Comparison between Cystatin C- and Creatinine-Based Estimated Glomerular Filtration Rate in the Follow-Up of Patients Recovering from a Stage-3 AKI in ICU
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Definitions—Acute Kidney Injury Criteria and Calculations
2.3. Serum Creatinine and Cystatin C Measurement
2.4. Evaluation of Glomerular Filtration Rate
2.5. Outcomes
2.6. Statistical Analyses
3. Results
3.1. Patients
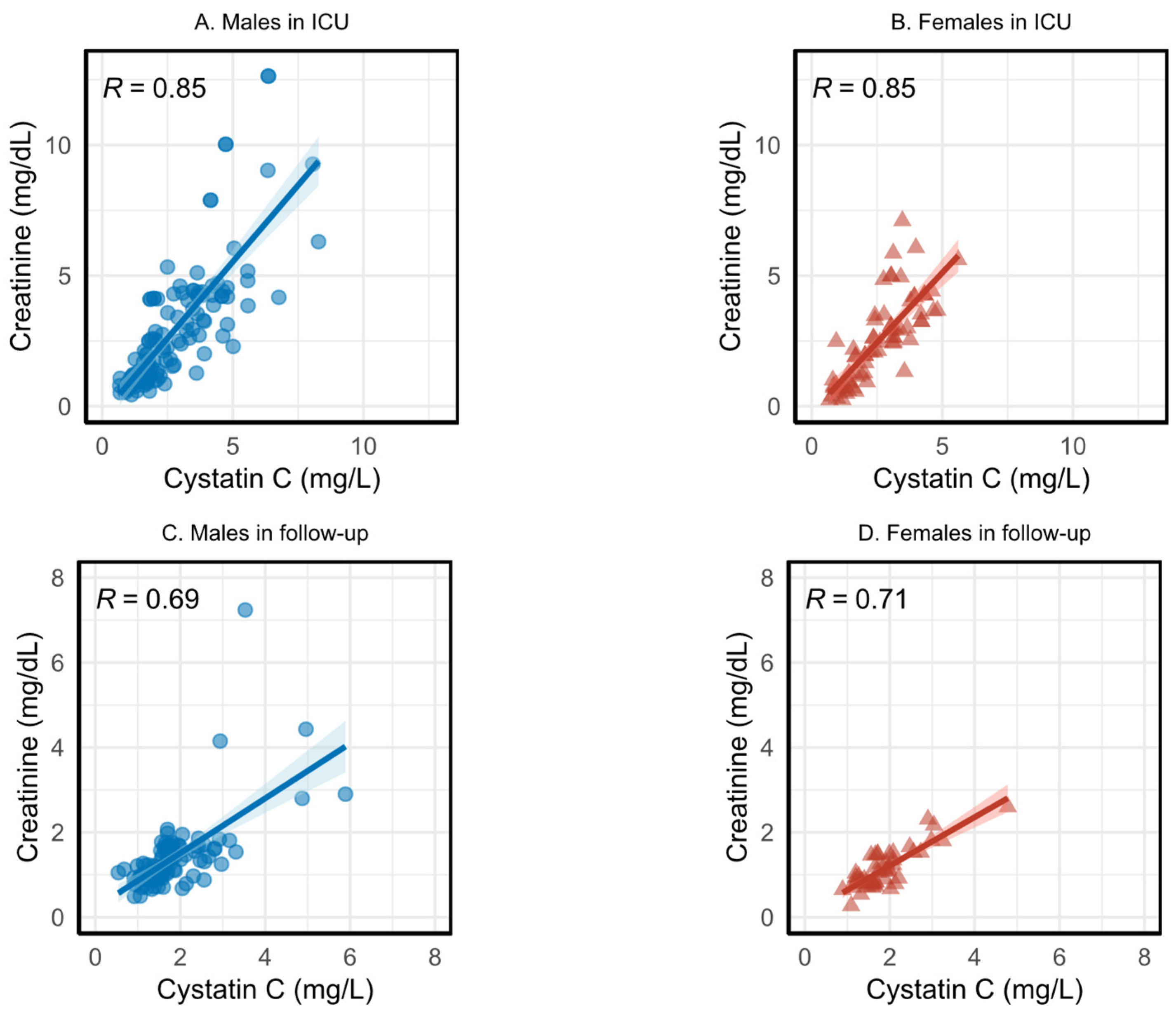
3.2. Correlation between Serum Creatinine and Cystatin C
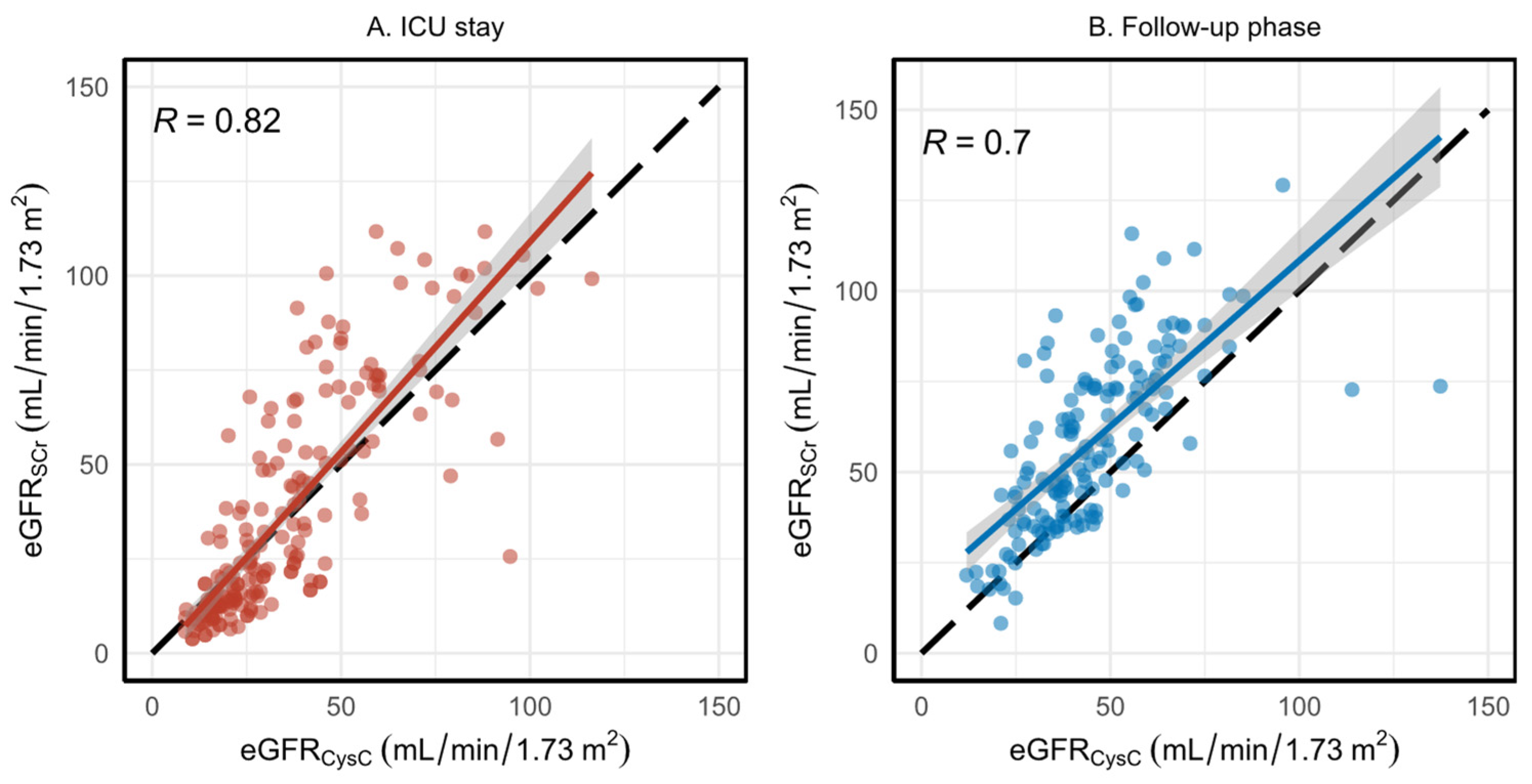
3.3. Evaluation of eGFR Using Serum Creatinine and Cystatin C
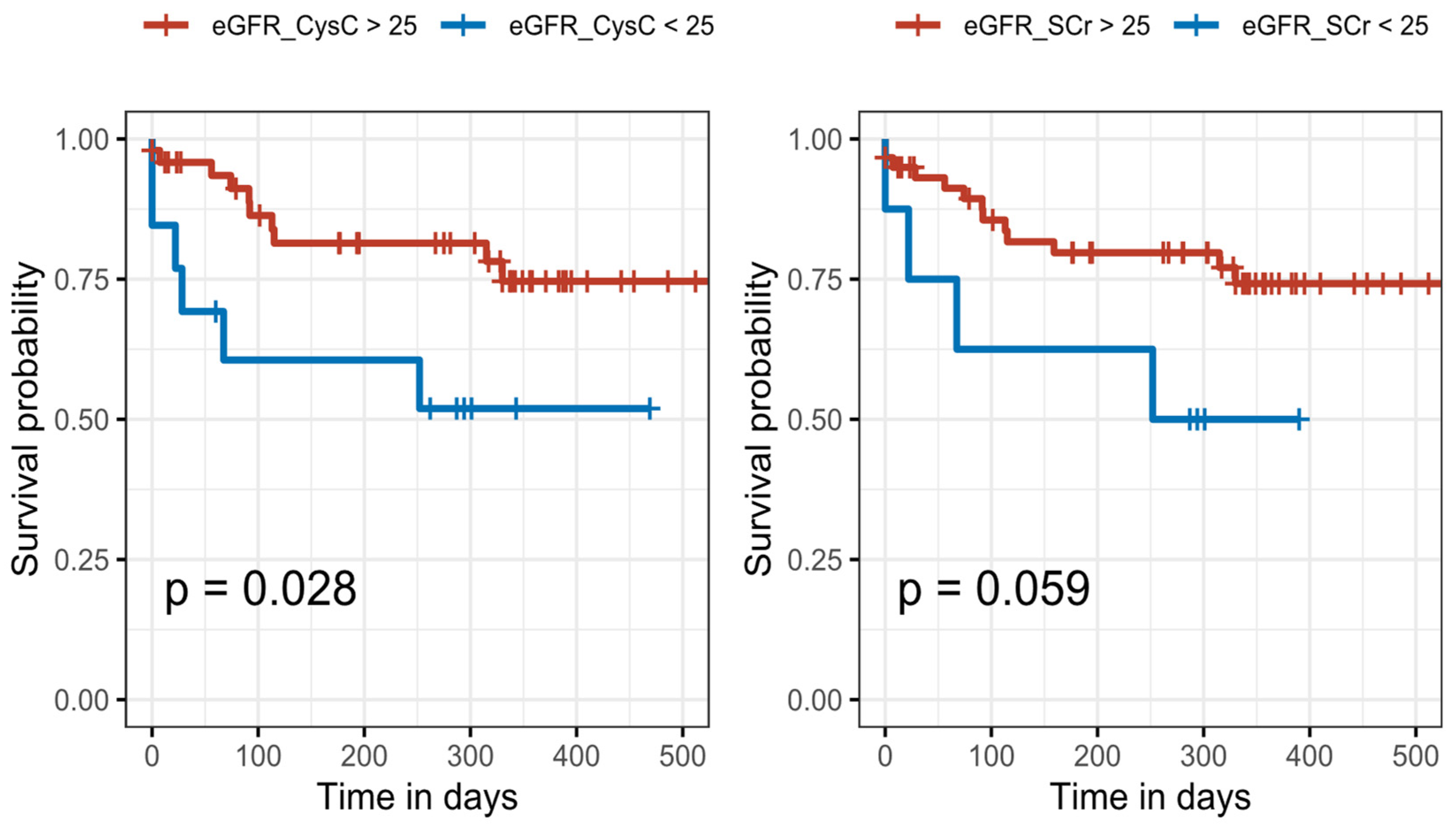
3.4. The Associations between eGFR and Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouchard, J.; Mehta, R.L. Acute Kidney Injury in Western Countries. Kidney Dis. 2016, 2, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef]
- Cerdá, J.; Mohan, S.; Garcia-Garcia, G.; Jha, V.; Samavedam, S.; Gowrishankar, S.; Bagga, A.; Chakravarthi, R.; Mehta, R. Acute Kidney Injury Recognition in Low- and Middle-Income Countries. Kidney Int. Rep. 2017, 