Mid-Term Outcome of Ventricular Arrhythmias Catheter Ablation in Patients with Chronic Coronary Total Occlusion Compared to Ischemic and Non-Ischemic Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
- −
- High burden of premature ventricular contractions (PVCs) (≥25% of total beats in 24 h ECG Holter monitoring) or non-sustained ventricular tachycardia (NSVT) during 24 h ECG Holter monitoring or exercise maximal stress test;
- −
- Sustained ventricular tachycardia (VT) or ventricular fibrillation (VF).
2.2. 3D-Electroanatomic Ventricular Mapping
2.3. Ventricular Arrhythmias Catheter Ablation
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
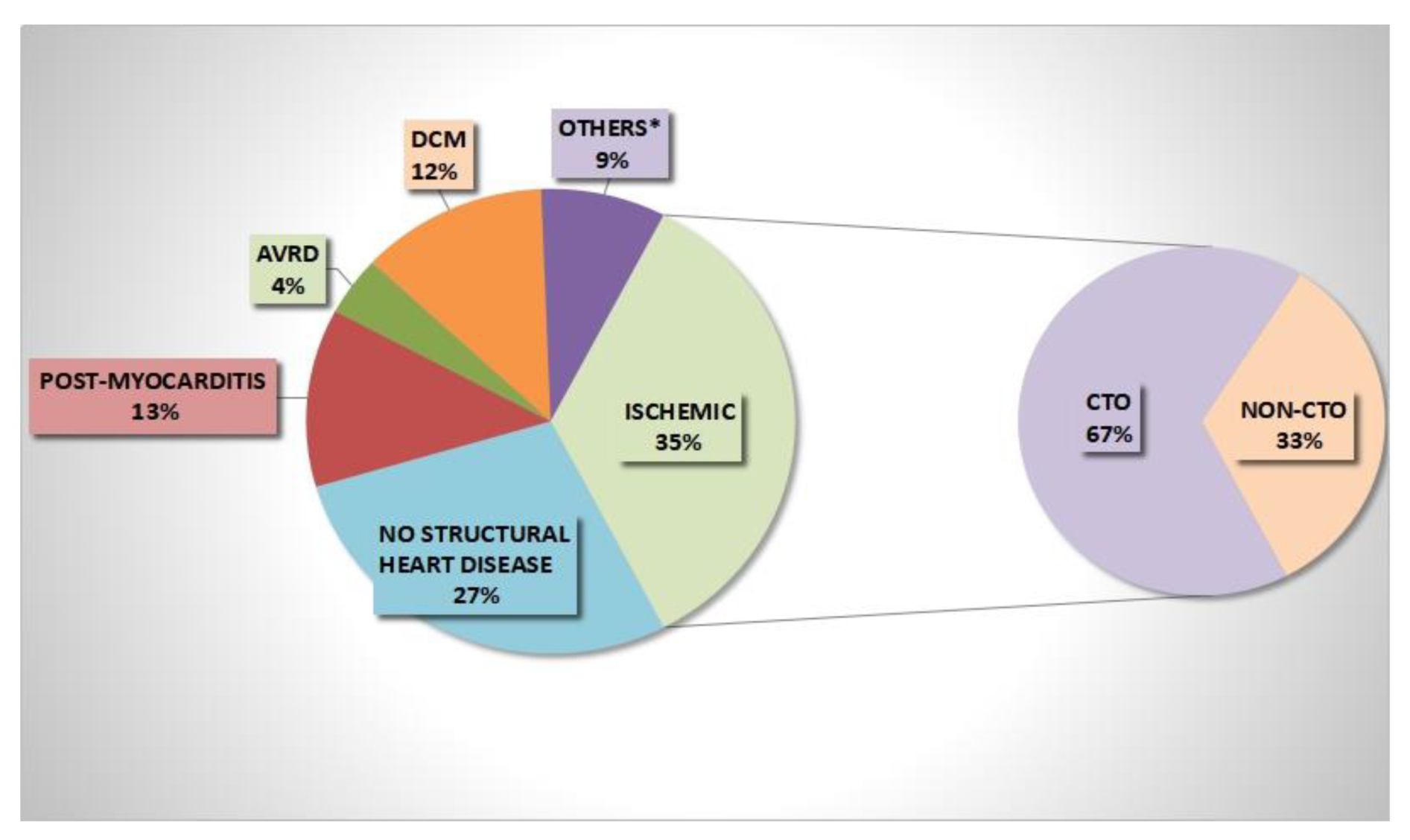
3.1. Baseline Findings
3.2. 3D Electroanatomic Mapping and Catheter Ablation Findings
3.3. Follow-Up Findings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTO | chronic total occlusions |
| 3D-EAM | three-dimension electroanatomic mapping |
| EP | electrophysiology |
| IRA | infarct-related artery |
| LAD | left anterior descending artery |
| LCA | left circumflex artery |
| LVOT | left ventricular outflow tract |
| LAVA | local abnormal ventricular activities |
| LP | late potentials |
| LVEF | left ventricular ejection fraction |
| MI | myocardial infarction |
| NSVT | non-sustained ventricular tachycardia |
| PVCs | premature ventricular contractions |
| RCA | right coronary artery |
| SVT | sustained ventricular tachycardia |
| VAs | ventricular arrhythmias |
| VF | ventricular fibrillation |
References
- Arenal, Á.; Hernández, J.; Calvo, D.; Ceballos, C.; Atéa, L.; Datino, T.; Atienza, F.; González-Torrecilla, E.; Eídelman, G.; Miracle, Á.; et al. Safety, long-term results, and predictors of recurrence after complete endocardial ventricular tachycardia substrate ablation in patients with previous myocardial infarction. Am. J. Cardiol. 2013, 111, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.K.; Gong, M.; Bazoukis, G.; Yan, B.P.; Letsas, K.P.; Liu, T.; Baranchuk, A.; Nombela-Franco, L.; Dong, M.; Tse, G. Impact of Coronary Artery Chronic Total Occlusion on Arrhythmic and Mortality Outcomes: A Systematic Review and Meta-Analysis. JACC Clin. Electrophysiol. 2018, 4, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Nombela-Franco, L.; Mitroi, C.D.; Fernández-Lozano, I.; García-Touchard, A.; Toquero, J.; Castro-Urda, V.; Fernández-Diaz, J.A.; Perez-Pereira, E.; Beltrán-Correas, P.; Segovia, J.; et al. Ventricular arrhythmias among implantable cardioverter-defibrillator recipients for primary prevention: Impact of chronic total coronary occlusion (VACTO Primary Study). Circ. Arrhythm. Electrophysiol. 