The Impact of Late Follicular Phase Progesterone Elevation on Cumulative Live Birth Rate and Embryo Quality in 4072 Freeze-All Cycles
Abstract
1. Introduction
2. Materials and Methods
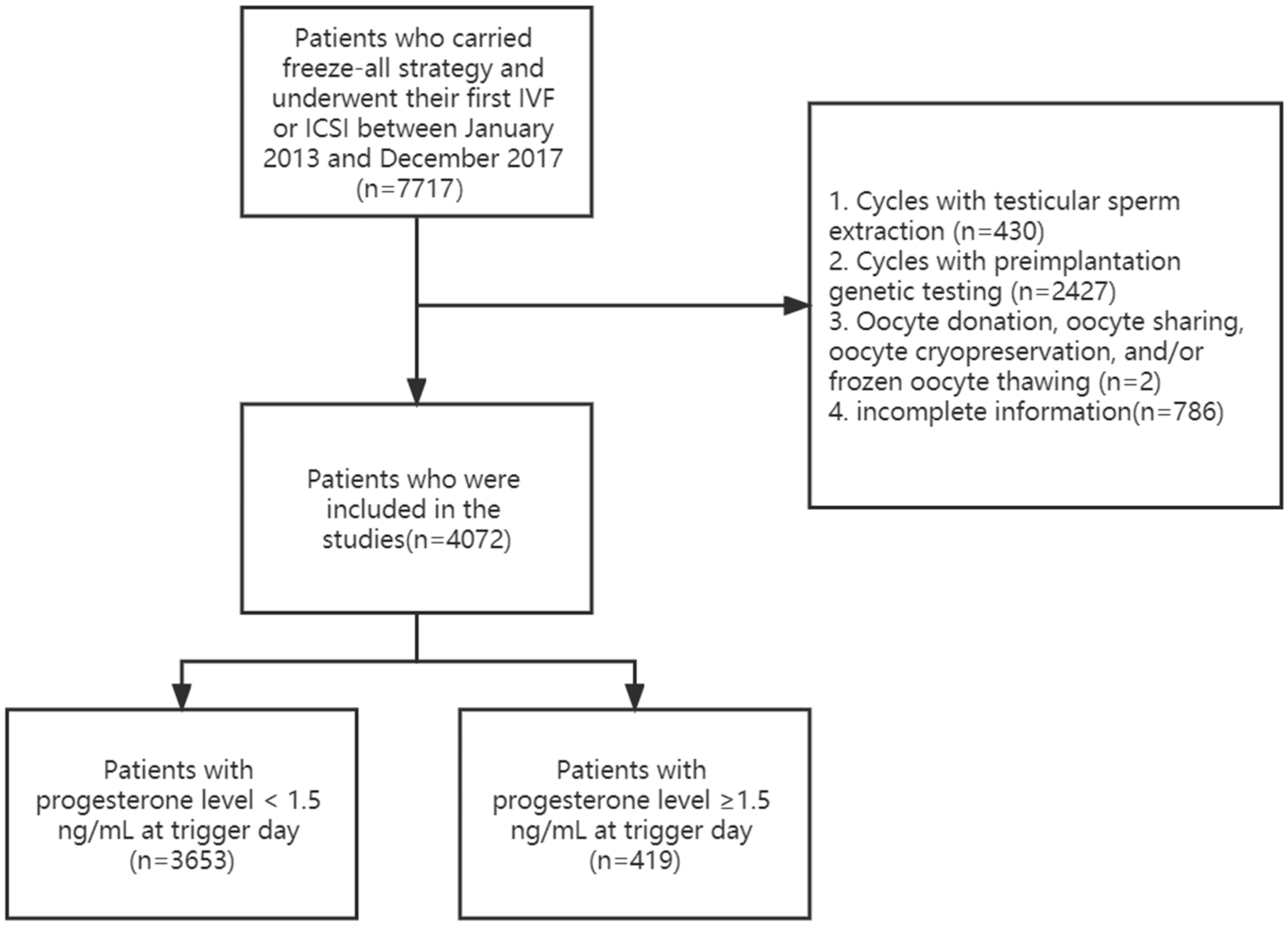
2.1. Patients Population
2.2. Hormone Assays
2.3. Controlled Ovarian Stimulation Protocol
2.4. Freeze-All Strategy
2.5. Vitrification and Preparation of the Frozen Embryo Transfer Cycle
2.6. Definition of High-Quality Embryos
2.7. Outcome Measures
2.8. Statistical Analysis
3. Results
3.1. Study Population
3.1.1. Baseline Characteristics and Outcome in Groups with P level < 1.5 ng/mL and ≥1.5 ng/mL on the Trigger Day
3.1.2. Association between P level on the Trigger Day and Cumulative Live Birth Rate
3.1.3. Association between P level on the Trigger Day and Embryo Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esteves, S.C.; Khastgir, G.; Shah, J.; Murdia, K.; Gupta, S.M.; Rao, D.G.; Dash, S.; Ingale, K.; Patil, M.; Moideen, K.; et al. Association Between Progesterone Elevation on the Day of Human Chronic Gonadotropin Trigger and Pregnancy Outcomes After Fresh Embryo Transfer in In Vitro Fertilization/Intracytoplasmic Sperm Injection Cycles. Front. Endocrinol. 2018, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Yding Andersen, C.; Bungum, L.; Nyboe Andersen, A.; Humaidan, P. Preovulatory progesterone concentration associates significantly to follicle number and LH concentration but not to pregnancy rate. Reprod. Biomed. Online 2011, 23, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Oktem, O.; Akin, N.; Bildik, G.; Yakin, K.; Alper, E.; Balaban, B.; Urman, B. FSH Stimulation promotes progesterone synthesis and output from human granulosa cells without luteinization. Hum. Reprod. 2017, 32, 643–652. [Google Scholar] [CrossRef]
- Racca, A.; De Munck, N.; Santos-Ribeiro, S.; Drakopoulos, P.; Errazuriz, J.; Galvao, A.; Popovic, B.; Mackens, S.; De Vos, M.; Verheyen, G.; et al. Do we need to measure progesterone in oocyte donation cycles? A retrospective analysis evaluating cumulative live birth rates and embryo quality. Hum. Reprod. 2020, 35, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Healy, M.W.; Yamasaki, M.; Patounakis, G.; Richter, K.S.; Devine, K.; DeCherney, A.H.; Hill, M.J. The slow growing embryo and premature progesterone elevation: Compounding factors for embryo-endometrial asynchrony. Hum. Reprod. 2017, 32, 362–367. [Google Scholar] [CrossRef]
- Bu, Z.; Zhao, F.; Wang, K.; Guo, Y.; Su, Y.; Zhai, J.; Sun, Y. Serum progesterone elevation adversely affects cumulative live birth rate in different ovarian responders during in vitro fertilization and embryo transfer: A large retrospective study. PLoS ONE 2014, 9, e100011. [Google Scholar] [CrossRef]
