Bone Metastases from Gastric Cancer Resembling Paget’s Disease: A Case Report
Abstract
:1. Introduction
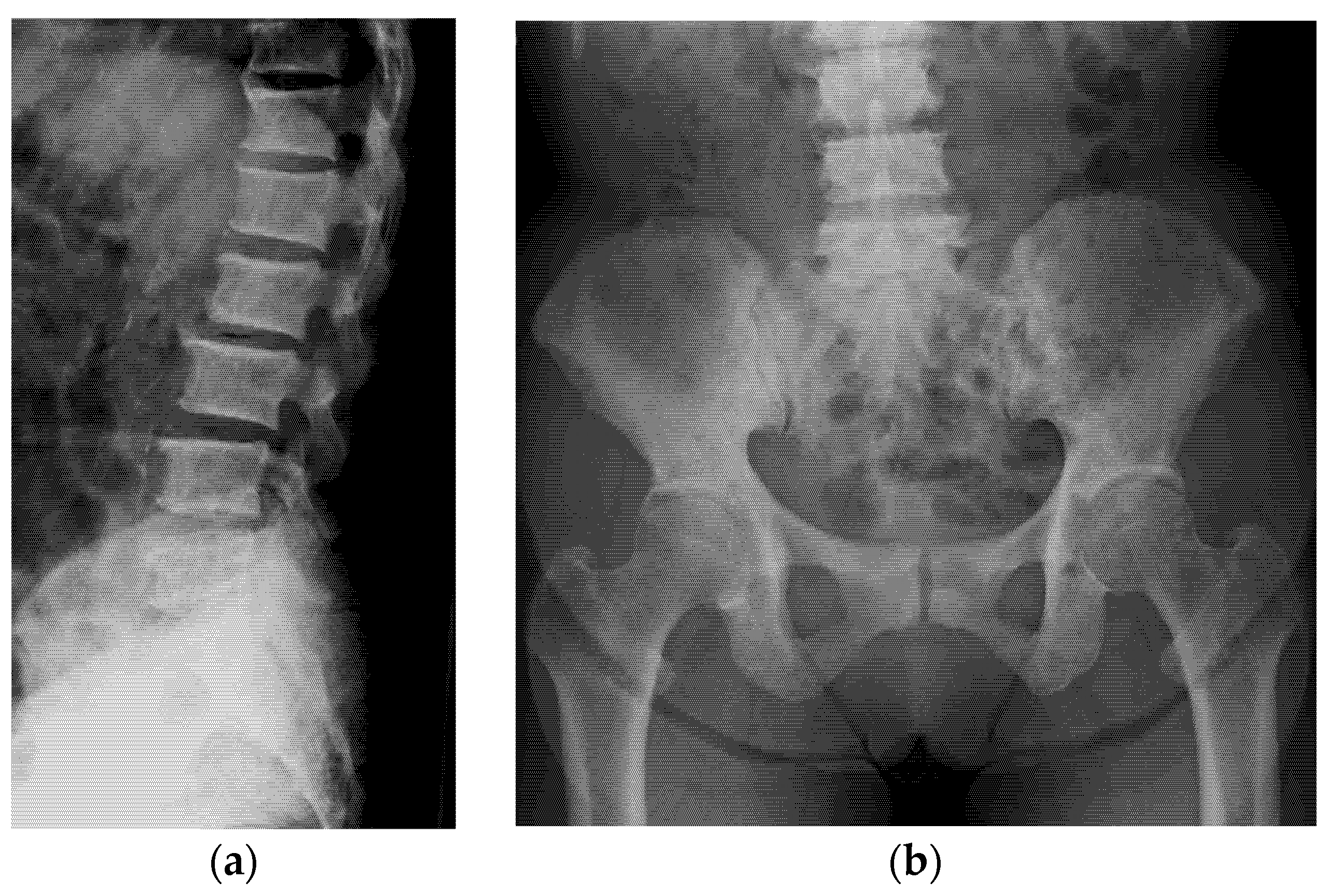
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.J.; Kim, S.H.; Kim, J.W.; Lee, J.-O.; Kim, J.H.; Bang, S.-M.; Lee, J.S.; Lee, K.-W. Gastric cancer with initial bone metastasis: A distinct group of diseases with poor prognosis. Eur. J. Cancer 2014, 50, 2810–2821. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Chui, S. Gastric carcinoma presenting with extensive bone metastases and marrow infiltration causing extradural spinal haemorrhage. Br. J. Radiol. 2006, 79, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Kitaoka, H. Bone metastasis of gastric cancer. Surg. Today 1983, 13, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Turkoz, F.P.; Solak, M.; Kilickap, S.; Ulas, A.; Esbah, O.; Oksuzoglu, B.; Yalcin, S. Bone Metastasis from Gastric Cancer: The Incidence, Clinicopathological Features, and Influence on Survival. J. Gastric Cancer 2014, 14, 164–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudo, H.; Takagi, Y.; Katayanagi, S.; Hoshino, S.; Suda, T.; Hibi, Y.; Ito, K.; Tsutida, A.; Aoki, T. Bone metastasis of gastric cancer. Gan Kagaku Ryoho (Cancer Chemother.) 2006, 33, 1058–1060. [Google Scholar]
- De Felice, F.; Piccioli, A.; Musio, D.; Tombolini, V. The role of radiation therapy in bone metastases management. Oncotarget 2017, 8, 25691–25699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okazaki, J.; Muguruma, N.; Kitamura, S.; Kimura, T.; Okamoto, K.; Miyamoto, H.; Kishi, K.; Bando, Y.; Kondo, T.; Endo, I.; et al. Paraneoplastic Hypocalcemia Developed in Gastric Cancer Accompanied by Osteoblastic Metastasis. Intern. Med. 2017, 56, 1345–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raskin, P.; McClain, C.J.; Medsger, T.A. Hypocalcemia associated with metastatic bone disease. A retrospective study. Arch. Intern. Med. 1973, 132, 539–543. [Google Scholar] [CrossRef]
- Iguchi, H. Recent aspects for disseminated carcinomatosis of the bone marrow associated with gastric cancer: What has been done for the past, and what will be needed in future? World J. Gastroenterol. 2015, 21, 12249–12260. [Google Scholar] [CrossRef]