2, 530–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Hoste, L.; Dubourg, L.; Selistre, L.; De Souza, V.C.; Ranchin, B.; Hadj-Aïssa, A.; Cochat, P.; Martens, F.; Pottel, H. A new equation to estimate the glomerular filtration rate in children, adolescents and young adults. Nephrol. Dial. Transplant. 2014, 29, 1082–1091. [Google Scholar] [CrossRef] [Green Version]
- Knight, E.L.; Verhave, J.C.; Spiegelman, D.; Hillege, H.L.; De Zeeuw, D.; Curhan, G.C.; De Jong, P.E. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004, 65, 1416–1421. [Google Scholar] [CrossRef] [Green Version]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Pottel, H.; Delanaye, P.; Schaeffner, E.; Dubourg, L.; Eriksen, B.O.; Melsom, T.; Lamb, E.J.; Rule, A.D.; Turner, S.T.; Glassock, R.J.; et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol. Dial. Transplant. 2017, 32, 497–507. [Google Scholar] [CrossRef]
- Grubb, A.; Horio, M.; Hansson, L.O.; Björk, J.; Nyman, U.; Flodin, M.; Larsson, A.; Bökenkamp, A.; Yasuda, Y.; Blufpand, H.; et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin. Chem. 2014, 60, 974–986. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604. [Google Scholar] [CrossRef]
