Abstract
Previous epidemiological studies have reported that the use of statins is associated with a decreased risk of gastric cancer, although the beneficial effects of statins on the reduction of gastric cancer remain unclear. Therefore, we conducted a systematic review and meta-analysis to investigate the association between the use of statins and the risk of gastric cancer. Electronic databases such as PubMed, EMBASE, Scopus, and Web of Science were searched between 1 January 2000 and 31 August 2022. Two authors used predefined selection criteria to independently screen all titles, abstracts, and potential full texts. Observational studies (cohort and case-control) or randomized control trials that assessed the association between statins and gastric cancer were included in the primary and secondary analyses. The pooled effect sizes were calculated using the random-effects model. The Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines were followed to conduct this study. The total sample size across the 20 included studies was 11,870,553. The use of statins was associated with a reduced risk of gastric cancer (RRadjusted: 0.72; 95%CI: 0.64–0.81, p < 0.001). However, the effect size of statin use on the risk of gastric cancer was lower in Asian studies compared to Western studies (RRAsian: 0.62; 95%CI: 0.53–0.73 vs. RRwestern: 0.88; 95%CI: 0.79–0.99). These findings suggest that the use of statins is associated with a reduced risk of gastric cancer. This reverse association was even stronger among Asian people than Western individuals.
1. Introduction
Gastric cancer is a common public health problem associated with a substantial healthcare burden [1]. Gastric cancer ranks fourth in terms of incidence, and is the fifth leading cause of cancer mortality worldwide, with an estimated approximately 1.1 million new cases and 770,000 deaths in 2020 [2,3]. Since a considerable proportion of gastric cancer patients are diagnosed at advanced stages, the five-year survival rate is only 32% [4]. Even though the prevalence and mortality of gastric cancer have been declining in some parts of the world, meaningful prevention strategies are needed to treat it early and reduce the overall healthcare burden. Previous studies have reported that several modifiable (e.g., tobacco smoking, alcohol consumption, obesity, gastroesophageal reflux, and Helicobacter pylori (H. pylori) infection) and non-modifiable (e.g., age, gender, and ethnicity) risk factors are associated with gastric cancer [5,6,7,8].
Statins are the most commonly prescribed medications and are considered to be effective for lowering cholesterol and protecting against cardiovascular diseases [9,10]. Previous epidemiological studies have highlighted the beneficial effects of statins against cancer [11,12,13,14]. The impact of statin therapy on the risk of gastric cancer, and the relevant biological relationships, which are not entirely understood, have also gained significant attention [15]. Previous studies have reported that statins profoundly reduce tumor growth and mitigation by blocking HMG-CoA reductase [16]. Statins also suppress the expression of genes directly linked to gastric cancer cells and increase apoptosis in early gastric cancer cells [17].
Although statins showed the potential benefits of reducing the risk of gastric cancer, no updated systematic review and meta-analysis of their association has been conducted. This study, therefore, conducted an updated systematic review and investigated the relationship between statin use and the risk of gastric cancer. The evidence of this study will provide adequate answers to this topic and may assist clinicians in weighing the benefits of statins in gastric cancer.
2. Methods
This study was based on two main questions. Question 1 was defined as follows: Are patients treated with statins associated with a reduced risk of gastric cancer? Question 2 was defined as follows: Do subgroup analyses support the effect size of the association between statin use and gastric cancer risk? The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and the guidelines for Meta-analysis Of Observational Studies in Epidemiology (MOOSE) were properly followed to conduct this study [18,19] (Supplementary Table S1).
2.1. Databases and Search Strategy
We conducted a comprehensive search on the most popular databases for scientific literatures such as PubMed, EMBASE, Scopus, and Web of Science to obtain all English-language studies published between 1 January 2000 and 31 August 2022. We considered studies that evaluated the association between statin use and the risk of gastric cancer. The following search terms were used: “statin/s” or “hydroxymethylglutaryl-CoA reductase inhibitors” or “lipid-lowering drugs” or “atorvastatin” or “fluvastatin” or “lovastatin” or “pitavastatin” or “pravastatin” or “rosuvastatin” or “simvastatin” and “gastric cancer” or “gastric carcinoma” or “gastric neoplasm” or “stomach cancer” or “stomach carcinoma” or “stomach adenocarcinoma” or “stomach neoplasm” or “cancer” (Supplementary Table S2). Additional searches were conducted in the bibliographies of relevant articles to obtain missing articles.