- Van Vaerenbergh, I.; Fatemi, H.M.; Blockeel, C.; Van Lommel, L.; In’t Veld, P.; Schuit, F.; Kolibianakis, E.M.; Devroey, P.; Bourgain, C. Progesterone rise on HCG day in GnRH antagonist/rFSH stimulated cycles affects endometrial gene expression. Reprod. Biomed. Online 2011, 22, 263–271. [Google Scholar] [CrossRef]
- Labarta, E.; Martínez-Conejero, J.A.; Alamá, P.; Horcajadas, J.A.; Pellicer, A.; Simón, C.; Bosch, E. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: A functional genomics analysis. Hum. Reprod. 2011, 26, 1813–1825. [Google Scholar] [CrossRef]
- Yovel, I.; Yaron, Y.; Amit, A.; Peyser, M.R.; David, M.P.; Kogosowski, A.; Lessing, J.B. High progesterone levels adversely affect embryo quality and pregnancy rates in in vitro fertilization and oocyte donation programs. Fertil. Steril. 1995, 64, 128–131. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Li, A.; Yang, N.; Cui, N.; Hao, G.; Gao, B.L. Impact of Elevated Progesterone in Late Follicular Phase on Early Pregnancy Outcomes and Live Birth Rate After Fresh Embryo Transfers. Front. Cell Dev. Biol. 2022, 10, 855455. [Google Scholar] [CrossRef]
- Hill, M.J.; Healy, M.W.; Richter, K.S.; Parikh, T.; Devine, K.; DeCherney, A.H.; Levy, M.; Widra, E.; Patounakis, G. Defining thresholds for abnormal premature progesterone levels during ovarian stimulation for assisted reproduction technologies. Fertil. Steril. 2018, 110, 671–679.e672. [Google Scholar] [CrossRef] [PubMed]
- Boynukalin, F.K.; Yarkiner, Z.; Gultomruk, M.; Turgut, N.E.; Ecemis, S.; Findikli, N.; Bahceci, M. Elevation of progesterone on the trigger day exerts no carryover effect on live birth in freeze-all cycles. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2020, 37, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Lawrenz, B.; Labarta, E.; Fatemi, H.; Bosch, E. Premature progesterone elevation: Targets and rescue strategies. Fertil. Steril. 2018, 109, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Santistevan, A.; Hunter Cohn, K.; Copperman, A.; Nulsen, J.; Miller, B.T.; Widra, E.; Westphal, L.M.; Yurttas Beim, P. Freeze-only versus fresh embryo transfer in a multicenter matched cohort study: Contribution of progesterone and maternal age to success rates. Fertil. Steril. 2017, 108, 254–261.e254. [Google Scholar] [CrossRef] [PubMed]
- Venetis, C.A.; Kolibianakis, E.M.; Bosdou, J.K.; Tarlatzis, B.C. Progesterone elevation and probability of pregnancy after IVF: A systematic review and meta-analysis of over 60 000 cycles. Hum. Reprod. Update 2013, 19, 433–457. [Google Scholar] [CrossRef] [PubMed]