- Pottel, H.; Hoste, L.; Dubourg, L.; Ebert, N.; Schaeffner, E.; Eriksen, B.O.; Melsom, T.; Lamb, E.J.; Rule, A.D.; Turner, S.T. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol. Dial. Transplant. 2016, 31, 798–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pottel, H.; Björk, J.; Courbebaisse, M.; Couzi, L.; Ebert, N.; Eriksen, B.O.; Dalton, R.N.; Dubourg, L.; Gaillard, F.; Garrouste, C.; et al. Development and validation of a modified full age spectrum creatinine-based equation to estimate glomerular filtration rate. Ann. Intern. Med. 2021, 174, 183–191. [Google Scholar] [CrossRef]
- Kar, S.; Paglialunga, S.; Islam, R. Cystatin C Is a More Reliable Biomarker for Determining eGFR to Support Drug Development Studies. J. Clin. Pharmacol. 2018, 58, 1239–1247. [Google Scholar] [CrossRef]
- Delanaye, P.; Cavalier, E.; Morel, J.; Mehdi, M.; Maillard, N.; Claisse, G.; Lambermont, B.; Dubois, B.E.; Damas, P.; Krzesinski, J.M.; et al. Detection of decreased glomerular filtration rate in intensive care units: Serum cystatin C versus serum creatinine. BMC Nephrol. 2014, 15, 9. [Google Scholar] [CrossRef]
