Genetic Factors Causing Thyroid Dyshormonogenesis as the Major Etiologies for Primary Congenital Hypothyroidism: Clinical and Genetic Characterization of 33 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Ethical Approval
2.2. Clinical Evaluation
2.3. Targeted NGS Analysis
2.4. Sanger Sequencing
2.5. Bioinformatic Analysis
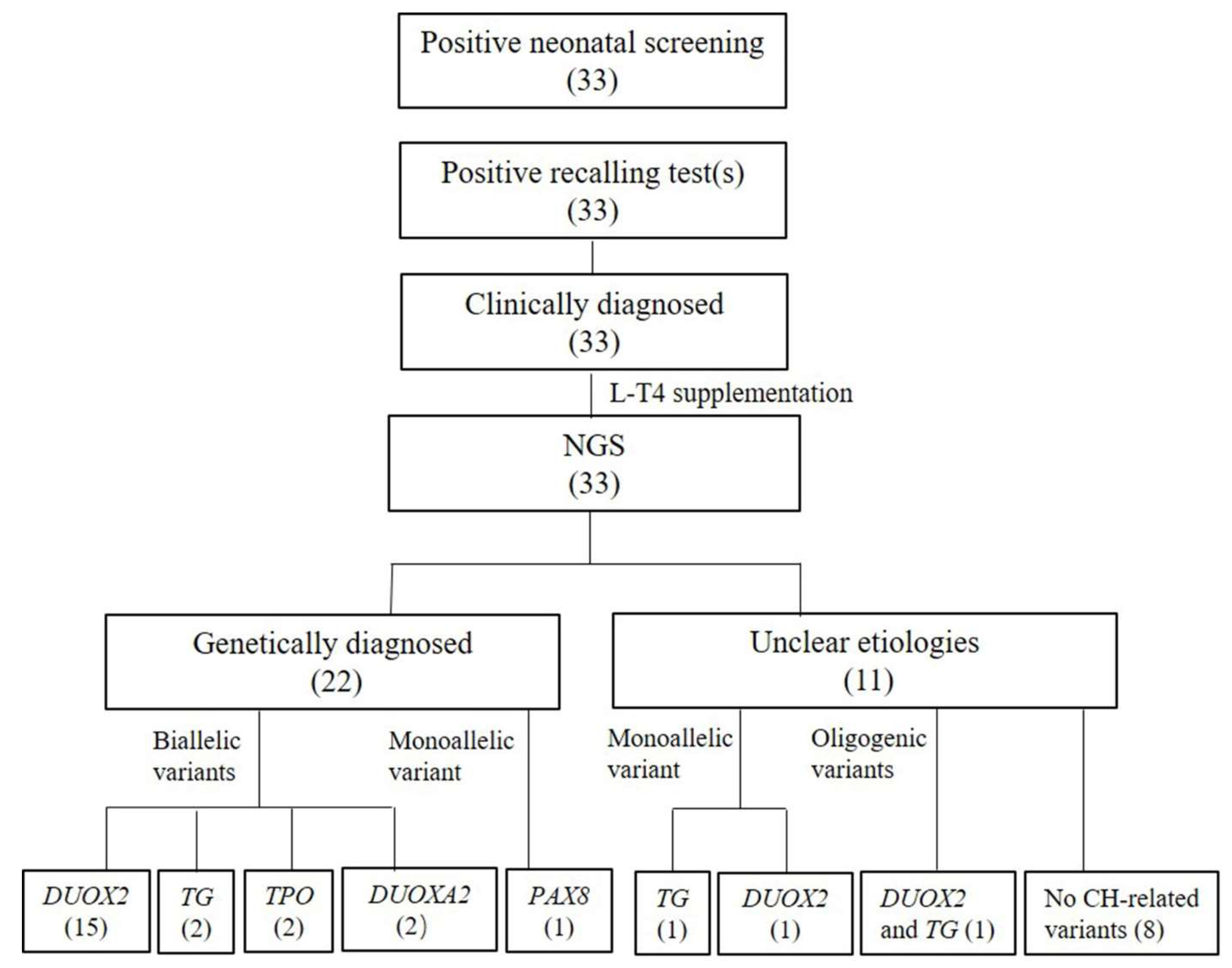
3. Results
3.1. Results of Genetic Testing
3.2. Bioinformatic Findings
3.3. Clinical Presentations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deladoey, J.; Ruel, J.; Giguere, Y.; Van Vliet, G. Is the incidence of congenital hypothyroidism really increasing? A 20-year retrospective population-based study in Quebec. J. Clin. Endocrinol. Metab. 2011, 96, 2422–2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, D.A. Second International Conference on Neonatal thyroid screening: Progress report. J. Pediatr. 1983, 1025, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; van Trotsenburg AS, P.; Schoenmakers, N. Diagnosis of Endocrine Disease: Congenital hypothyroidism: Update and perspectives. Eur. J. Endocrinol. 2018, 179, R297–R317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szinnai, G. Clinical genetics of congenital hypothyroidism. Endocr. Dev. 2014, 26, 60–78. [Google Scholar]
- Kostopoulou, E.; Miliordos, K.; Spiliotis, B. Genetics of primary congenital hypothyroidism-a review. Hormones 2021, 20, 225–236. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Nakamura, A.; Nakayama, K.; Hishimura, N.; Morikawa, S.; Ishizu, K.; Tajima, T. Targeted Next-Generation Sequencing for Congenital Hypothyroidism with Positive Neonatal TSH Screening. J. Clin. Endocrinol. Metab. 2020, 105, dgaa308. [Google Scholar] [CrossRef]
- Persani, L.; Rurale, G.; de Filippis, T.; Galazzi, E.; Muzza, M.; Fugazzola, L. Genetics and management of congenital hypothyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 387–396. [Google Scholar] [CrossRef]
- Cherella, C.E.; Wassner, A.J. Congenital hypothyroidism: Insights into pathogenesis and treatment. Int. J. Pediatr. Endocrinol. 2017, 2017, 11. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, J.; Yang, C.; Gao, G.; Zhu, W.; Han, B.; Zhang, L.; Wan, Y.; Ye, X.; Ma, Y.; et al. The Genetic Characteristics of Congenital Hypothyroidism in China by Comprehensive Screening of 21 Candidate Genes. Eur. J. Endocrinol. 2018, 178, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Huang, Y.; Jiang, X.; Li, P.; Tang, C.; Jia, X.; Chen, Q.; Chen, W.; Sheng, H.; Feng, Y.; et al. The Prevalence, Clinical, and Molecular Characteristics of Congenital Hypothyroidism Caused by DUOX2 Mutations: A Population-Based Cohort Study in Guangzhou. Horm. Metab. Res. 2016, 48, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Niu, D.M.; Hwang, B.; Chu, Y.K.; Liao, C.J.; Wang, P.L.; Lin, C.Y. High prevalence of a novel mutation (2268 insT) of the thyroid peroxidase gene in Taiwanese patients with total iodide organification defect, and evidence for a founder effect. J. Clin. Endocrinol. Metab. 2002, 87, 4208–4212. [Google Scholar] [CrossRef] [PubMed]