- Cummins, J.M.; Breen, T.M.; Harrison, K.L.; Shaw, J.M.; Wilson, L.M.; Hennessey, J.F. A formula for scoring human embryo growth rates in in vitro fertilization: Its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J. Vitr. Fertil. Embryo Transf. IVF 1986, 3, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Lane, M.; Schoolcraft, W.B. Physiology and culture of the human blastocyst. J. Reprod. Immunol. 2002, 55, 85–100. [Google Scholar] [CrossRef]
- Vermey, B.G.; Chua, S.J.; Zafarmand, M.H.; Wang, R.; Longobardi, S.; Cottell, E.; Beckers, F.; Mol, B.W.; Venetis, C.A.; D’Hooghe, T. Is there an association between oocyte number and embryo quality? A systematic review and meta-analysis. Reprod. Biomed. Online 2019, 39, 751–763. [Google Scholar] [CrossRef]
- Scheffer, J.B.; Carvalho, R.F.; Aguiar, A.P.S.; Machado, I.J.M.; Franca, J.B.; Lozano, D.M.; Fanchin, R. Which ovarian reserve marker relates to embryo quality on day 3 and blastocyst; age, AFC, AMH? JBRA Assist. Reprod. 2021, 25, 109–114. [Google Scholar] [CrossRef]
- Malizia, B.A.; Hacker, M.R.; Penzias, A.S. Cumulative live-birth rates after in vitro fertilization. N. Engl. J. Med. 2009, 360, 236–243. [Google Scholar] [CrossRef]
- Malchau, S.S.; Henningsen, A.A.; Forman, J.; Loft, A.; Nyboe Andersen, A.; Pinborg, A. Cumulative live birth rate prognosis based on the number of aspirated oocytes in previous ART cycles. Hum. Reprod. 2019, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, Y.; Wu, D.; Fu, M.; Zhang, Q.; Ren, Y.; Yang, Z.; Shen, H.; Han, H. A Premature Rise of Luteinizing Hormone Is Associated with a Reduced Cumulative Live Birth Rate in Patients ≥37 Years Old Undergoing GnRH Antagonist In Vitro Fertilization Cycles. Front. Endocrinol. 2021, 12, 722655. [Google Scholar] [CrossRef] [PubMed]
- Vanni, V.S.; Somigliana, E.; Reschini, M.; Pagliardini, L.; Marotta, E.; Faulisi, S.; Paffoni, A.; Vigano, P.; Vegetti, W.; Candiani, M.; et al. Top quality blastocyst formation rates in relation to progesterone levels on the day of oocyte maturation in GnRH antagonist IVF/ICSI cycles. PLoS ONE 2017, 12, e0176482. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ren, X.; Wu, L.; Zhu, L.; Xu, B.; Li, Y.; Ai, J.; Jin, L. Elevated Progesterone Levels on the Day of Oocyte Maturation May Affect Top Quality Embryo IVF Cycles. PLoS ONE 2016, 11, e0145895. [Google Scholar] [CrossRef]
- Hernandez-Nieto, C.; Lee, J.A.; Alkon-Meadows, T.; Luna-Rojas, M.; Mukherjee, T.; Copperman, A.B.; Sandler, B. Late follicular phase progesterone elevation during ovarian stimulation is not associated with decreased implantation of chromosomally screened embryos in thaw cycles. Hum. Reprod. 2020, 35, 1889–1899. [Google Scholar] [CrossRef]
- Roque, M.; Valle, M.; Guimarães, F.; Sampaio, M.; Geber, S. Freeze-all policy: Fresh vs. frozen-thawed embryo transfer. Fertil. Steril. 2015, 103, 1190–1193. [Google Scholar] [CrossRef]
