollast, R.; Fossion, P.; Kotsou, I.; Rebrassé, A.; Leys, C. Interoceptive Awareness and Anorexia Nervosa: When Emotions Influence the Perception of Physiological Sensations. J. Nerv. Ment. Dis. 2022, 210, 390–393. [Google Scholar] [CrossRef]
- Yamamotova, A.; Papezova, H.; Uher, R. Modulation of thermal pain perception by stress and sweet taste in women with bulimia nervosa. Neuro Endocrinol. Lett. 2009, 30, 237–244. [Google Scholar]
- Zopf, R.; Contini, E.; Fowler, C.; Mondraty, N.; Williams, M.A. Body distortions in Anorexia Nervosa: Evidence for changed processing of multisensory bodily signals. Psychiatry Res. 2016, 245, 473–481. [Google Scholar] [CrossRef]
- Martin, E.; Dourish, C.; Rotshtein, P.; Spetter, M.; Higgs, S. Interoception and disordered eating: A systematic review. Neurosci. Biobehav. Rev. 2019, 107, 166–191. [Google Scholar] [CrossRef]
- Schandry, R. Heart Beat Perception and Emotional Experience. Psychophysiology 1981, 18, 483–488. [Google Scholar] [CrossRef]
- Mayer, J.D.; Allen, J.P.; Beauregard, K. Mood inductions for four specific moods: A procedure employing guided imagery vignettes with music. J. Ment. Imag. 1995, 19, 151–159. [Google Scholar]
- Craig, A.D. How do you feel—Now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Case, L.K.; Laubacher, C.M.; Olausson, H.; Wang, B.; Spagnolo, P.A.; Bushnell, M.C. Encoding of touch intensity but not pleasantness in human primary somatosensory cortex. J. Neurosci. 2016, 36, 5850–5860. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.; Voos, A.C.; Bennett, R.H.; Bolling, D.Z.; Pelphrey, K.A.; Kaiser, M.D. Brain mechanisms for processing affective touch. Hum. Brain Mapp. 2011, 34, 914–922. [Google Scholar] [CrossRef]
- McGlone, F.; Wessberg, J.; Olausson, H. Discriminative and Affective Touch: Sensing and Feeling. Neuron 2014, 82, 737–755. [Google Scholar] [CrossRef] [PubMed]
- McGlone, F.; Vallbo, A.B.; Olausson, H.; Loken, L.; Wessberg, J. Discriminative touch and emotional touch. Can. J. Exp. Psychol. 2007, 61, 173–183. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. ‘Sniffin’sticks’: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Kallert, S.; Renner, B.; Stiassny, K.; Temmel, A.F.P.; Hummel, T.; Kobal, G. Quantitative assessment of gustatory function in a clinical context using impregnated “taste strips”. Rhinol. J. 2003, 41, 2–6. [Google Scholar]
- Cardini, F.; Haggard, P.; Ladavas, E. Seeing and feeling for self and other: Proprioceptive spatial location determines multisensory enhancement of touch. Cognition 2013, 127, 84–92. [Google Scholar] [CrossRef]
- Pavani, F.; Spence, C.; Driver, J. Visual Capture of Touch: Out-of-the-Body Experiences with Rubber Gloves. Psychol. Sci. 2000, 11, 353–359. [Google Scholar] [CrossRef]
- Botvinick, M.; Cohen, J. Rubber hands ‘feel’ touch that eyes see. Nature 1998, 391, 756. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, M.; Haggard, P. The Rubber Hand Illusion Revisited: Visuotactile Integration and Self-Attribution. J. Exp. Psychol. Hum. Percept. Perform. 2005, 31, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.R.; Schüür, F.; Kammers, M.P.; Tsakiris, M.; Haggard, P. What is embodiment? A psychometric approach. Cognition 2008, 107, 978–998. [Google Scholar] [CrossRef] [PubMed]
- Berner, L.A.; Simmons, A.N.; Wierenga, C.E.; Bischoff-Grethe, A.; Paulus, M.P.; Bailer, U.F.; Ely, A.V.; Kaye, W.H. Altered interoceptive activation before, during, and after aversive breathing load in women remitted from anorexia nervosa. Psychol. Med. 2017, 48, 142–154. [Google Scholar] [CrossRef]