- Stoupa, A.; Kariyawasam, D.; Carre, A.; Polak, M. Update of Thyroid Developmental Genes. Endocrinol. Metab. Clin. N. Am. 2016, 45, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Jiang, X.; Dou, Z.; Rakeman, M.A.; Zhang, M.; O’Donnell, K.; Ma, T.; Amette, K.; DeLong, N.; DeLong, G.R. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N. Engl. J. Med. 1994, 331, 1739–1744. [Google Scholar] [CrossRef]
- Fu, C.; Luo, S.; Zhang, S.; Wang, J.; Zheng, H.; Yang, Q.; Xie, B.; Hu, X.; Fan, X.; Luo, J.; et al. Next-generation sequencing analysis of DUOX2 in 192 Chinese subclinical congenital hypothyroidism (SCH) and CH patients. Clin. Chim. Acta 2016, 458, 30–34. [Google Scholar] [CrossRef]
- Lu, J.T.; Campeau, P.M.; Lee, B.H. Genotype–Phenotype Correlation—Promiscuity in the Era of Next-Generation Sequencing. N. Engl. J. Med. 2014, 371, 593–596. [Google Scholar] [CrossRef]
- Rastogi, M.V.; LaFranchi, S.H. Congenital hypothyroidism. Orphanet J. Rare Dis. 2010, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, C.; Luo, S.; Li, Y.; Li, Q.; Hu, X.; Li, M.; Zhang, Y.; Su, J.; Hu, X.; Chen, Y.; et al. The incidence of congenital hypothyroidism (CH) in Guangxi, China and the predictors of permanent and transient CH. Endocr. Connect. 2017, 6, 926–934. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.H.; Shen, Y.; Gong, J.M.; Meng, Y.; Su, L.; Zhang, X. Newborn screening for congenital hypothyroidism in Henan province, China. Clin. Chim. Acta 2016, 452, 58–60. [Google Scholar] [CrossRef]
- Wassner, A.J. Congenital hypothyroidism. Clin. Perinatol. 2018, 45, 1–18. [Google Scholar] [CrossRef]
- Wassner, A.J. Pediatric Hypothyroidism: Diagnosis and Treatment. Paediatr. Drugs 2017, 19, 291–301. [Google Scholar] [CrossRef]
- van Trotsenburg, P.; Stoupa, A.; Leger, J.; Rohrer, T.; Peters, C.; Fugazzola, L.; Cassio, A.; Heinrichs, C.; Beauloye, V.; Pohlenz, J.; et al. Congenital Hypothyroidism: A 2020–2021 Consensus Guidelines Update-An ENDO-European Reference Network Initiative Endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid 2021, 31, 387–419. [Google Scholar] [CrossRef] [PubMed]
- Group of Endocrinology and Inborn Metabolic Diseases. Consensus of diagnosis and treatment of congenital hypothyroidism. Chin. J. Pediatr. 2011, 49, 421–424. [Google Scholar]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009, 4, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods 2014, 11, 361–362. [Google Scholar] [CrossRef]
- Choi, Y.; Chan, A.P. PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 2015, 31, 2745–2747. [Google Scholar] [CrossRef] [Green Version]
- Bacalhau, M.; Pratas, J.; Simoes, M.; Mendes, C.; Ribeiro, C.; Santos, M.J.; Diogo, L.; Macario, M.C.; Grazina, M. In silico analysis for predicting pathogenicity of five unclassified mitochondrial DNA mutations associated with mitochondrial cytopathies’ phenotypes. Eur. J. Med. Genet. 2017, 60, 172–177. [Google Scholar] [CrossRef]