2.2. Inclusion and Exclusion Criteria
All observational studies (e.g., cohort or case-control studies) and randomized control trials (RCTs) that met the following criteria were included: (1) studies that carried out at least one analysis assessing the effect of statins use on gastric cancer, (2) patients with an established diagnosis of gastric cancer by accepted clinical and/or histologic criteria, (3) studies published in English, and (4) studies that provided sufficient information to calculate the pooled effect size. We excluded studies if they were reviews, letters, case reports, or editorials.
2.3. Data Extraction
Two authors (CCW and MMI) independently extracted information from all selected studies using piloted data extraction sheets. Extracted data included (1) demographics: author name, publication year, country, (2) population: age, gender, percentage of male, number of statin users, number of gastric cancer patients, (3) methods: study design, inclusion and exclusion criteria, and (4) results: effect sizes (hazard ratio, odds ratio). Any disagreement during the study screening process was resolved by discussing with the third author.
2.4. Study Quality Assessment
The risk of bias of RCTs was assessed using the Cochrane Collaboration tool [20], which is comprised of the following domains: (a) sequence generation, (b) allocation concealment, (c) blinding of participants, (d) incomplete outcome data, (e) selective reporting, and (f) other risk of bias. Moreover, the Newcastle-Ottawa Scale was used to assess the methodological quality of the observational studies [21]. The NOS scale is recommended by the Cochrane Handbook for Systematic Reviews of Interventions, and it is divided into three categories such as (a) study selection, (b) comparability, and (c) the ascertainment of exposure (for case-control studies) or the outcome of interest (for cohort studies).
2.5. Statistical Analysis
We used ORs and HRs to calculate the overall pooled effect sizes of statin use on the risk of gastric cancer. The pooled RRs were calculated using random-effects models. We calculated heterogeneity across studies using the Q-statistic and quantified using the inconsistency I2. The I2 values were classified into four groups: of 0~29%, 30~49%, 50~74%, and 75~100%, representing very low, low, medium, and high inconsistency, respectively [22,23,24]. We also conducted subgroup analyses to assess the potential impact of study design, region, quality of observational studies (NOS ≤ 7 vs. NOS > 7), duration, and statin types. In the sensitivity analysis, the impact of each study on the summary statistics was evaluated by excluding one study at a time from the meta-analysis. A forest plot was drawn to visually represent the effect size of each study and the pooled analyses. Finally, funnel plots and the Egger regression test of funnel plot asymmetry were used to calculate overall publication bias. The statistical analyses were performed using comprehensive meta-analysis software (CMA). A p < 0.05 was considered statistically significant.
3. Results
3.1. Study Selection and Characteristics
Figure 1 shows the PRISMA diagram of the selection of studies. The search identified 6976 studies. However, 3700 studies were excluded for duplications and 3247 studies were excluded after screening the titles and abstracts. In total, 29 studies were selected for full-text screening based on prespecified selection criteria. Finally, 20 studies were selected for the meta-analysis [17,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43].
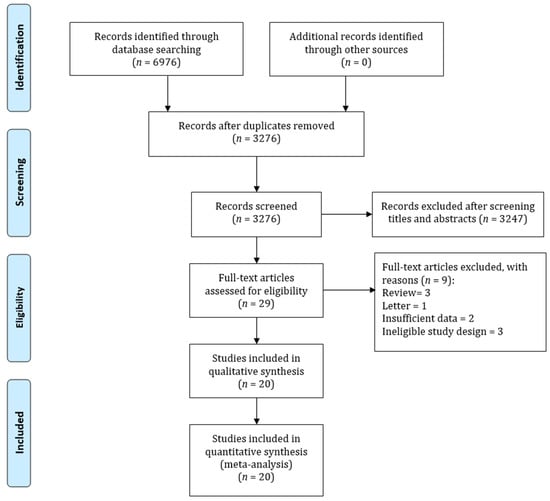
Figure 1.
PRISMA flowchart for summarizing study identification and selection.
These 20 studies consisted of 9 cohort, 8 case-control, and 3 RCTs studies, which comprised of 11,870,553 participants. Twelve studies were conducted in Asia and eight studies were conducted in Western countries. A total of 17 out of 20 studies used administrative databases to identify statin users and gastric cancer patients. More than half of observational studies were of good quality, reflected by a Newcastle-Ottawa score of at least 8. Table 1 presents the characteristics of the included studies, intervention, outcomes, included and excluded criteria, and study quality.

Table 1.
Basic characteristics of included studies.
3.2. Statin Use and the Risk of Gastric Cancer
Twenty studies (three RCTs and 17 observational studies) reporting the effect of statins on gastric cancer were included in the meta-analysis. When all adjusted effect sizes were pooled, the summary RR in statin users compared with statin nonusers from the random-effects models was 0.72 (95%CI: 0.64–0.81). There was significant heterogeneity across studies (I2 = 92.63, Q = 272.69, τ2 = 0.05) in the random-effects model. Figure 2 shows the forest plot of the association between statin use and the risk of gastric cancer.