- Yan, J.; Qin, Y.; Zhao, H.; Sun, Y.; Gong, F.; Li, R.; Sun, X.; Ling, X.; Li, H.; Hao, C.; et al. Live Birth with or without Preimplantation Genetic Testing for Aneuploidy. N. Engl. J. Med. 2021, 385, 2047–2058. [Google Scholar] [CrossRef]
- Pabby, V.; Oza, S.S.; Dodge, L.E.; Hacker, M.R.; Moragianni, V.A.; Correia, K.; Missmer, S.A.; Fox, J.H.; Ibrahim, Y.; Penzias, A.; et al. In Vitro Fertilization Is Successful in Women with Ulcerative Colitis and Ileal Pouch Anal Anastomosis. Am. J. Gastroenterol. 2015, 110, 792–797. [Google Scholar] [CrossRef]
- Ghobara, T.; Gelbaya, T.A.; Ayeleke, R.O. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst. Rev. 2017, 7, Cd003414. [Google Scholar] [CrossRef]
| Progesterone Level < 1.5 ng/mL (n = 3653) | Progesterone Level ≥ 1.5 ng/mL (n = 419) | p Value | |
|---|---|---|---|
| Female age (years) | 32 (28–35) | 32 (30–36) | 0.001 a,* |
| Duration of infertility (years) | 3 (2–6) | 4 (2–6) | 0.187 a |
| Body mass index (kg/m2) | 20.90 (19.40–23.00) | 20.40 (19.10–22.31) | <0.001 a,* |
| Type of infertility | |||
| Primary | 1756 (48.1%) | 191 (45.6%) | 0.335 b |
| Secondary | 1897 (51.9%) | 228 (54.4%) | |
| Gravidity | 1 (0–2) | 1 (0–2) | 0.525 a |
| Parity | 0 (0–0) | 0 (0–0) | 0.637 a |
| Times of previous miscarriage | 0 (0–1) | 0 (0–1) | 0.831 a |
| Insemination method | |||
| IVF | 2554 (69.9%) | 308 (73.5%) | 0.127 b |
| ICSI | 1099 (30.1%) | 111 (26.5%) | |
| Antral follicle count | 11 (7–17) | 11 (8–15) | 0.977 a |
| basal FSH | 5.32 (4.51–6.50) | 5.33 (4.54–6.36) | 0.945 a |
| Stimulation protocol | |||
| Long GnRH agonist protocol | 2549 (69.8%) | 339 (80.9%) | <0.001 b,* |
| GnRH antagonist protocol | 1104 (30.2%) | 80 (19.1%) | |
| Duration of stimulation (days) | 10 (9–12) | 11 (10–12) | <0.001 a,* |
| Total dose of gonadotropins (IU) | 1950 (1425–2700) | 2350 (1762.5–3000) | <0.001 a,* |
| LH level on day of HCG administration (mIU/mL) | 0.97 (0.65–1.48) | 0.66 (0.48–1.25) | <0.001 a,* |
| Estrogen level on day of HCG administration | 3147 (1528.5–4936) | 4209 (2936–5000) | <0.001 a,* |
| Progesterone level on day of HCG administration (ng/mL) | 0.7 (0.5–0.9) | 1.7 (1.5–2.0) | <0.001 a,* |
| The endometrial thickness on the HCG trigger day | 11 (9.25–13) | 11 (9–13) | 0.094 |
| Number of oocytes retrieved | 15 (7–24) | 18 (12–24) | <0.001 a,* |
| Number of oocytes fertilized | 9 (4–15) | 11 (8–16) | <0.001 a,* |
| Number of viable embryos | 5 (2–8) | 6 (4–9) | < 0.001 a,* |
| Number of high-quality embryos | 4 (1–7) | 5 (2–7) | <0.001 a,* |
| Cumulative live birth rate | 1894 (51.8%) | 238 (56.8%) | 0.054 b |
| Adjusted ORs | 95% CI | p Value | |
|---|---|---|---|