- Wang, F.; Lu, K.; Yang, Z.; Zhang, S.; Lu, W.; Zhang, L.; Liu, S.; Yan, S. Genotypes and phenotypes of congenital goitre and hypothyroidism caused by mutations in dual oxidase 2 genes. Clin. Endocrinol. 2014, 81, 452–457. [Google Scholar] [CrossRef]
- Bruellman, R.J.; Watanabe, Y.; Ebrhim, R.S.; Creech, M.K.; Abdullah, M.A.; Dumitrescu, A.M.; Refetoff, S.; Weiss, R.E. Increased Prevalence of TG and TPO Mutations in Sudanese Children with Congenital Hypothyroidism. J. Clin. Endocrinol. Metab. 2020, 105, 1564–1572. [Google Scholar] [CrossRef]
- Lee, J.; Di Jeso, B.; Arvan, P. Maturation of thyroglobulin protein region I. J. Biol. Chem. 2011, 286, 33045–33052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vono-Toniolo, J.; Rivolta, C.M.; Targovnik, H.M.; Medeiros-Neto, G.; Kopp, P. Naturally occurring mutations in the thyroglobulin gene. Thyroid 2005, 15, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Li, G.Y.; Wu, X.J.; Jia, Q.; Lyu, G.T.; Wang, M.L.; Wang, J. Novel non-synonymous mutations of PAX8 in a cohort of Chinese with congenital hypothyroidism. Chin. Med. J. 2019, 132, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Magliano, M.P.; di Lauro, R.D.; Zannini, M. Pax8 has a key role in thyroid cell differentiation. Proc. Natl. Acad. Sci. USA 2000, 97, 13144–13149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwahashi-Odano, M.; Fujisawa, Y.; Ogata, T.; Nakashima, S.; Muramatsu, M.; Narumi, S. Identification and functional characterization of a novel PAX8 mutation (p.His39Pro) causing familial thyroid hypoplasia. Clin. Pediatr. Endocrinol. 2020, 29, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Sorapipatcharoen, K.; Tim-Aroon, T.; Mahachoklertwattana, P.; Chantratita, W.; Iemwimangsa, N.; Sensorn, I.; Panthan, B.; Jiaranai, P.; Noojarern, S.; Khlairit, P.; et al. DUOX2 variants are a frequent cause of congenital primary hypothyroidism in Thai patients. Endocr. Connect. 2020, 9, 1121–1134. [Google Scholar] [CrossRef]
- Lof, C.; Patyra, K.; Kuulasmaa, T.; Vangipurapu, J.; Undeutsch, H.; Jaeschke, H.; Pajunen, T.; Kero, A.; Krude, H.; Biebermann, H.; et al. Detection of Novel Gene Variants Associated with Congenital Hypothyroidism in a Finnish Patient Cohort. Thyroid 2016, 26, 1215–1224. [Google Scholar] [CrossRef]
- Narumi, S.; Muroya, K.; Asakura, Y.; Aachi, M.; Hasegawa, T. Molecular basis of thyroid dyshormonogenesis: Genetic screening in population-based Japanese patients. J. Clin. Endocrinol. Metab. 2011, 96, E1838–E1842. [Google Scholar] [CrossRef]