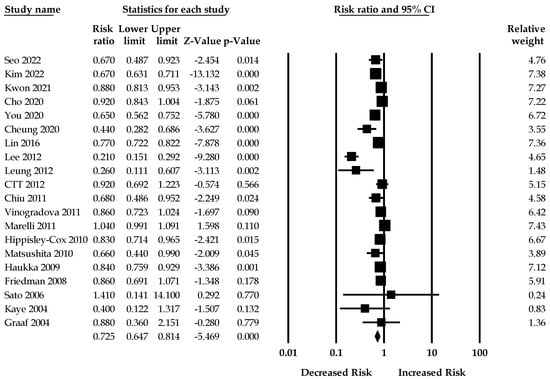
Figure 2.
Association between statin use and the risk of gastric cancer [17,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43].
3.3. Sensitivity and Subgroup Analyses
We also conducted a sensitivity analysis to assess changing the overall effect sizes of the use of statin on gastric cancer and the presence of heterogeneity by omitting one study from the main analysis (Supplementary Table S3). The overall effect size and heterogeneity did not change after sensitivity analyses.
The pooled RRs from the random-effects model based on data from cohort studies, case-control studies, and RCTs were 0.77 (95%CI: 0.66–0.90), 0.61 (95%CI: 0.48–0.77), and 0.82 (95%CI: 0.65–1.04), respectively. However, there was significant heterogeneity across the studies (Table 2).

Table 2.
Subgroup analyses for the association between statin use and risk of gastric cancer.
The pooled RRs for the studies from Asia and Western countries were 0.62 (95%CI: 0.53–0.73; I2 = 92.67, Q = 150.22, τ2 = 0.05) and 0.88 (95%CI: 0.79–0.99, I2 = 72.27, Q = 25.25, τ2 = 0.01), respectively.
The pooled RRs for male and female patients were 0.80 (95%CI: 0.68–0.94), and 0.72 (95%CI: 0.55–0.94), respectively. However, there was moderate heterogeneity across the studies.
3.4. Dose–Response Association between Statins and Gastric Cancer
Kim et al. [26] evaluated the risk of gastric acid among statin users who received <1460 and ≥1460 cumulative defined daily doses (cDDDs). After propensity score matching, the effect size for the association between gastric cancer risk and statin users with <1460 cDDDs and ≥1460 cDDDs were 0.98 (95%CI: 0.91–1.06) and 0.22 (95%CI: 0.19–0.26), respectively. Cho et al. [27] classified the cumulative duration of statin use into four categories such as <182.5, 182.5–365.0, 365.0–547.5, and 547.5–730.0. The risk of gastric cancer decreased as cDDDs increased among statin users [HR0.96 (95%CI: 0.89–1.04), 0.85 (95%CI: 0.74–0.96), 0.78 (95%CI: 0.68–0.90), and 0.89 (95%CI: 0.82–0.96)]. Finally, Chiu et al. [34] examined the risk of gastric cancer among various statin users, and they observed a significant trend of reducing gastric cancer with increasing cumulative dose. The adjusted ORs were 0.90 (95% CI = 0.60–1.36) for the group with cumulative statin use <134.25 DDDs and 0.49 (95% CI = 0.30–0.79) for the group with cumulative statin use of ≥134.25 DDDs.
3.5. Duration–Response Association between Statins and Gastric Cancer
Cheung et al. [29] invested the duration–response relationship and categorized statin users into two groups: (i) <5 years and (ii) ≥5 years. Lower risk of gastric cancer was observed among patients who used statins longer (0.46 [95%CI:0.25–0.86] for <5 years of use and 0.43 [95%CI:0.29–0.66] for ≥5 years of use). Le et al. [31] also evaluated the impact of duration of statin use on gastric cancer risk. Diabetes patients who received statins less than 1 year before the index date had an effect size of 0.45; however, the risk of gastric cancer was even lower among patients with statin use of more than 2 years (0.154 [95%CI: 0.09–0.26]).
3.6. Publication Bias
Figure 3 shows the funnel plot for publication bias. Out of 20 data points, six lay outside the triangle, and only one lay on the left side of the triangle altitude. The Egger regression test of the funnel asymmetry showed no publication bias (p = 0.09).