2012, 5, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nombela-Franco, L.; Iannaccone, M.; Anguera, I.; Amat-Santos, I.J.; Sanchez-Garcia, M.; Bautista, D.; Calvelo, M.N.; Di Marco, A.; Moretti, C.; Pozzi, R.; et al. Impact of Chronic Total Coronary Occlusion on Recurrence of Ventricular Arrhythmias in Ischemic Secondary Prevention Implantable Cardioverter-Defibrillator Recipients (VACTO Secondary Study) Insights from Coronary Angiogram and Electrogram Analysis. Cardiovasc. Interv. 2017, 10, 879–888. [Google Scholar]
- Di Marco, A.; Sanjuan, T.O.; Paglino, G.; Baratto, F.; Vergara, P.; Bisceglia, C.; Trevisi, N.; Sala, S.; Marzi, A.; Gulletta, S.; et al. Late potentials abolition reduces ventricular tachycardia recurrence after ablation especially in higher-risk patients with a chronic total occlusion in an infarct-related artery. J. Cardiovasc. Electrophysiol. 2018, 29, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, A.; Paglino, G.; Oloriz, T.; Maccabelli, G.; Baratto, F.; Vergara, P.; Bisceglia, C.; Anguera, I.; Sala, S.; Sora, N.; et al. Impact of a chronic total occlusion in an infarct-related artery on the long-term outcome of ventricular tachycardia ablation. J. Cardiovasc. Electrophysiol. 2015, 26, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Tajti, P.; Brilakis, E.S. Chronic total occlusion percutaneous coronary intervention: Evidence and controversies. J. Am. Heart Assoc. 2018, 7, e006732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; A Barrabés, J.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867l. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faga, V.; Anguera, I.; Oloriz, T.; Nombela-Franco, L.; Teruel, L.; Dallaglio, P.; Guerrero, A.P.; Salazar, C.H.; Escaned, J.; Abadía, A.A.; et al. Improved prediction of electrical storm in patients with prior myocardial infarction and implantable cardioverter defibrillator. Int. J. Cardiol. 2022, 355, 9–14. [Google Scholar] [CrossRef]
- Raja, V.; Wiegn, P.; Obel, O.; Christakopoulos, G.; Christopoulos, G.; Rangan, B.V.; Roesle, M.; Abdullah, S.M.; Luna, M.; Addo, T.; et al. Impact of chronic total occlusions and coronary revascularization on all-cause mortality and the incidence of ventricular arrhythmias in patients with ischemic cardiomyopathy: The study was presented at the SCAI 2014 Scientific Sessions, Las Vegas, Nevada. Am. J. Cardiol. 2015, 116, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