- Park, K.J.; Park, H.K.; Kim, Y.J.; Lee, K.R.; Park, J.H.; Park, J.H.; Park, H.D.; Lee, S.Y.; Kim, J.W. DUOX2 Mutations Are Frequently Associated with Congenital Hypothyroidism in the Korean Population. Ann. Lab. Med. 2016, 36, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Long, W.; Lu, G.; Zhou, W.; Yang, Y.; Zhang, B.; Zhou, H.; Jiang, L.; Yu, B. Targeted next-generation sequencing of thirteen causative genes in Chinese patients with congenital hypothyroidism. Endocr. J. 2018, 65, 1019–1028. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Yang, L.; Sun, C.; Wu, J.; Luo, F.; Zhou, W.; Lu, W. Genotype and phenotype correlation in a cohort of Chinese congenital hypothyroidism patients with DUOX2 mutations. Ann. Transl. Med. 2020, 8, 1649. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhang, S.; Su, J.; Luo, S.; Zheng, H.; Wang, J.; Qin, H.; Chen, Y.; Shen, Y.; Hu, X.; et al. Mutation screening of DUOX2 in Chinese patients with congenital hypothyroidism. J. Endocrinol. Investig. 2015, 38, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zang, Y.; Li, M.; Liu, W.; Wang, Y.; Yu, X.; Li, H.; Wang, F.; Liu, S. DUOX2 and DUOXA2 Variants Confer Susceptibility to Thyroid Dysgenesis and Gland-in-situ With Congenital Hypothyroidism. Front. Endocrinol. 2020, 11, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Long, W.; Yang, Y.; Wang, Y.; Jiang, L.; Cai, Z.; Wang, H. Newborn Screening and Molecular Profile of Congenital Hypothyroidism in a Chinese Population. Front. Genet. 2018, 9, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narumi, S.; Muroya, K.; Asakura, Y.; Adachi, M.; Hasegawa, T. Transcription factor mutations and congenital hypothyroidism: Systematic genetic screening of a population-based cohort of Japanese patients. J. Clin. Endocrinol. Metab. 2010, 95, 1981–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, M.; Fagman, H. Mechanisms of thyroid development and dysgenesis: An analysis based on developmental stages and concurrent embryonic anatomy. Curr. Top. Dev. Biol. 2013, 106, 123–170. [Google Scholar]
- De Marco, G.; Agretti, P.; Montanelli, L.; Di Cosmo, C.; Bagattini, B.; De Servi, M.; Ferrarini, E.; Dimida, A.; Freitas Ferreira, A.C.; Molinaro, A.; et al. Identification and functional analysis of novel dual oxidase 2 (DUOX2) mutations in children with congenital or subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2011, 96, E1335–E1339. [Google Scholar] [CrossRef] [Green Version]
- Maruo, Y.; Nagasaki, K.; Matsui, K.; Mimura, Y.; Mori, A.; Fukami, M.; Takeuchi, Y. Natural course of congenital hypothyroidism by dual oxidase 2 mutations from the neonatal period through puberty. Eur. J. Endocrinol. 2016, 174, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Aminzadeh, M. Higher prevalence of permanent congenital hypothyroidism in the Southwest of Iran mostly caused by dyshormonogenesis: A five-year follow-up study. Arch. Endocrinol. Metab. 2018, 62, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Cangul, H.; Aycan, Z.; Kendall, M.; Bas, V.N.; Saglam, Y.; Barrett, T.G.; Maher, E.R. A truncating DUOX2 mutation (R434X) causes severe congenital hypothyroidism. J. Pediatr. Endocrinol. Metab. 2014, 27, 323–327. [Google Scholar] [CrossRef]
| Patients | MOI |
Causative Genes | The Allele Frequency in gnomAD | Genotypes | ||