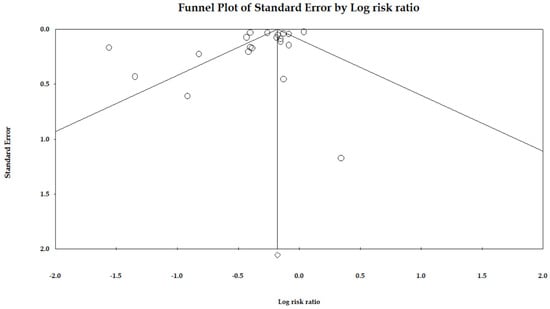
Figure 3.
Funnel plot of the association between statin use and risk of gastric cancer.
4. Discussion
In this updated systematic review and meta-analysis, we evaluated the association between statin use and the risk of gastric cancer, which is currently unclear. Our findings are similar to previous evidence [15,44,45,46,47] that showed statin use is associated with a significantly reduced risk of gastric cancer. The strength of this association varied among study designs, and no association was observed in the RCTs. In addition, a more protective effect was observed in Asian people compared to Western individuals.
The possible biological mechanisms as to how statin use reduces the risk of gastric cancer is still unclear. However, several biological pathways may help to understand the process of their association. Statin reduces the production of cholesterol, dolichol, and coenzyme Q10 by inhibiting HMG-CoA reductase in the mevalonate pathway [48,49]. Previous studies highlighted that statin increases apoptosis, suppress angiogenesis, and changes the tumor microenvironment downstream of the mevalonate pathway [50,51,52]. Moreover, suppression of the mevalonate pathway due to statin use can reduce radiosensitization or chemosensitization [53,54]. Statins interrupt the production of primary geranylgeranyl pyrophosphate (GGPP) and farnesylpyrophophosphate (FPP) and delay the growth of malignant cells, eventually leading to apoptosis [55]. Statins alter the activation of the proteasome pathway, inhibiting the breakdown of both p21 and p27 [56]. Finally, statins allow p21 and p27 molecules to utilize their growth-inhibitory effects and try to slow down gastric cancer cell mitosis [57].
The effect size of the relationship between statin use and the risk of gastric cancer was different in Asian and Western population. Statin use significantly showed a reduction of gastric cancer in Asian populations, but no association was observed in Western populations. Previous studies assessed statin responses between Asians and Westerners (European Americans), showing a difference in pharmacokinetic and pharmacodynamic effects [58,59]. Studies highlighted that statin responses were significantly different even after adjustment for potential confounding factors such as age, comorbidities, and/or socioeconomic status. Body size differences between Asians and Westerners may also contribute to pharmacokinetic variation (<10%) [60,61]. Moreover, genetic variation among Asians can influence statin pharmacokinetics and pharmacodynamics [62].
The findings of our study also showed that the reduction of gastric cancer among statin users was higher in the case-control studies compared to cohort and RCTs. The inherent risk of bias among case-control studies is consistently high due to potential confounding factors. In the cohort studies, maximum efforts are given to reduce the possible biases, although it does demonstrate a causal relationship. In the clinical-decision making, RCTs are considered the gold standard of study design, revealing the causal relationship between a drug and gastric cancer risk. On the other hand, more chemo-preventive effects of statins were observed in the low-quality studies, which may overestimate their true effect. Low-quality studies often contain a lack of random patient allocation and a lack of adjusting for numerous covariates; therefore, it is not possible to eliminate the possibility of residual confounding factors. Included studies also demonstrated that statins are even more protective against gastric cancer if patients take a higher dose over a longer duration of time [26,27,28]. Kwon et al. [17] showed that a significant reduction in gastric cancer was observed among patients taking hydrophilic statins. Our subgroup analyses showed that pravastatin (hydrophilic) was associated with an insignificant reduction of gastric cancer, whereas rosuvastatin showed an increased risk of gastric cancer. More studies with controlled confounding factors and larger follow-up periods are needed to provide supporting evidence that demonstrates the effectiveness of statins against gastric cancer.
There are several strengths in this study. First, this is the most updated systematic review and meta-analysis so far that evaluated the relationship between statin use and gastric cancer risk. We included both RCTs and observational studies to summarize overall effect size that ensures the best available evidence through a meta-analytic approach. Second, this meta-analysis included a large sample size and the quality of included studies were high. Therefore, the findings of this study were more reliable. Third, we could identify statin as an independent risk factor for gastric cancer since we adjusted with potential confounding factors to estimate the pooled summary size.
This study also has several limitations. First, most studies were observational studies with a highly heterogeneous population with different patient characteristics. The heterogeneity among the studies was high, although this can be explained by study design, region, and study quality. Second, there was limited information regarding the types of statins, indication, follow-up, and duration of statins; therefore, we were unable to pool effect size based on dosage, duration, and follow-up. Third, several potential covariates/confounders related to gastric cancer risk have not been adjusted in the included study. Future studies should adjust all possible confounders to pool the effect sizes and to show a stronger association between statin use and gastric cancer risk.