- Helmersson-Karlqvist, J.; Lipcsey, M.; Ärnlöv, J.; Bell, M.; Ravn, B.; Dardashti, A.; Larsson, A. Cystatin C predicts long term mortality better than creatinine in a nationwide study of intensive care patients. Sci. Rep. 2021, 11, 5882. [Google Scholar] [CrossRef] [PubMed]
- Gharaibeh, K.A.; Hamadah, A.M.; El-Zoghby, Z.M.; Lieske, J.C.; Larson, T.S.; Leung, N. Cystatin C Predicts Renal Recovery Earlier Than Creatinine Among Patients With Acute Kidney Injury. Kidney Int. Rep. 2018, 3, 337. [Google Scholar] [CrossRef] [Green Version]
- Rimes-Stigare, C.; Ravn, B.; Awad, A.; Torlén, K.; Martling, C.R.; Bottai, M.; Mårtensson, J.; Bell, M. Creatinine- and Cystatin C-Based Incidence of Chronic Kidney Disease and Acute Kidney Disease in AKI Survivors. Crit. Care Res. Pract. 2018, 2018, 7698090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunn, A.; Korpela, M. Crossdating in dplR. 2013. Available online: https://mran.microsoft.com/snapshot/2019-04-29/web/packages/dplR/vignettes/xdate-dplR.pdf (accessed on 2 October 2022).