|---|---|---|---|---|---|---|
| Index Patient | Father | Mother | ||||
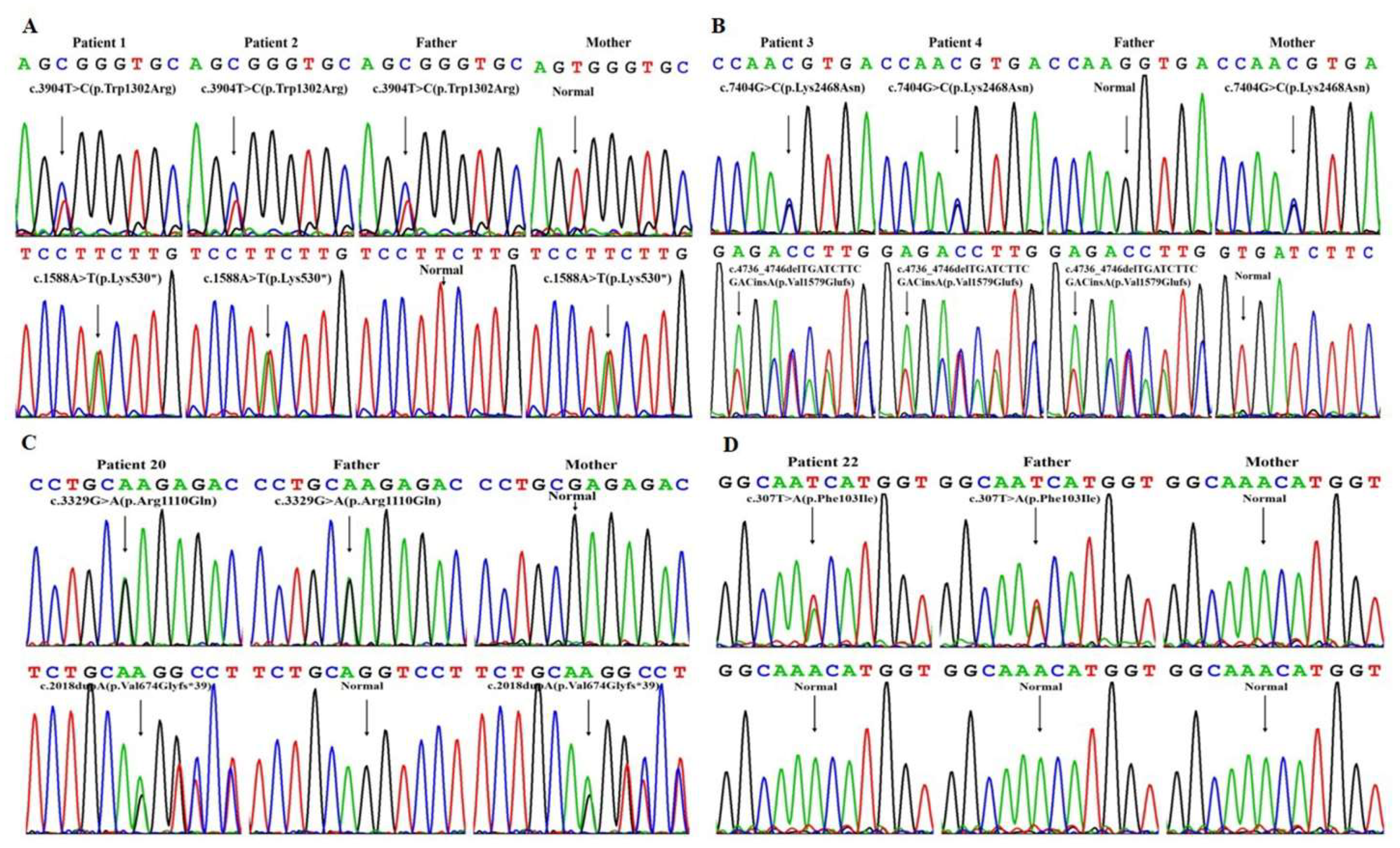
| 1 | AR | DUOX2 | 0.00398‰/0.0119‰ | c.3904T > C (p.Trp1302Arg)/c.1588A > T (p.Lys530*) | c.3904T > C (p.Trp1302Arg)/- | c.1588A > T (p.Lys530*)/- |
| 2 | AR | DUOX2 | 0.00398‰/0.0119‰ | c.3904T > C (p.Trp1302Arg)/c.1588A > T (p.Lys530*) | c.3904T > C(p.Trp1302Arg)/- | c.1588A > T (p.Lys530*)/- |
| 3 | AR | TG | NA/0.00398‰ | c.4736_4746delTGATCTTCGACinsA (p.Val1579Glufs)/c.7404G > C (p.Lys2468Asn) | c.4736_4746delTGATCTTCGACinsA (p.Val1579Glufs)/- | c.7404G > C (p.Lys2468Asn)/- |
| 4 | AR | TG | NA/0.00398‰ | c.4736_4746delTGATCTTCGACinsA (p.Val1579Glufs)/c.7404G > C (p.Lys2468Asn) | c.4736_4746delTGATCTTCGACinsA (p.Val1579Glufs)/- | c.7404G > C (p.Lys2468Asn)/- |
| 5 | AR | TPO | 0.117‰ | c.2268dupT (p.Glu757*)/c c.2268dupT (p.Glu757*) | c.2268dupT (p.Glu757*)/- | c.2268dupT (p.Glu757*)/- |
| 6 | AR | DUOX2 |
0.598‰; 0.343‰/ 0.417‰ |
[c.4027C > T (p.Leu1343Phe); c.2048G > T (p.Arg683Leu)]/ c.2654G > T (p.Arg885Leu) | [c.4027C > T (p.Leu1343Phe); c.2048G > T (p.Arg683Leu)]/- | c.2654G > T (p.Arg885Leu)/- |
| 7 | AR | DUOX2 | 0.417‰/0.113‰ | c.2654G > T (p.Arg885Leu)/c.2654G > A (p.Arg885Gln) | c.2654G > T (p.Arg885Leu)/- | c.2654G > A (p.Arg885Gln)/- |
| 8 | AR | DUOX2 |
0.343‰; 0.598‰/ 0.191‰; NA |
[c.2048G > T (p.Arg683Leu); c.4027C > T (p.Leu1343Phe/ [c.3329G > A (p.Arg1110Gln); c.1094A > G (p.Gln365Arg)] | [c.2048G > T (p.Arg683Leu); c.4027C > T (p.Leu1343Phe)]/- | [c.3329G > A (p.Arg1110Gln); c.1094A > G (p.Gln365Arg)]/- |
| 9 | AR | DUOX2 |
0.0119‰/ 0.598‰; 0.343‰ |
c.1588A > T (p.Lys530*)/ [c.4027C > T (p.Leu1343Phe); c.2048G > T (p.Arg683Leu)] | c.1588A > T (p.Lys530*)/- | [c.4027C > T (p.Leu1343Phe); c.2048G > T (p.Arg683Leu)]/- |
| 10 | AR | DUOX2 | 0.191‰/0.0191‰ | c.3329G > A (p.Arg1110Gln)/c.1588A > T (p.Lys530*) | c.3329G > A (p.Arg1110Gln)/- | c.1588A > T (p.Lys530*)/- |
| 11 | AR | DUOX2 |
0.0119‰/ 0.343‰; 0.598‰ |
c.1588A > T (p.Lys530*)/ [c.2048G > T (p.Arg683Leu); c.4027C > T (p.Leu1343Phe)] | c.1588A > T (p.Lys530*)/- | [c.2048G > T (p.Arg683Leu); c.4027C > T (p.Leu1343Phe)]/- |
| 12 | AR | DUOX2 |
0.019‰/ 0.343‰; 0.598‰ |
c.1588A > T (p.Lys530*)/ [c.2048G > T (p.Arg683Leu); c.4027C > T (p.Leu1343Phe)] | c.1588A > T (p.Lys530*)/- | [c.2048G > T (p.Arg683Leu); c.4027C > T (p.Leu1343Phe)]/- |
| 13 | AR | DUOXA2 | 0.231‰/0.143‰ | c.413dupA (p.Tyr138*)/c.738C > G (p.Tyr246*) | c.413dupA (p.Tyr138*)/- | c.738C > G (p.Tyr246*)/- |
| 14 | AR | DUOX2 | 0.417‰/0.113‰ | c.2654G > T (p.Arg885Leu)/c.3693 + 1G > T | c.2654G > T (p.Arg885Leu)/- | c.3693 + 1G > T/- |
| 15 | AR | DUOXA2 | 0.231‰/0.00402‰ | c.413dupA (p.Tyr138*)/c.515dupA (p. Tyr173Valfs*57) | c.413dupA (p.Tyr138*)/- | c.515dupA (p. Tyr173Valfs*57)/- |
| 16 | AR | DUOX2 | 0.0707‰/0.113‰ | c.2104_2106delGGA (p.Gly702del)/c.3693 + 1G > T | c.2104_2106delGGA (p.Gly702del)/- | c.3693 + 1G > T/- |