- Beggs, S.A.S.; Jhund, P.S.; Jackson, C.E.; McMurray, J.J.V.; Gardner, R.S. Non-ischaemic cardiomyopathy, sudden death and implantable defibrillators: A review and meta-analysis. Heart 2018, 104, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Yap, S.-C.; Sakhi, R.; Theuns, D.A.; Yasar, Y.E.; Bhagwandien, R.E.; Diletti, R.; Zijlstra, F.; Szili-Torok, T. Increased risk of ventricular arrhythmias in survivors of out-of-hospital cardiac arrest with chronic total coronary occlusion. Heart Rhythm 2018, 15, 124–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baks, T.; van Geuns, R.-J.; Duncker, D.J.; Cademartiri, F.; Mollet, N.R.; Krestin, G.P.; Serruys, P.W.; de Feyter, P.J. Prediction of left ventricular function after drug-eluting stent implantation for chronic total coronary occlusions. J. Am. Coll. Cardiol. 2006, 47, 721–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-W.; Lee, P.H.; Ahn, J.-M.; Park, D.-W.; Yun, S.-C.; Han, S.; Kang, H.; Kang, S.-J.; Kim, Y.-H.; Lee, C.W.; et al. Randomized Trial Evaluating Percutaneous Coronary Intervention for the Treatment of Chronic Total Occlusion: The DECISION-CTO Trial. Circulation 2019, 139, 1674–1683. [Google Scholar] [CrossRef] [PubMed]



| Clinical Data | No CTO pts (92) | CTO pts (28) | p Value |
|---|---|---|---|
| Men, n (%) | 63 (68%) | 27 (96%) | 0.002 |
| Age, y, mean ± SD | 56 ± 16 | 68 ± 8 | <0.001 |
| Hypertension, n (%) | 44 (47%) | 24 (86%) | <0.001 |
| Diabetes mellitus, n (%) | 24 (26%) | 9 (32%) | 0.37 |
| Smoke, n (%) | 13 (14%) | 0 (0) | 0.07 |
| COPD/OSAS, n (%) | 16 (17%) | 6 (21%) | 0.27 |
| Previous MI, n (%) | 10 (11%) | 27 (96%) | <0.001 |
| Sustained VAs on admission | 49 (53%) | 27 (96%) | <0.001 |
| Chronic kidney disease, n (%) | 25 (27%) | 16 (57%) | 0.005 |
| NYHA class, n (%) | 32 (35%) | 18 (64%) | 0.06 |
| ICD carriers, n (%) | 41 (44%) | 23 (82%) | 0.005 |
| Electrical storm, n (%) | 29 (31%) | 11 (39%) | 0.14 |
| Atrial fibrillation, n (%) | 31 (34%) | 11 (39%) | 0.15 |
| AAD on admission, n (%) | |||
| B-blockers | 36 (39%) | 15 (53%) | 0.09 |
| Amiodarone | 17 (19%) | 12 (42%) | 0.003 |
| Echocardiography Data | No CTO pts (92) | CTO pts (28) | p Value |
|---|---|---|---|
| LVEF, mean, % ± SD | 48 ± 13 | 36 ± 10 | <0.001 |
| Moderate/Severe LV dysfunction LVEF < 40%, n (%) | 28 (30%) | 18 (64%) | 0.006 |
| RV dysfunction (TAPSE < 17 mm), n (%) | 15 (16%) | 3 (11%) | 0.81 |
| Akinetic ventricular areas, n (%) | 20 (22%) | 21 (75%) | <0.001 |
| EAM Data | No CTO pts (92) | CTO pts (28) | p Value |
|---|---|---|---|
| LV Endocardial 3D-EAM, n (%) | 67 (73%) | 27 (96%) | <0.001 |
| Total volume mL, n ± SD | 214 ± 101 | 212 ± 68 | 0.864 |
| Unipolar scar, n (%) | 33 (36%) | 14 (50%) | 0.117 |
| Unipolar scar area, cm2, mean ± SD | 99 ± 96 | 85 ± 68 | 0.457 |
| Bipolar scar, n pts (%) | 21 (23%) | 11 (39%) | 0.058 |
| Bipolar scar area, cm2, mean ± SD | 36 ± 40 | 33 ± 28 | 0.992 |