5. Conclusions
The findings of this updated meta-analysis suggest that statin use is associated with a reduced risk of gastric cancer. Although the pooled effect size of observational studies showed a possible role of statin therapy in the prevention of gastric cancer, the pooled effect size of the clinical trials and the risk of the bias of observational studies do not encourage physicians to consider statin use to achieve a reduction in gastric cancer. More RCTs with larger populations, longer follow-up, and standardized methods are required to consider statin use as a strategy for reducing gastric cancer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11237180/s1, Table S1: PRISMA checklist; Table S2: Search strategy; Table S3: Sensitivity analyses for the association between statin use and the risk of gastric cancer. Reference [63] has been cited in Supplementary Materials.
Author Contributions
Conceptualization: C.-H.S., G.J. and C.-C.W.; methodology: C.-H.S. and M.M.I.; software: M.M.I.; validation: C.-C.W. and G.J.; formal analysis: M.M.I.; investigation: C.-C.W.; resources: M.M.I.; data curation: M.M.I.; writing—original draft preparation: M.M.I.; writing—review and editing: C.-C.W.; visualization: M.M.I.; supervision: C.-C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Islam, M.M.; Poly, T.N.; Walther, B.A.; Lin, M.-C.; Li, Y.-C. Artificial intelligence in gastric cancer: Identifying gastric cancer using endoscopic images with convolutional neural network. Cancers 2021, 13, 5253. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.; Vignat, J.; Laversanne, M. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–2040: A population-based modelling study. EClinicalMedicine 2022, 47, 101404. [Google Scholar] [PubMed]
- Liao, X.-L.; Liang, X.-W.; Pang, H.-Y.; Yang, K.; Chen, X.-Z.; Chen, X.-L.; Liu, K.; Zhao, L.-Y.; Zhang, W.-H.; Hu, J.-K. Safety and efficacy of laparoscopic versus open gastrectomy in patients with advanced gastric cancer following neoadjuvant chemotherapy: A meta-analysis. Front. Oncol. 2021, 11, 704244. [Google Scholar] [CrossRef] [PubMed]
- Sasako, M.; Sakuramoto, S.; Katai, H.; Kinoshita, T.; Furukawa, H.; Yamaguchi, T.; Nashimoto, A.; Fujii, M.; Nakajima, T.; Ohashi, Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011, 29, 4387–4393. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, Y.; Chen, B.; Wan, H.-W.; Jia, G.-Q.; Bai, H.-L.; Wu, X.-T. Overweight, obesity and gastric cancer risk: Results from a meta-analysis of cohort studies. Eur. J. Cancer 2009, 45, 2867–2873. [Google Scholar]
- Ladeiras-Lopes, R.; Pereira, A.K.; Nogueira, A.; Pinheiro-Torres, T.; Pinto, I.; Santos-Pereira, R.; Lunet, N. Smoking and gastric cancer: Systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008, 19, 689–701. [Google Scholar] [CrossRef]
- Sung, N.; Choi, K.; Park, E.; Park, K.; Lee, S.; Lee, A.; Choi, I.; Jung, K.; Won, Y.; Shin, H. Smoking, alcohol and gastric cancer risk in Korean men: The National Health Insurance Corporation Study. Br. J. Cancer 2007, 97, 700–704. [Google Scholar] [CrossRef]
- Conteduca, V.; Sansonno, D.; Lauletta, G.; Russi, S.; Ingravallo, G.; Dammacco, F.H. pylori infection and gastric cancer: State of the art. Int. J. Oncol. 2013, 42, 5–18. [Google Scholar] [CrossRef]
- Wu, C.-C.; Islam, M.M.; Lee, A.-J.; Su, C.-H.; Weng, Y.-C.; Yeh, C.-Y.; Lee, H.-H.; Lin, M.-C. Association between Statin Use and Risk of Parkinson’s Disease: Evidence from 18 Observational Studies Comprising 3.7 Million Individuals. J. Pers. Med. 2022, 12, 825. [Google Scholar] [CrossRef]
- Wu, C.-C.; Lee, A.-J.; Su, C.-H.; Huang, C.-Y.; Islam, M.M.; Weng, Y.-C. Statin use is associated with a decreased risk of mortality among patients with COVID-19. J. Clin. Med. 2021, 10, 1450. [Google Scholar] [CrossRef]
- Islam, M.M.; Poly, T.N.; Walther, B.A.; Yang, H.-C.; Li, Y.-C. Statin use and the risk of hepatocellular carcinoma: A meta-analysis of observational studies. Cancers 2020, 12, 671. [Google Scholar] [CrossRef] [PubMed]