| 17 | AR | DUOX2 | 0.0119‰/0.0679‰ | c.1588A > T(p.Lys530*)/c.1946C > A(p.Ala649Glu) | c.1588A > T(p.Lys530*)/- | c.1946C > A (p.Ala649Glu)/- |
| 18 | AR | DUOX2 | 0.191‰/0.0119‰ | c.3329G > A(p.Arg1110Gln)/c.1588A > T(p.Lys530*) | c.3329G > A(p.Arg1110Gln)/- | c.1588A > T (p.Lys530*)/- |
| 19 | AR | DUOX2 | 0.0119‰/0.0278‰ | c.1588A > T (p.Lys530*)/c.3478_3480del (p.Leu1160del) | c.1588A > T (p.Lys530*)/- | c.3478_3480del (p.Leu1160del)/- |
| 20 | AR | DUOX2 | 0.191‰/0.00398‰ | c.3329G > A (p.Arg1110Gln)/c.2018dupA (p.Val674Glyfs*39) | c.3329G > A (p.Arg1110Gln)/- | c.2018dupA (p.Val674Glyfs*39)/- |
| 21 | AR | TPO | 0.117‰/NA | c.2268dupT (p.Glu757*)/c.1804C > T (p.Arg602Cys) | c.2268dupT (p.Glu757*)/- | c.1804C > T (p.Arg602Cys)/- |
| 22 | AD | PAX8 | NA/- | c.307T > A (p.Phe103Ile)/- | c.307T > A (p.Phe103Ile)/- | -/- |
| Patients | Sex | GA(Weeks) | BW(g) | Delivery Mode | Chief Complaint | Family History | ThyroidMorphology/Age at Latest Examination (y) | OtherPresentations | Clinical Outcomes/Assessment Ages (y) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 40+2 | 2700 | Vaginal delivery | Abnormal NS | N | Normal/3.0 | None | Normal DQ/3.0 |
| 2 | Male | 39+3 | 2580 | Vaginal delivery | Abnormal NS | His sister had CH | Normal/0.1 | None | Normal DQ/0.5 |
| 3 | Female | 41 | 4500 | Caesarean | Abnormal NS | N | Increased/9.3 | Macrosomia | Normal school performance/10.0 |
| 4 | Male | 40 | 3500 | Caesarean | Abnormal NS | His sister had CH | Normal/2.7 | None | Normal school performance/7.3 |
| 5 | Male | 40+4 | 2900 | Vaginal delivery | Abnormal NS | Mother hadHashimoto’s thyroiditis | Normal/3.3 | None | Normal DQ/6.7 |
| 6 | Male | 40 | 3800 | Caesarean | Abnormal NS | N | Normal/3.8 | None | Normal DQ/3.7 |
| 7 | Male | 38 | 2450 | Vaginal delivery | Abnormal NS | N | Normal/2.3 | Neonatal jaundice | Normal DQ/4.3 |
| 8 | Male | 40+1 | 3000 | Vaginal delivery | Abnormal NS | N | Normal/0.1 | Neonatal jaundice | Normal DQ/0.8 |
| 9 | Female | 42+3 | 3000 | Caesarean | Abnormal NS | N | Normal/0.1 | None | Normal DQ/0.9 |
| 10 | Female | 39+1 | 3300 | Caesarean | Abnormal NS | N | Increased/0.1 | Neonatal jaundice | Normal DQ/2.0 |
| 11 | Female | 38 | 2400 | Vaginal delivery | Abnormal NS | N | Normal/3.7 | Neonatal jaundice | Normal DQ/4.4 |
| 12 | Male | 40 | 3250 | Vaginal delivery | Abnormal NS | His sister had CH | Normal/2.6 | None | Normal DQ/6.3 |
| 13 | Female | 38 | 2970 | Vaginal delivery | Abnormal NS | Her brother had CH | Normal/9.2 | None | Normal DQ/4.8 |
| 14 | Male | 37+5 | 2590 | Caesarean | Abnormal NS | N | Normal/2.5 | One of the monozygotic twins, Full term | Normal DQ/3.9 |
| 15 | Male | 38+5 | 3150 | Vaginal delivery | Abnormal NS | N | Normal/1.5 | Neonatal jaundice | Normal DQ/3.0 |
| 16 | Female | 39 | 3500 | Caesarean | Abnormal NS | Mother hadHashimoto’s thyroiditis | Normal/3.5 | Neonatal jaundice | Normal DQ/4.4 |
| 17 | Female | 39+1 | 3400 | Vaginal delivery | Abnormal NS | N | Normal/0.3 | Neonatal jaundice | Normal DQ/4.0 |