- Keizer, A.; Smeets, M.A.; Postma, A.; van Elburg, A.; Dijkerman, H.C. Does the experience of ownership over a rubber hand change body size perception in anorexia nervosa patients? Neuropsychologia 2014, 62, 26–37. [Google Scholar] [CrossRef]
- Paulus, M.P.; Stein, M.B. Interoception in anxiety and depression. Brain Struct. Funct. 2010, 214, 451–463. [Google Scholar] [CrossRef]
- Merwin, R.M.; Moskovich, A.A.; Wagner, H.R.; Ritschel, L.A.; Craighead, L.W.; Zucker, N.L. Emotion regulation difficulties in anorexia nervosa: Relationship to self-perceived sensory sensitivity. Cogn. Emot. 2013, 27, 441–452. [Google Scholar] [CrossRef]
- Brener, J.; Ring, C. Towards a psychophysics of interoceptive processes: The measurement of heartbeat detection. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160015. [Google Scholar] [CrossRef]
- Murphy, J.; Brewer, R.; Hobson, H.; Catmur, C.; Bird, G. Is alexithymia characterised by impaired interoception? Further evidence, the importance of control variables, and the problems with the Heartbeat Counting Task. Biol. Psychol. 2018, 136, 189–197. [Google Scholar] [CrossRef]
- Khalsa, S.S.; Lapidus, R.C. Can Interoception Improve the Pragmatic Search for Biomarkers in Psychiatry? Front. Psychiatry 2016, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Lautenbacher, S.; Pauls, A.M.; Strian, F.; Pirke, K.-M.; Krieg, J.-C. Pain sensitivity in anorexia nervosa and bulimia nervosa. Biol. Psychiatry 1991, 29, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- de Zwaan, M.; Biener, D.; Bach, M.; Wiesnagrotzki, S.; Stacher, G. Pain sensitivity, alexithymia, and depression in patients with eating disorders: Are they related? J. Psychosom. Res. 1996, 41, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Papezová, H.; Yamamotová, A.; Nedvídková, J. Pain modulation role of melatonin in eating disorders. Eur. Psychiatry 2001, 16, 68–70. [Google Scholar] [CrossRef]
- Yamamotová, A.; Papezova, H. Does the pain perception depend on the type of vegetative reactivity? Comparison of healthy women with eating disorders patients. Homeost. Praha 2000, 40, 134–136. [Google Scholar]
- Scharner, S.; Stengel, A. Alterations of brain structure and functions in anorexia nervosa. Clin. Nutr. Exp. 2019, 28, 22–32. [Google Scholar] [CrossRef]
- Strigo, I.A.; Matthews, S.C.; Simmons, A.N.; Oberndorfer, T.; Klabunde, M.; Bsc, L.E.R.; Kaye, W.H. Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: Evidence of interoceptive dysregulation. Int. J. Eat. Disord. 2012, 46, 23–33. [Google Scholar] [CrossRef]
- Yamamotova, A.; Bulant, J.; Bocek, V.; Papezova, H. Dissatisfaction with own body makes patients with eating disorders more sensitive to pain. J. Pain Res. 2017, 10, 1667–1675. [Google Scholar] [CrossRef]
- Schmahl, C.; Meinzer, M.; Zeuch, A.; Fichter, M.; Cebulla, M.; Kleindienst, N.; Ludäscher, P.; Steil, R.; Bohus, M. Pain sensitivity is reduced in borderline personality disorder, but not in posttraumatic stress disorder and bulimia nervosa. World J. Biol. Psychiatry 2010, 11, 364–371. [Google Scholar] [CrossRef]