- Archibugi, L.; Arcidiacono, P.G.; Capurso, G. Statin use is associated to a reduced risk of pancreatic cancer: A meta-analysis. Dig. Liver Dis. 2019, 51, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.P.; Singh, A.G.; Murad, M.H.; Sanchez, W. Statins are associated with a reduced risk of hepatocellular cancer: A systematic review and meta-analysis. Gastroenterology 2013, 144, 323–332. [Google Scholar] [CrossRef]
- Bansal, D.; Undela, K.; D’Cruz, S.; Schifano, F. Statin use and risk of prostate cancer: A meta-analysis of observational studies. PLoS ONE 2012, 7, e46691. [Google Scholar] [CrossRef]
- Singh, P.; Singh, S. Statins are associated with reduced risk of gastric cancer: A systematic review and meta-analysis. Ann. Oncol. 2013, 24, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; Casaburi, I.; Avena, P.; Trotta, F.; De Luca, A.; Rago, V.; Pezzi, V.; Sirianni, R. Cholesterol and its metabolites in tumor growth: Therapeutic potential of statins in cancer treatment. Front. Endocrinol. 2019, 9, 807. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kang, H.S.; Kim, J.-H.; Kim, J.H.; Kim, S.H.; Kim, N.Y.; Nam, E.S.; Min, K.-W.; Choi, H.G. Association between statin use and gastric cancer: A nested case-control study using a national health screening cohort in Korea. Pharmaceuticals 2021, 14, 1283. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Rajasekhar, A.; Lottenberg, R.; Lottenberg, L.; Liu, H.; Ang, D. Pulmonary embolism prophylaxis with inferior vena cava filters in trauma patients: A systematic review using the meta-analysis of observational studies in epidemiology (MOOSE) guidelines. J. Thromb. Thrombolysis 2011, 32, 40–46. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Iqbal, U.; Walther, B.; Atique, S.; Dubey, N.K.; Nguyen, P.-A.; Poly, T.N.; Masud, J.H.B.; Li, Y.-C.J.; Shabbir, S.-A. Benzodiazepine use and risk of dementia in the elderly population: A systematic review and meta-analysis. Neuroepidemiology 2016, 47, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Poly, T.N.; Islam, M.M.; Yang, H.C.; Lin, M.C.; Jian, W.-S.; Hsu, M.-H.; Li, Y.-C.J. Obesity and mortality among patients diagnosed with COVID-19: A systematic review and meta-analysis. Front. Med. 2021, 8, 620044. [Google Scholar] [CrossRef] [PubMed]
- Poly, T.N.; Islam, M.; Yang, H.-C.; Li, Y.-C.J. Non-steroidal anti-inflammatory drugs and risk of Parkinson’s disease in the elderly population: A meta-analysis. Eur. J. Clin. Pharmacol. 2019, 75, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.I.; Park, C.H.; Kim, T.J.; Bang, C.S.; Kim, J.Y.; Lee, K.J.; Kim, J.; Kim, H.H.; You, S.C.; Shin, W.G. Aspirin, metformin, and statin use on the risk of gastric cancer: A nationwide population-based cohort study in Korea with systematic review and meta-analysis. Cancer Med. 2022, 11, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-s.; Kim, H.J.; Ahn, H.S. Statins and the risk of gastric, colorectal, and esophageal cancer incidence and mortality: A cohort study based on data from the Korean national health insurance claims database. J. Cancer Res. Clin. Oncol. 2022, 148, 2855–2865. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Yoo, T.G.; Jeong, S.-M.; Shin, D.W. Association of Aspirin, Metformin, and Statin Use with Gastric Cancer Incidence and Mortality: A Nationwide Cohort StudyCardiovascular Drugs and Gastric Cancer. Cancer Prev. Res. 2021, 14, 95–104. [Google Scholar] [CrossRef] [PubMed]
- You, H.-S.; You, N.; Lee, J.-W.; Lim, H.-J.; Kim, J.; Kang, H.-T. Inverse association between statin use and stomach cancer incidence in individuals with hypercholesterolemia, from the 2002–2015 NHIS-HEALS Data. Int. J. Environ. Res. Public Health 2020, 17, 1054. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.; Chen, L.; Seto, W.-K.; Wong, I.C.; Leung, W.K. Statins were associated with a reduced gastric cancer risk in patients with eradicated Helicobacter pylori infection: A territory-wide propensity score matched study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 493–499. [Google Scholar] [CrossRef]