- Kusumoto, H.; Haraguchi, M.; Nozuka, Y.; Oda, Y.; Tsuneyoshi, M.; Iguchi, H. Characteristic features of disseminated carcinomatosis of the bone marrow due to gastric cancer: The pathogenesis of bone destruction. Oncol. Rep. 2006, 16, 735–740. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Mu, E.; Wei, Y.; Riethdorf, S.; Yang, Q.; Yuan, M.; Yan, J.; Hua, Y.; Tiede, B.J.; Lu, X.; et al. VCAM-1 Promotes Osteolytic Expansion of Indolent Bone Micrometastasis of Breast Cancer by Engaging α4β1-Positive Osteoclast Progenitors. Cancer Cell 2011, 20, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xia, F.; Wei, Y.; Wei, X. Molecular mechanisms and clinical management of cancer bone metastasis. Bone Res. 2020, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Vičić, I.; Belev, B. The pathogenesis of bone metastasis in solid tumors: A review. Croat. Med. J. 2021, 62, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Paget, J. On a Form of Chronic Inflammation of Bones (Osteitis Deformans). Med.-Chir. Trans. 1877, 60, 37. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, J.; Ohno, I.; Nakatsuka, K.; Yoshimura, N.; Takata, S.; Zamma, M.; Yabe, H.; Abe, S.; Terada, M.; Yoh, K. Prevalence and clinical features of Paget’s disease of bone in Japan. J. Bone Miner. Metab. 2006, 24, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Hosking, D.J. Paget’s disease of bone. Br. Med. J. (Clin. Res. Ed.) 1981, 283, 686–688. [Google Scholar] [CrossRef] [Green Version]
- Seitz, S.; Priemel, M.; Zustin, J.; Beil, F.T.; Semler, J.; Minne, H.; Schinke, T.; Amling, M. Paget’s Disease of Bone: Histologic Analysis of 754 Patients. J. Bone Miner. Res. 2009, 24, 62–69. [Google Scholar] [CrossRef]
- Guañabens, N.; Filella, X.; Florez, H.; Ruiz-Gaspá, S.; Conesa, A.; Peris, P.; Monegal, A.; Torres, F. Tartrate-resistant acid phosphatase 5b, but not periostin, is useful for assessing Paget’s disease of bone. Bone 2019, 124, 132–136. [Google Scholar] [CrossRef]
- Al Nofal, A.A.; Altayar, O.; BenKhadra, K.; Qasim Agha, O.Q.; Asi, N.; Nabhan, M.; Prokop, L.J.; Tebben, P.; Murad, M.H. Bone turnover markers in Paget’s disease of the bone: A Systematic review and meta-analysis. Osteoporos. Int. 2015, 26, 1875–1891. [Google Scholar] [CrossRef]
- Sohn, W.; Simiens, M.A.; Jaeger, K.; Hutton, S.; Jang, G. The pharmacokinetics and pharmacodynamics of denosumab in patients with advanced solid tumours and bone metastases: A systematic review. Br. J. Clin. Pharmacol. 2014, 78, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Miller, P.D. Denosumab: Anti-RANKL antibody. Curr. Osteoporos. Rep. 2009, 7, 18–22. [Google Scholar] [CrossRef]