- Garner, D.M.; Garfinkel, P.E. Body image in anorexia nervosa: Measurement, theory and clinical implications. Int. J. Psychiatry Med. 1982, 11, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Connan, F.; Campbell, I.C.; Katzman, M.; Lightman, S.L.; Treasure, J. A neurodevelopmental model for anorexia nervosa. Physiol. Behav. 2003, 79, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.; Kaye, W.H.; Matsunaga, H.; Myers, D.; Orbach, I.; Har-Even, D.; Frank, G.; Rao, R. Pain perception in recovered bulimia nervosa patients. Int. J. Eat. Disord. 2003, 34, 331–336. [Google Scholar] [CrossRef]
- Pollatos, O.; Georgiou, E. Normal interoceptive accuracy in women with bulimia nervosa. Psychiatry Res. 2016, 240, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Serino, S.; Dakanalis, A.; Gaudio, S.; Carrà, G.; Cipresso, P.; Clerici, M.; Riva, G. Out of body, out of space: Impaired reference frame processing in eating disorders. Psychiatry Res. 2015, 230, 732–734. [Google Scholar] [CrossRef]
- Guardia, D.; Carey, A.; Cottencin, O.; Thomas, P.; Luyat, M. Disruption of Spatial Task Performance in Anorexia Nervosa. PLoS ONE 2013, 8, e54928. [Google Scholar] [CrossRef] [PubMed]
- Gaudio, S.; Quattrocchi, C.C. Neural basis of a multidimensional model of body image distortion in anorexia nervosa. Neurosci. Biobehav. Rev. 2012, 36, 1839–1847. [Google Scholar] [CrossRef]
- Riva, G.; Gaudio, S. Locked to a wrong body: Eating disorders as the outcome of a primary disturbance in multisensory body integration. Conscious. Cogn. 2018, 59, 57–59. [Google Scholar] [CrossRef]
- Culham, J.C.; Cavina-Pratesi, C.; Singhal, A. The role of parietal cortex in visuomotor control: What have we learned from neuroimaging? Neuropsychologia 2006, 44, 2668–2684. [Google Scholar] [CrossRef]
- Ehrsson, H.H.; Holmes, N.P.; Passingham, R.E. Touching a Rubber Hand: Feeling of Body Ownership Is Associated with Activity in Multisensory Brain Areas. J. Neurosci. 2005, 25, 10564–10573. [Google Scholar] [CrossRef]
- Ehrsson, H.H.; Spence, C.; Passingham, R.E. That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science 2004, 305, 875–877. [Google Scholar] [CrossRef]
- Holmes, N.P.; Snijders, H.J.; Spence, C. Reaching with alien limbs: Visual exposure to prosthetic hands in a mirror biases proprioception without accompanying illusions of ownership. Percept. Psychophys. 2006, 68, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Mohr, C.; Porter, G.; Benton, C. Psychophysics reveals a right hemispheric contribution to body image distortions in women but not men. Neuropsychologia 2007, 45, 2942–2950. [Google Scholar] [CrossRef]
- Grunwald, M.; Weiss, T.; Krause, W.; Beyer, L.; Rost, R.; Gutberlet, I.; Gertz, H.-J. Theta power in the EEG of humans during ongoing processing in a haptic object recognition task. Cogn. Brain Res. 2001, 11, 33–37. [Google Scholar] [CrossRef]
- Spitoni, G.F.; Serino, A.; Cotugno, A.; Mancini, F.; Antonucci, G.; Pizzamiglio, L. The two dimensions of the body representation in women suffering from Anorexia Nervosa. Psychiatry Res. 2015, 230, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Dakanalis, A.; Riva, G. Current considerations for eating and body-related disorders among men. In Handbook on Body Image: Gender Differences, Sociocultural Influences and Health Implications; Nova Publishers: New York, NY, USA, 2013; pp. 195–216. [Google Scholar]