| 18 | Male | 38 | 2500 | Caesarean | Abnormal NS | N | Normal/3.5 | None | Normal DQ/3.5 |
| 19 | Female | 39 | 3400 | Vaginal delivery | Abnormal NS | N | Normal/0.1 | None | Normal DQ/0.8 |
| 20 | Female | 39+1 | 3100 | Vaginal delivery | Abnormal NS | Her sister had CH | Normal/0.1 | Neonatal jaundice | Normal DQ/1.2 |
| 21 | Male | 41 | 3500 | Vaginal delivery | Abnormal NS | N | Normal/0.7 | Neonatal jaundice | Low DQ/4.5 |
| 22 | Female | 37 | 2740 | Vaginal delivery | Abnormal NS | Her father had a history of hypothyroidism | Normal/0.1 | None | Normal DQ/2.2 |
| 23 | Female | 39+4 | 2780 | Vaginal delivery | Abnormal NS | N | Not done | Neonatal jaundiceAtrial septal defect, Pierre-Robin syndrome | Normal DQ/0.9 |
| 24 | Female | 40+4 | 3000 | Vaginal delivery | Abnormal NS | Aunt had hyperthyroidism | Thyroid agenesis/0.1 | None | Normal DQ/2.5 |
| 25 | Male | 39+6 | 2700 | Vaginal delivery | Abnormal NS | Mother had hyperthyroidism in late pregnancy | Not done | None | Microcephaly and poor learning ability and school performance/6.3 |
| 26 | Female | 37+3 | 900 | Caesarean | Abnormal NS | N | Not done | One of the monozygotic twins, Full term, Small for gestational age | Normal developmental milestones/0.8 |
| 27 | Female | 39 | 3100 | Vaginal delivery | Abnormal NS | N | Thyroid agenesis/0.1 | Neonatal jaundice | Normal DQ/6.1 |
| 28 | Female | 40+6 | 2900 | Vaginal delivery | Abnormal NS | N | Thyroid ectopy/4.7 | Neonatal jaundice | Normal DQ/3.0 |
| 29 | Male | 38 | 3450 | Caesarean | Abnormal NS | Mother had hyperthyroidism in pregnancy | Normal/0.1 | Neonatal jaundice | Normal DQ/0.3 |
| 30 | Female | 38+5 | 3330 | Caesarean | Abnormal NS | N | Thyroid ectopy/0.1 | None | Normal DQ/2.8 |
| 31 | Male | 40 | 3370 | Vaginal delivery | Abnormal NS | Mother had hypothyroidism | Normal/0.8 | Neonatal jaundice | Normal DQ/2.6 |
| 32 | Female | 41 | 3350 | Caesarean | Abnormal NS | Mother had hyperthyroidism | Thyroid agenesis/2.4 | Neonatal jaundice | Normal DQ/1.6 |
| 33 | Female | 41 | 3870 | Caesarean | Abnormal NS | N | Thyroid agenesis/1.1 | Neonatal jaundice | Normal DQ/1.1 |
| Patients | Age When L-T4 Initiated (days) | Diagnostic Evaluation | Initial L-T4 Dosage (µg/kg/day) | Days of Age at Normalization of Thyroid Function (Days) | Maintenance L-T4 Dosage (µg/kg/day)/ the Latest Ages (Years) | ||||
|---|---|---|---|---|---|---|---|---|---|
| FT3 (3.5–10 pmol/L) | FT4 (12–27 pmol/L) | T3 (1.3–3.8 nmol/L) | T4 (79–192 nmol/L) | TSH (0.27–4.2 mIU/L) | |||||
| 1 | 21 | 2.15 | <5.15 | - | - | >100 | 8.3 | 56 | 2.8/2.9 |
| 2 | 20 | 1.76 | 6.63 | 1.76 | 30.80 | >100 | 11.7 | 37 | 3.7/0.5 |
| 3 | 21 | - | - | - | - | >100 | 11.1 | - | 1.8/10.0 |
| 4 | 22 | 3.65 | 5.13 | 1.78 | 46.75 | >100 | 9.4 | 3(y) | 3.5/7.3 |
| 5 | 27 | - | - | 0.31 | - | >150 | 10.7 | 57 | 3.2/6.7 |
| 6 | 21 | 5.20 | 11.80 | 1.90 | 87.82 | 22.42 | 8.5 | 90 | 1.0/3.7 |