- Forni, L.G.; Darmon, M.; Ostermann, M.; Oudemans-van Straaten, H.M.; Pettilä, V.; Prowle, J.R.; Schetz, M.; Joannidis, M. Renal recovery after acute kidney injury. Intensive Care Med. 2017, 43, 855. [Google Scholar] [CrossRef] [PubMed]
- Prowle, J.R.; Kolic, I.; Purdell-Lewis, J.; Taylor, R.; Pearse, R.M.; Kirwan, C.J. Article Serum Creatinine Changes Associated with Critical Illness and Detection of Persistent Renal Dysfunction after AKI. Clin. J. Am. Soc. Nephrol. 2014, 9, 1015–1023. [Google Scholar] [CrossRef] [Green Version]
- Shemesh, O.; Golbetz, H.; Kriss, J.P.; Myers, B.D. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985, 28, 830–838. [Google Scholar] [CrossRef]
- Chen, Y.; Zelnick, L.R.; Wang, K.; Katz, R.; Hoofnagle, A.N.; Becker, J.O.; Hsu, C.-Y.; Go, A.S.; Feldman, H.I.; Mehta, R.C.; et al. Association of tubular solute clearances with the glomerular filtration rate and complications of chronic kidney disease: The Chronic Renal Insufficiency Cohort study. Nephrol. Dial. Transplant. 2021, 36, 1271. [Google Scholar] [CrossRef] [PubMed]
- Tidman, M.; Sjöström, P.; Jones, I. A Comparison of GFR estimating formulae based upon s-cystatin C and s-creatinine and a combination of the two. Nephrol. Dial. Transplant 2008, 23, 154–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, L.A.; Zhang, Y.; Schmid, C.H. Evaluating the performance of equations for estimating glomerular filtration rate. J. Nephrol. 2008, 21, 797–807. [Google Scholar]
- Fan, L.; Levey, A.S.; Gudnason, V.; Eiriksdottir, G.; Andresdottir, M.B.; Gudmundsdottir, H.; Indridason, O.S.; Palsson, R.; Mitchell, G.; Inker, L.A. Comparing GFR Estimating Equations Using Cystatin C and Creatinine in Elderly Individuals. J. Am. Soc. Nephrol. 2015, 26, 1982–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristics | All Patients (N = 101) |
|---|---|
| Demographics | |
| Female sex, n (%) | 37 (36.6%) |
| Age, years | |
| Body weight, kg | |
| Body mass index, kg/m2 | 27.7 (17–57) |
| ICU types | |
| MICU, n (%) | 81 (80%) |
| SICU, n (%) | 17 (16.8%) |
| Trauma, n (%) | 3 (2.9%) |
| Admission diagnosis (%) | |
| Community-acquired pneumonia (CAP) | 10% |
| Cardiac disease | 9% |
| Acute respiratory failure | 8% |
| Sepsis | 7% |
| Aspiration pneumonia | 6% |
| Pulmonary edema | 4% |
| Other diagnosis | 56% |
| Results | |
| Length of stay in ICU, days | 14.5 (1–160) |
| Cystatin C (mg/L) at ICU admission | 1.94 (0.67–8.06) |
| Creatinine (mg/dL) at ICU admission | 1.98 (0.31–12.64) |