- Prentice, F.; Hobson, H.; Spooner, R.; Murphy, J. Gender differences in interoceptive accuracy and emotional ability: An explanation for incompatible findings. Neurosci. Biobehav. Rev. 2022, 141, 104808. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Wiederhold, B.K.; Chirico, A.; di Lernia, D.; Mantovani, F.; Gaggioli, A. Brain and Virtual Reality: What Do They Have in Common and How to Exploit Their Potential. Annu. Rev. Cybertherapy Telemed. 2018, 2018, 3–8. [Google Scholar]
- Riva, G.; Wiederhold, B.K.; di Lernia, D.; Chirico, A.; Riva, E.F.M.; Mantovani, F.; Cipresso, P.; Gaggioli, A. Virtual Reality Meets Artificial Intelligence: The Emergence of Advanced Digital Therapeutics and Digital Biomarkers. Annu. Rev. Cybertherapy Telemed. 2019, 17, 3–7. [Google Scholar]
- Riva, G.; Serino, S.; Di Lernia, D.; Pagnini, F. Regenerative Virtual Therapy: The Use of Multisensory Technologies and Mindful Attention for Updating the Altered Representations of the Bodily Self. Front. Syst. Neurosci. 2021, 15, 749268. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S.; Freeman, W. The Bayesian brain: Phantom percepts resolve sensory uncertainty. Neurosci. Biobehav. Rev. 2014, 44, 4–15. [Google Scholar] [CrossRef]
- Friston, K. The freeenergy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Schoeller, F.; Haar, A.; Jain, A.; Maes, P. Enhancing human emotions with interoceptive technologies. Phys. Life Rev. 2019, 31, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Björnsdotter, M.; Morrison, I.; Olausson, H. Feeling good: On the role of C fiber mediated touch in interoception. Exp. Brain Res. 2010, 207, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Di Lernia, D.; Lacerenza, M.; Ainley, V.; Riva, G. Altered Interoceptive Perception and the Effects of Interoceptive Analgesia in Musculoskeletal, Primary, and Neuropathic Chronic Pain Conditions. J. Pers. Med. 2020, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Serino, S.; Di Lernia, D.; Pavone, E.F.; Dakanalis, A. Embodied Medicine: Mens Sana in Corpore Virtuale Sano. Front. Hum. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, B.K.; Riva, G. Virtual Reality Therapy: Emerging Topics and Future Challenges. Cyberpsychol. Behav. Soc. Netw. 2019, 22, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Iodice, P.; Porciello, G.; Bufalari, I.; Barca, L.; Pezzulo, G. An interoceptive illusion of effort induced by false heart-rate feedback. Proc. Natl. Acad. Sci. USA 2019, 116, 13897–13902. [Google Scholar] [CrossRef]
- Barańska, K.; Różańska, A.; Maćkowska, S.; Rojewska, K.; Spinczyk, D. Determining the Intensity of Basic Emotions among People Suffering from Anorexia Nervosa Based on Free Statements about Their Body. Electronics 2022, 11, 138. [Google Scholar] [CrossRef]
- Rojewska, K.; Maćkowska, S.; Maćkowski, M.; Różańska, A.; Barańska, K.; Dzieciątko, M.; Spinczyk, D. Natural Language Processing and Machine Learning Supporting the Work of a Psychologist and Its Evaluation on the Example of Support for Psychological Diagnosis of Anorexia. Appl. Sci. 2022, 12, 4702. [Google Scholar] [CrossRef]
- Coppersmith, G.; Leary, R.; Crutchley, P.; Fine, A. Natural language processing of social media as screening for suicide risk. Biomed. Inform. Insights 2018, 10, 1–11. [Google Scholar] [CrossRef]
- Yan, H.; Fitzsimmons-Craft, E.E.; Goodman, M.; Krauss, M.; Das, S.; Cavazos-Rehg, P. Automatic detection of eating disorder-related social media posts that could benefit from a mental health intervention. Int. J. Eat. Disord. 2019, 52, 1150–1156. [Google Scholar] [CrossRef]

| Authors and Year | Sample | Gender | Diagnosis | Task/Instrument | Constructs Measured | Primary Outcomes |