| LV VA exit by 3D-EAM, n (%) | 51 (55%) | 24 (86%) | 0.001 |
| RV Endocardial 3D-EAM, n (%) | 51 (55%) | 5 (18%) | <0.001 |
| Total volume, mL, n ± SD | 105 ± 40 | 130 ± 2 | 0.839 |
| Unipolar scar, n (%) | 12 (13%) | 0 | - |
| Unipolar scar area, cm2, mean ± SD | 27 ± 26 | 0 | - |
| Bipolar scar, n (%) | 6 (6.5%) | 0 | - |
| Bipolar scar area, cm2, mean ± SD | 20 ± 20 | 0 | - |
| Endocardial LAVA, n (%) | 23 (25%) | 20 (71%) | <0.001 |
| Epicardial 3D-EAM, n (%) | 7 (7%) | 2 (7%) | 0.786 |
| Bipolar scar, n (%) | 6 (6%) | 2 (7%) | 0.571 |
| Bipolar scar area, cm2, ± SD | 105 ± 42 | 119 ± 3 | 0.593 |
| Epicardial LAVA n (%) | 5 (5%) | 2 (7%) | 0.391 |
| Variables | HR (95% CI) | p Value | HR (95% CI) | p Value |
|---|---|---|---|---|
| Age | 1 (0.9–1.03) | 0.5 | ||
| Sex | 0.9 (0.5–1.8) | 0.9 | ||
| Hypertension | 1.4 (0.8–2.4) | 0.1 | ||
| Type II DM | 0.7 (0.4–1.4) | 0.3 | ||
| Previous MI | 1.5 (0.9–2.5) | 0.1 | ||
| Presence of CTO | 1.8 (1.03–3.1) | 0.04 | 1.5 (0.9–2.5) | 0.13 |
| IRA CTO | 1.6 (0.53–1.9) | 0.4 | ||
| Number of CTO | 1.2 (0.83–1.8) | 0.2 | ||
| LAD CTO | 2.1 (0.9–5) | 0.07 | ||
| LCA CTO | 0.9 (0.2–3.8) | 0.9 | ||
| RCA CTO | 1.5 (0.8–2.8) | 0.2 | ||
| LVEF < 35% | 2.6 (1.5–4.6) | 0.001 | 3.1 (1.5–6.4) | 0.002 |
| Akinetic areas | 0.9 (0.4–1.7) | 0.7 | ||
| NYHA class > II | 1.3 (1.05–1.7) | 0.02 | ||
| ICD carriers | 1.7 (1.3–2.4) | 0.001 | ||
| Endocardial LAVA/LP | 4 (1.8–9) | 0.001 | ||
| Beta-blockers at FUP | 0.65 (0.2–1.6) | 0.37 | ||
| Amiodarone at FUP | 0.87 (0.5–1.4) | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narducci, M.L.; Niccoli, G.; Flore, F.; Perna, F.; Bencardino, G.; Montone, R.A.; Pelargonio, G.; Crea, F. Mid-Term Outcome of Ventricular Arrhythmias Catheter Ablation in Patients with Chronic Coronary Total Occlusion Compared to Ischemic and Non-Ischemic Patients. J. Clin. Med. 2022, 11, 7181. https://doi.org/10.3390/jcm11237181
Narducci ML, Niccoli G, Flore F, Perna F, Bencardino G, Montone RA, Pelargonio G, Crea F. Mid-Term Outcome of Ventricular Arrhythmias Catheter Ablation in Patients with Chronic Coronary Total Occlusion Compared to Ischemic and Non-Ischemic Patients. Journal of Clinical Medicine. 2022; 11(23):7181. https://doi.org/10.3390/jcm11237181
Chicago/Turabian StyleNarducci, Maria Lucia, Giampaolo Niccoli, Francesco Flore, Francesco Perna, Gianluigi Bencardino, Rocco Antonio Montone, Gemma Pelargonio, and Filippo Crea. 2022. "Mid-Term Outcome of Ventricular Arrhythmias Catheter Ablation in Patients with Chronic Coronary Total Occlusion Compared to Ischemic and Non-Ischemic Patients" Journal of Clinical Medicine 11, no. 23: 7181. https://doi.org/10.3390/jcm11237181