- Gouldthorpe, C.; Quinton, R.; Wakefield, D. Denosumab-induced hypocalcaemia in metastatic gastric cancer. BMJ Support. Palliat. Care, 2020; Epub ahead of printing. [Google Scholar] [CrossRef]



| Name of Items | Value | Normal Range | Units |
|---|---|---|---|
| AST | 18 | 13–30 | IU/L |
| ALT | 9 | 7–23 | IU/L |
| LDH | 219 | 124–222 | U/L |
| Creatinine | 0.54 | 0.46–0.79 | mg/dL |
| Na | 144 | 138–145 | mEq/L |
| K | 4.8 | 3.6–4.8 | mEq/L |
| Ca | 9.7 | 8.8–10.1 | mg/dL |
| Cl | 108 | 101–108 | mEq/L |
| WBC | 4580 | 3300–8600 | /mm3 |
| Hb | 10.9 | 11.6–14.8 | g/dL |
| Platelet | 18.4 | 15.8–34.8 | ×104/mm3 |
| CA19-9 | 44.1 | 0.0–37.0 | U/mL |
| CEA | 1.4 | 0.0–5.0 | ng/mL |
| K | 4.8 | 3.6–4.8 | mEq/L |
| ALP | 270 | 38–113 | U/L |
| ALP1 | 0 | 0–0 | % |
| ALP2 | 22 | 36–74 | % |
| ALP3 | 78 | 25–59 | % |
| ALP4 | 0 | 0–0 | % |
| ALP5 | 0 | 0–0 | % |
| P1NP | 1200 | 26–98 | ng/mL |
| TRAP-5b | 3440 | 120–420 | mU/dL |
| sIL-2 | 288 | 122–496 | U/mL |
| PTH-intact | 53 | 10–65 | pg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiba, H.; Nakazato, T.; Matsuo, H.; Kimura, H.; Saito, S.; Sakai, T.; Murakami, H.; Kawai, J.; Kawasaki, S.; Imamura, Y. Bone Metastases from Gastric Cancer Resembling Paget’s Disease: A Case Report. J. Clin. Med. 2022, 11, 7306. https://doi.org/10.3390/jcm11247306
Aiba H, Nakazato T, Matsuo H, Kimura H, Saito S, Sakai T, Murakami H, Kawai J, Kawasaki S, Imamura Y. Bone Metastases from Gastric Cancer Resembling Paget’s Disease: A Case Report. Journal of Clinical Medicine. 2022; 11(24):7306. https://doi.org/10.3390/jcm11247306
Chicago/Turabian StyleAiba, Hisaki, Tomoharu Nakazato, Hideo Matsuo, Hiroaki Kimura, Shiro Saito, Takao Sakai, Hideki Murakami, Jun Kawai, Shingo Kawasaki, and Yasuhiro Imamura. 2022. "Bone Metastases from Gastric Cancer Resembling Paget’s Disease: A Case Report" Journal of Clinical Medicine 11, no. 24: 7306. https://doi.org/10.3390/jcm11247306
APA StyleAiba, H., Nakazato, T., Matsuo, H., Kimura, H., Saito, S., Sakai, T., Murakami, H., Kawai, J., Kawasaki, S., & Imamura, Y. (2022). Bone Metastases from Gastric Cancer Resembling Paget’s Disease: A Case Report. Journal of Clinical Medicine, 11(24), 7306. https://doi.org/10.3390/jcm11247306