|---|---|---|---|---|---|---|
| (Ambrosecchia et al., 2017 [74]) | AN: 24 HC: 25 | F | AN | Heartbeat perception task | Interoception | Results showed no differences between AN and HCs in heartbeat perception task. |
| (Aschenbrenner et al., 2009 [75]) | AN:16 BN: 24 HC: 23 | F | AN BN | “Sniffin’ Sticks” Test Battery and ‘‘Taste Strip” Test Kit | Interoception | Compared to HC and BN, individuals with AN showed lowered olfactory and gustatory sensitivities. |
| (Bär et al., 2006 [76]) | AN: 15 HC: 15 | F | AN | Heat Pain Thresholds | Interoception | The heat pain thresholds were significantly increased in the acute state of AN and decreased after weight had been regained for 6 months. |
| (Bellard et al., 2022 [77]) | AN: 27 RAN: 29 HC: 35 | F | AN | Affective touch | Interoception | AN and RAN did not differ in their pleasantness ratings to touch for another compared to HC, but when evaluating touch for self, both AN and RAN rated CT-optimal touch as less pleasant than HCs. |
| (Brown et al., 2022 [78]) | AN: 10 HC: 10 | F | AN | Behavioral Water Load Task | Interoception | Participants with AN drank significantly less water than HC, but reported greater increases in negative affects pre-to-post-Water Load Task. |
| (Case et al., 2011 [79]) | AN:10 HC: 10 | F | AN | Size Weight Illusion | Proprioception | Results showed a reduction in size weight illusion in individuals with AN compared to controls. |
| (Crucianelli et al. 2016 [80]) | AN: 25 HC: 30 | F | AN | Affective touch | Interoception | Results showed less pleasure in people with AN regarding affective touch compared to HCs. |
| (Crucianelli et al., 2020 [81]) | AN: 27 RAN: 24 HC: 27 | F | AN | Affective Touch | Interoception | Both AN and RAN anticipated tactile experiences and rated delivered tactile stimuli as less pleasant than HCs. |
| (Demartini et al., 2017 [82]) | AN: 20 FMS: 20 HC: 20 | F | AN; FMCS (Functional Motor Symptoms) | Heartbeat Perception Task | Interoception | Results showed no differences between people with AN and HC in interoceptive sensitivity and interoceptive awareness. |
| (Di Lernia et al., 2019 [83]) | AN: 1 (single case) HC: 4 | F | AN | Heartbeat Perception Task; Metacognitive Confidence in Heartbeat Task Perception; Interoceptive Buffer Saturation Index | Interoception | The patient with AN showed a dissociation of interceptive axes with widespread perceptional deficits. |
| (Epstein et al., 2001 [84]) | AN:20 HC: 20 | F | AN | ‘‘Proprioception Test’’ and ‘‘Right-Left Orientation Test’’ | Proprioception | People with AN showed significantly lower scores in the ‘‘right-left orientation test’’ at pre-treatment assessment as compared to HCs. |
| (Fontana et al., 2009 [85]) | AN: 15 BN: 15 HC: 11 | F | AN BN | Kinematics (or segmental) Method | Vestibular Signals | Patients with BN were more unstable than HCs, showing significant differences in anteroposterior center of mass (CoM) excursions and length of the path, while individuals with AN showed no significant differences from HCs. |
| (Goldzak-Kunik et al., 2012 [71]) | AN: 15 HC: 15 | F | AN | Interoception: Cold Pain, VAS for Cold, Unpleasantness, and Pain. Proprioception: Kinesthesia task | Interoception and Proprioception | Patients with AN and HCs did not differ in cold pain responses and at the kinaesthesia task. |