- Lin, C.-J.; Liao, W.-C.; Lin, H.-J.; Hsu, Y.-M.; Lin, C.-L.; Chen, Y.-A.; Feng, C.-L.; Chen, C.-J.; Kao, M.-C.; Lai, C.-H. Statins attenuate Helicobacter pylori CagA translocation and reduce incidence of gastric cancer: In vitro and population-based case-control studies. PLoS ONE 2016, 11, e0146432. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.H.; Hur, K.Y.; Woo, S.Y.; Kim, S.W.; Kang, W.K. Statins and the risk of gastric cancer in diabetes patients. BMC Cancer 2012, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.W.; Chan, A.L.; Lo, D.; Leung, J.H.; Chen, H.-L. Common cancer risk and statins: A population-based case–control study in a Chinese population. Expert Opin. Drug Saf. 2013, 12, 19–27. [Google Scholar] [CrossRef]
- Collaboration, C.T.T. Lack of effect of lowering LDL cholesterol on cancer: Meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS ONE 2012, 7, e29849. [Google Scholar]
- Chiu, H.-F.; Ho, S.-C.; Chang, C.-C.; Wu, T.-N.; Yang, C.-Y. Statins are associated with a reduced risk of gastric cancer: A population-based case–control study. Off. J. Am. Coll. Gastroenterol. ACG 2011, 106, 2098–2103. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, Y.; Coupland, C.; Hippisley-Cox, J. Exposure to statins and risk of common cancers: A series of nested case-control studies. BMC Cancer 2011, 11, 409. [Google Scholar] [CrossRef]
- Marelli, C.; Gunnarsson, C.; Ross, S.; Haas, S.; Stroup, D.F.; Cload, P.; Clopton, P.; DeMaria, A.N. Statins and risk of cancer: A retrospective cohort analysis of 45,857 matched pairs from an electronic medical records database of 11 million adult Americans. J. Am. Coll. Cardiol. 2011, 58, 530–537. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C. Unintended effects of statins in men and women in England and Wales: Population based cohort study using the QResearch database. BMJ 2010, 340, c2197. [Google Scholar] [CrossRef]
- Matsushita, Y.; Sugihara, M.; Kaburagi, J.; Ozawa, M.; Iwashita, M.; Yoshida, S.; Saito, H.; Hattori, Y. Pravastatin use and cancer risk: A meta-analysis of individual patient data from long-term prospective controlled trials in Japan. Pharmacoepidemiol. Drug Saf. 2010, 19, 196–202. [Google Scholar] [CrossRef]
- Haukka, J.; Sankila, R.; Klaukka, T.; Lonnqvist, J.; Niskanen, L.; Tanskanen, A.; Wahlbeck, K.; Tiihonen, J. Incidence of cancer and statin usage—Record linkage study. Int. J. Cancer 2010, 126, 279–284. [Google Scholar] [CrossRef]
- Friedman, G.D.; Flick, E.D.; Udaltsova, N.; Chan Pharm, D.J.; Quesenberry, C.P., Jr.; Habel, L.A. Screening statins for possible carcinogenic risk: Up to 9 years of follow-up of 361 859 recipients. Pharmacoepidemiol. Drug Saf. 2008, 17, 27–36. [Google Scholar] [CrossRef]
- Sato, S.; Ajiki, W.; Kobayashi, T.; Awata, N. Pravastatin use and the five-year incidence of cancer in coronary heart disease patients: From the prevention of coronary sclerosis study. J. Epidemiol. 2006, 16, 201–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaye, J.; Jick, H. Statin use and cancer risk in the General Practice Research Database. Br. J. Cancer 2004, 90, 635–637. [Google Scholar] [CrossRef]
- Graaf, M.R.; Beiderbeck, A.B.; Egberts, A.C.; Richel, D.J.; Guchelaar, H.-J. The risk of cancer in users of statins. J. Clin. Oncol. 2004, 22, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-D.; Zeng, K.; Xue, F.-Q.; Chen, J.-H.; Chen, Y.-Q. Statins are associated with reduced risk of gastric cancer: A meta-analysis. Eur. J. Clin. Pharmacol. 2013, 69, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, W.; Jin, G.; Chu, P.; Li, H. Effect of statins on gastric cancer incidence: A meta-analysis of case control studies. J. Cancer Res. Ther. 2014, 10, 859. [Google Scholar]