| 7 | 22 | 5.70 | 7.25 | 2.39 | 44.94 | 89.46 | 12.5 | 37 | 1.6/4.3 |
| 8 | 27 | 7.13 | 6.06 | 3.24 | 35.94 | >100 | 9.4 | 46 | 2.0/0.8 |
| 9 | 17 | 2.89 | <5.15 | 1.06 | 24.14 | >100 | 10.7 | 31 | 1.9/0.9 |
| 10 | 20 | 4.89 | <5.15 | 1.81 | 26.60 | >100 | 15 | 34 | 1.9/2.0 |
| 11 | 36 | 6.33 | 9.01 | - | - | 68.54 | 4.9 | 97 | 1.0/8.5 |
| 12 | 17 | 6.96 | 10.17 | - | - | 46.41 | 6.8 | 60 | 0/4.5 |
| 13 | 17 | 1.62 | 2.96 | - | - | >150 | 10.4 | 38 | 0/3.5 |
| 14 | 14 | 5.68 | 7.34 | - | - | 100 | 12.5 | 30 | 2.2/3.5 |
| 15 | 25 | 1.89 | 1.80 | - | - | 100 | 8.5 | 45 | 2.2/4.3 |
| 16 | 17 | 3.91 | 2.83 | - | - | 100 | 9.4 | 34 | 1.5/4.4 |
| 17 | 14 | 3.79 | 4.89 | - | - | 100 | 9.8 | 32 | 0.4/4.0 |
| 18 | 27 | 5.07 | 6.05 | - | - | 99.77 | 11.4 | 45 | 1.8/4.0 |
| 19 | 14 | 3.70 | 5.79 | - | - | 100 | 13.0 | 39 | 3.7/0.8 |
| 20 | 16 | 1.86 | 1.54 | - | - | 100 | 10.7 | 37 | 3.4/1.6 |
| 21 | 36 | 0.59 | 1.29 | - | - | 100 | 8.3 | 60 | 3.1/4.9 |
| 22 | 17 | 2.93 | 2.96 | - | - | 100 | 7.8 | 30 | 1.0/2.2 |
| 23 | 46 | 5.87 | 5.93 | - | - | 145.97 | 9.8 | 70 | 3.9/0.9 |
| 24 | 21 | 4.3 | 8.07 | 1.63 | 59.25 | >100 | 7.4 | 71 | 3.3/2.5 |
| 25 | 90 | - | - | - | - | >100 | 5.0 | - | 1.7/6.3 |
| 26 | 12 | 3.68 | 10.83 | 1.33 | 92.25 | 42.41 | 10 | 19 | 13.8/0.2 |
| 27 | 14 | 4.05 | 9.40 | - | - | >150 | 7.0 | 90 | 2.1/8.3 |
| 28 | 22 | - | 4.03 | 1.1 | - | 223.1 | 7.6 | 50 | 1.6/8.3 |
| 29 | 15 | 4.56 | 11.84 | 1.41 | 57.27 | 64.89 | 12.5 | 21 | 0/0.1 |
| 30 | 13 | 3.70 | 6.69 | - | - | 100 | 13.0 | 25 | 1.5/3.7 |
| 31 | 14 | 1.39 | 2.19 | - | - | 100 | 13.0 | 45 | 0/4.3 |
| 32 | 11 | 1.48 | 2.19 | - | - | 100 | 8.6 | 120 | 4.7/2.9 |
| 33 | 18 | 2.19 | 2.71 | - | - | 56.5 | 7.7 | 30 | 3.1/2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Tian, J.-L.; Huang, X.-L.; Song, Y.-Z. Genetic Factors Causing Thyroid Dyshormonogenesis as the Major Etiologies for Primary Congenital Hypothyroidism: Clinical and Genetic Characterization of 33 Patients. J. Clin. Med. 2022, 11, 7313. https://doi.org/10.3390/jcm11247313
Liu R, Tian J-L, Huang X-L, Song Y-Z. Genetic Factors Causing Thyroid Dyshormonogenesis as the Major Etiologies for Primary Congenital Hypothyroidism: Clinical and Genetic Characterization of 33 Patients. Journal of Clinical Medicine. 2022; 11(24):7313. https://doi.org/10.3390/jcm11247313
Chicago/Turabian StyleLiu, Rui, Jing-Li Tian, Xiao-Ling Huang, and Yuan-Zong Song. 2022. "Genetic Factors Causing Thyroid Dyshormonogenesis as the Major Etiologies for Primary Congenital Hypothyroidism: Clinical and Genetic Characterization of 33 Patients" Journal of Clinical Medicine 11, no. 24: 7313. https://doi.org/10.3390/jcm11247313
APA StyleLiu, R., Tian, J.-L., Huang, X.-L., & Song, Y.-Z. (2022). Genetic Factors Causing Thyroid Dyshormonogenesis as the Major Etiologies for Primary Congenital Hypothyroidism: Clinical and Genetic Characterization of 33 Patients. Journal of Clinical Medicine, 11(24), 7313. https://doi.org/10.3390/jcm11247313