| Female age (years) | 0.936 | 0.920–0.952 | <0.001 * |
| Body mass index (kg/m2) | 0.998 | 0.973–1.023 | 0.195 |
| Stimulation protocol | |||
| GnRH antagonist protocol (Ref) | 1 | - | |
| Long GnRH agonist protocol | 1.108 | 0.929–1.321 | 0.256 |
| Total dose of gonadotropins (IU) | 0.998 | 0.997–0.999 | 0.006 * |
| LH level on day of HCG administration (mIU/mL) | 0.922 | 0.873–0.974 | 0.004 * |
| Progesterone level on the day of HCG administration | 1.109 | 0.988–1.245 | 0.080 |
| Number of oocytes retrieved | 1.085 | 1.075–1.095 | <0.001 * |
| Number of Viable Embryos | p Value | Number of High-Quality Embryos | p Value | |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | |||
| Long GnRH agonist protocol | ||||
| Female age | −0.068 (−0.093 to −0.039) | <0.001 * | −0.016 (−0.042 to 0.012) | 0.267 |
| Progesterone level on the day of HCG administration (P < 1.5 ng/mL or ≥1.5 ng/mL) | 0.026 (−0.01 to 0.706) | 0.057 | −0.006 (−0.439 to 0.274) | 0.650 |
| Number of oocyte retrieved | 0.671 (0.281 to 0.305) | <0.001 * | 0.673 (0.273 to 0.297) | <0.001 * |
| GnRH antagonist protocol | ||||
| Female age | 0.016 (−0.036 to 0.028) | 0.783 | 0.011 (−0.041 to 0.025) | 0.623 |
| Progesterone level on the day of HCG administration (P < 1.5 ng/mL or ≥1.5 ng/mL) | −0.004 (−0.708 to 0.562) | 0.822 | −0.006 (−0.763 to 0.547) | 0.746 |
| Number of oocyte retrieved | 0.807 (0.326 to 0.359) | <0.001 * | 0.785 (0.304 to 0.339) | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Huang, S.; Wen, Y.; Zhang, X.; Hu, X.; Wu, R.; Chen, M.; Zhou, C. The Impact of Late Follicular Phase Progesterone Elevation on Cumulative Live Birth Rate and Embryo Quality in 4072 Freeze-All Cycles. J. Clin. Med. 2022, 11, 7300. https://doi.org/10.3390/jcm11247300
Huang L, Huang S, Wen Y, Zhang X, Hu X, Wu R, Chen M, Zhou C. The Impact of Late Follicular Phase Progesterone Elevation on Cumulative Live Birth Rate and Embryo Quality in 4072 Freeze-All Cycles. Journal of Clinical Medicine. 2022; 11(24):7300. https://doi.org/10.3390/jcm11247300
Chicago/Turabian StyleHuang, Ling, Sunxing Huang, Yangxing Wen, Xiubing Zhang, Xiaokun Hu, Rihan Wu, Minghui Chen, and Canquan Zhou. 2022. "The Impact of Late Follicular Phase Progesterone Elevation on Cumulative Live Birth Rate and Embryo Quality in 4072 Freeze-All Cycles" Journal of Clinical Medicine 11, no. 24: 7300. https://doi.org/10.3390/jcm11247300
APA StyleHuang, L., Huang, S., Wen, Y., Zhang, X., Hu, X., Wu, R., Chen, M., & Zhou, C. (2022). The Impact of Late Follicular Phase Progesterone Elevation on Cumulative Live Birth Rate and Embryo Quality in 4072 Freeze-All Cycles. Journal of Clinical Medicine, 11(24), 7300. https://doi.org/10.3390/jcm11247300