- Lou, D.; Fu, R.; Gu, L.; Su, H.; Guan, L. Association between statins’ exposure with incidence and prognosis of gastric cancer: An updated meta-analysis. Expert Rev. Clin. Pharmacol. 2022, 15, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Sun, G.; Yao, Q.; Wan, Z.; Huang, X. Statin use and risk of gastrointestinal cancer: A meta-analysis of cohort studies. Int. J. Clin. Exp. Med. 2018, 11, 1437–1447. [Google Scholar]
- Mortensen, S.; Leth, A.; Agner, E.; Rohde, M. Dose-related decrease of serum coenzyme Q10 during treatment with HMG-CoA reductase inhibitors. Mol. Asp. Med. 1997, 18, 137–144. [Google Scholar] [CrossRef]
- Marcheggiani, F.; Cirilli, I.; Orlando, P.; Silvestri, S.; Vogelsang, A.; Knott, A.; Blatt, T.; Weise, J.M.; Tiano, L. Modulation of Coenzyme Q10 content and oxidative status in human dermal fibroblasts using HMG-CoA reductase inhibitor over a broad range of concentrations. From mitohormesis to mitochondrial dysfunction and accelerated aging. Aging 2019, 11, 2565. [Google Scholar] [CrossRef]
- Pereira, M.; Matuszewska, K.; Glogova, A.; Petrik, J. Mutant p53, the Mevalonate Pathway and the Tumor Microenvironment Regulate Tumor Response to Statin Therapy. Cancers 2022, 14, 3500. [Google Scholar] [CrossRef]
- Göbel, A.; Rauner, M.; Hofbauer, L.C.; Rachner, T.D. Cholesterol and beyond-The role of the mevalonate pathway in cancer biology. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2020, 1873, 188351. [Google Scholar] [CrossRef] [PubMed]
- Likus, W.; Siemianowicz, K.; Bieńk, K.; Pakuła, M.; Pathak, H.; Dutta, C.; Wang, Q.; Shojaei, S.; Assaraf, Y.G.; Ghavami, S. Could drugs inhibiting the mevalonate pathway also target cancer stem cells? Drug Resist. Updates 2016, 25, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.M.; Hohl, R.J. Anti-cancer therapy: Targeting the mevalonate pathway. Curr. Cancer Drug Targets 2006, 6, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Aicher, A.; Vasa, M.; Mildner-Rihm, C.; Adler, K.; Tiemann, M.; Rütten, H.; Fichtlscherer, S.; Martin, H.; Zeiher, A.M. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J. Clin. Investig. 2001, 108, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.W.; Dimitroulakos, J.; Minden, M.; Penn, L. HMG-CoA reductase inhibitors and the malignant cell: The statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 2002, 16, 508–519. [Google Scholar] [CrossRef]
- Rao, S.; Porter, D.C.; Chen, X.; Herliczek, T.; Lowe, M.; Keyomarsi, K. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc. Natl. Acad. Sci. USA 1999, 96, 7797–7802. [Google Scholar] [CrossRef]
- Kusama, T.; Mukai, M.; Iwasaki, T.; Tatsuta, M.; Matsumoto, Y.; Akedo, H.; Inoue, M.; Nakamura, H. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors reduce human pancreatic cancer cell invasion and metastasis. Gastroenterology 2002, 122, 308–317. [Google Scholar] [CrossRef]
- Li, Y.-F.; Feng, Q.-Z.; Gao, W.-Q.; Zhang, X.-J.; Huang, Y.; Chen, Y.-D. The difference between Asian and Western in the effect of LDL-C lowering therapy on coronary atherosclerotic plaque: A meta-analysis report. BMC Cardiovasc. Disord. 2015, 15, 6. [Google Scholar] [CrossRef]
- Shah, R.R.; Gaedigk, A. Precision medicine: Does ethnicity information complement genotype-based prescribing decisions? Ther. Adv. Drug Saf. 2018, 9, 45–62. [Google Scholar] [CrossRef]
- Kim, K.; Johnson, J.A.; Derendorf, H. Differences in drug pharmacokinetics between East Asians and Caucasians and the role of genetic polymorphisms. J. Clin. Pharmacol. 2004, 44, 1083–1105. [Google Scholar] [CrossRef]
- Lee, E.; Ryan, S.; Birmingham, B.; Zalikowski, J.; March, R.; Ambrose, H.; Moore, R.; Lee, C.; Chen, Y.; Schneck, D. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin. Pharmacol. Ther. 2005, 78, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.I.; Kim, S.R.; Huh, W.; Ko, J.-W.; Lee, S.-Y. Association of genetic variations with pharmacokinetics and lipid-lowering response to atorvastatin in healthy Korean subjects. Drug Des. Dev. Ther. 2017, 11, 1135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).