| (Kinnaird et al., 2020 [86]) | AN: 37 HC: 37 | F | AN | Heartbeat Perception Task; Metacognitive Confidence in Heartbeat Task Perception | Interoception | Heartbeat perception performance was not found to be altered in the AN group compared to the HC group. However, confidence ratings in task performance in the AN group were lower compared to the HC group. |
| (Lutz et al., 2019 [87]) | AN: 20 HC: 20 | F | AN AN | Heartbeat Perception Task; Interoceptive Sensibility Task | Interoception | Results showed that people with AN and HCs did not differ significantly in interoceptive accuracy or interoceptive sensibility. |
| (Mergen et al., 2018 [88]) | AN: 27 HC: 40 | F | AN | One-Point-Localization Task | Proprioception | Results showed no difference between AN and HC in their performance since both groups showed alterations in the localization task. |
| (Pollatos et al., 2016 [89]) | AN: 15 HC: 15 | F | AN | Heartbeat Perception Task | Interoception | During the self-focus, individuals with AN showed lower Interoception accuracy compared to HCs. |
| (Richard et al., 2019 [90]) | AN: 37 HC: 39 | F | AN | Heartbeat Perception Task | Interoception | Results showed no evidence of lower heartbeat perception in people with AN compared to HCs. |
| (Wollast et al., 2022 [91]) | AN: 25 HC: 25 | F | AN | Heartbeat Perception Task | Interoception | A deficit in interoceptive accuracy was observed for the individuals suffering from AN at rest as well as when an emotional context was induced, compared to HCs. |
| (Yamamotova et al., 2009 [92]) | BN:21 HC: 21 | F | BN | Heat Pain Threshold using Analgesia Meterradiant Heat applied to 1 cm2 | Interoception | BN had a higher pain threshold than HCs in all six conditions. BN also had shorter tolerance latency of cold pressor than HCs. |
| (Zopf et al., 2016 [93]) | AN: 23 HC: 23 | F | AN | Rubber Hand Illusion; Proprioception Drift | Proprioception | Results showed the reduced influence of proprioceptive signals on hand location estimates in AN compared to HCs. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malighetti, C.; Sansoni, M.; Gaudio, S.; Matamala-Gomez, M.; Di Lernia, D.; Serino, S.; Riva, G. From Virtual Reality to Regenerative Virtual Therapy: Some Insights from a Systematic Review Exploring Inner Body Perception in Anorexia and Bulimia Nervosa. J. Clin. Med. 2022, 11, 7134. https://doi.org/10.3390/jcm11237134
Malighetti C, Sansoni M, Gaudio S, Matamala-Gomez M, Di Lernia D, Serino S, Riva G. From Virtual Reality to Regenerative Virtual Therapy: Some Insights from a Systematic Review Exploring Inner Body Perception in Anorexia and Bulimia Nervosa. Journal of Clinical Medicine. 2022; 11(23):7134. https://doi.org/10.3390/jcm11237134
Chicago/Turabian StyleMalighetti, Clelia, Maria Sansoni, Santino Gaudio, Marta Matamala-Gomez, Daniele Di Lernia, Silvia Serino, and Giuseppe Riva. 2022. "From Virtual Reality to Regenerative Virtual Therapy: Some Insights from a Systematic Review Exploring Inner Body Perception in Anorexia and Bulimia Nervosa" Journal of Clinical Medicine 11, no. 23: 7134. https://doi.org/10.3390/jcm11237134
APA StyleMalighetti, C., Sansoni, M., Gaudio, S., Matamala-Gomez, M., Di Lernia, D., Serino, S., & Riva, G. (2022). From Virtual Reality to Regenerative Virtual Therapy: Some Insights from a Systematic Review Exploring Inner Body Perception in Anorexia and Bulimia Nervosa. Journal of Clinical Medicine, 11(23), 7134. https://doi.org/10.3390/jcm11237134