| First Follow-Up | Second Follow-Up | Third Follow-Up | Fourth Follow-Up | |
|---|---|---|---|---|
| Number of survivors for follow-up | 77 − 2 = 75 | 61 | 48 | 40 |
| Number of dropouts | 7 | 7 | 3 | 5 |
| Median follow-up days | 37 | 142 | 229 | 337 |
| Number of patients with SCr values | 68 | 54 | 45 | 35 |
| Number of patients with CysC values | 62 | 39 | 34 | 26 |
| Number of patients with SCr and CysC | 60 | 39 | 34 | 25 |
| eGFRSCr | eGFRCysC | |||||
|---|---|---|---|---|---|---|
| Predictors | Estimates | Estimates | ||||
| Intercept | 57.25 | 50.50–64.00 | <0.001 | 37.85 | 33.8–42.27 | <0.001 |
| Slope | 0.003 | −0.01–0.02 | 0.7 | 0.041 | 0.01–0.07 | 0.004 |
| eGFRSCr | eGFRCysC | |||
|---|---|---|---|---|
| <60 | ≥60 | <60 | ≥60 | |
| Visit 1 (n = 60) | 34 | 26 | 52 | 8 |
| Visit 2 (n = 39) | 23 | 16 | 34 | 5 |
| Visit 3 (n = 34) | 20 | 14 | 29 | 5 |
| Visit 4 (n = 25) | 10 | 15 | 15 | 10 |
| Mortality in ICU | Mortality in Follow-Up | |||
|---|---|---|---|---|
| Variable | HR | 95% CI | HR | 95% CI |
| Age | 1.05 * | 1.01–1.09 | 1.03 | 0.99–1.07 |
| Gender (male) | 0.93 | 0.41–2.13 | 2.022 | 0.67–6.10 |
| LoS in ICU | - | - | 0.98 | 0.96–1.01 |
| Dialysis in ICU | - | - | 1.86 | 0.87–3.95 |
| Reduced eGFRCysC in the first follow-up | - | - | 3.32 * | 1.2–9.2 |
| Reduced eGFRSCr in the first follow-up | - | - | 2.8 | 0.91–8.65 |
| Average eGFRCysC in ICU | 1 | 0.97–1.01 | 1.00 | 0.97–1.02 |
| Average eGFRSCr in ICU | 0.98 | 0.96–1.01 | 1.00 | 0.98–1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nateghi Haredasht, F.; Viaene, L.; Vens, C.; Callewaert, N.; De Corte, W.; Pottel, H. Comparison between Cystatin C- and Creatinine-Based Estimated Glomerular Filtration Rate in the Follow-Up of Patients Recovering from a Stage-3 AKI in ICU. J. Clin. Med. 2022, 11, 7264. https://doi.org/10.3390/jcm11247264
Nateghi Haredasht F, Viaene L, Vens C, Callewaert N, De Corte W, Pottel H. Comparison between Cystatin C- and Creatinine-Based Estimated Glomerular Filtration Rate in the Follow-Up of Patients Recovering from a Stage-3 AKI in ICU. Journal of Clinical Medicine. 2022; 11(24):7264. https://doi.org/10.3390/jcm11247264
Chicago/Turabian StyleNateghi Haredasht, Fateme, Liesbeth Viaene, Celine Vens, Nico Callewaert, Wouter De Corte, and Hans Pottel. 2022. "Comparison between Cystatin C- and Creatinine-Based Estimated Glomerular Filtration Rate in the Follow-Up of Patients Recovering from a Stage-3 AKI in ICU" Journal of Clinical Medicine 11, no. 24: 7264. https://doi.org/10.3390/jcm11247264





