Effects of Rifaximin on Circulating Albumin Structures and Serum Ammonia Levels in Patients with Liver Cirrhosis: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Outcomes
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Patients
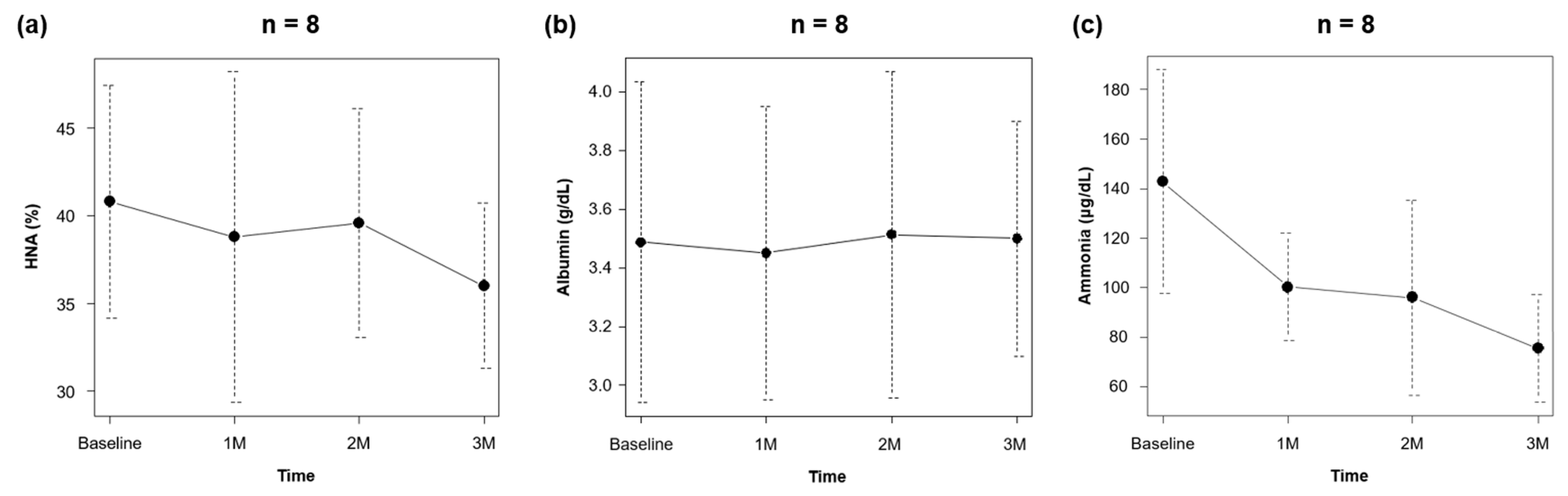
3.2. Effect of Rifaximin on Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, F.; Wong, N.K.; He, J.; Zhang, R.; Sun, R.; Xu, Y.; Liu, Y.; Li, W.; Koike, K.; et al. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J. Hepatol. 2019, 71, 212–221. [Google Scholar] [CrossRef]
- Moon, A.M.; Singal, A.G.; Tapper, E.B. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin. Gastroenterol. Hepatol. 2020, 18, 2650–2666. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.F.; Amodio, P.; Bajaj, J.S.; Dhiman, R.K.; Montagnese, S.; Taylor-Robinson, S.D.; Vilstrup, H.; Jalan, R. Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. J. Hepatol. 2020, 73, 1526–1547. [Google Scholar] [CrossRef]
- Hirode, G.; Vittinghoff, E.; Wong, R.J. Increasing Burden of hepatic encephalopathy among hospitalized adults: An analysis of the 2010-2014 national inpatient sample. Dig. Dis. Sci. 2019, 64, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Shiraki, M.; Watanabe, S.; Imai, K.; Suetsugu, A.; Takai, K.; Moriwaki, H.; Shimizu, M. Prognostic significance of minimal hepatic encephalopathy in patients with liver cirrhosis in Japan: A propensity score-matching analysis. J. Gastroenterol. Hepatol. 2019, 34, 1809–1816. [Google Scholar] [CrossRef]
- Miwa, T.; Hanai, T.; Nishimura, K.; Maeda, T.; Ogiso, Y.; Imai, K.; Suetsugu, A.; Takai, K.; Shiraki, M.; Shimizu, M. Handgrip strength stratifies the risk of covert and overt hepatic encephalopathy in patients with cirrhosis. J. Parenter. Enter. Nutr. 2021, 46, 858–866. [Google Scholar] [CrossRef]
- Kato, M.; Hughes, R.D.; Keays, R.T.; Williams, R. Electron microscopic study of brain capillaries in cerebral edema from fulminant hepatic failure. Hepatology 1992, 15, 1060–1066. [Google Scholar] [CrossRef]
- Sepehrinezhad, A.; Zarifkar, A.; Namvar, G.; Shahbazi, A.; Williams, R. Astrocyte swelling in hepatic encephalopathy: Molecular perspective of cytotoxic edema. Metab. Brain Dis. 2020, 35, 559–578. [Google Scholar] [CrossRef]
- Claeys, W.; Van Hoecke, L.; Lefere, S.; Geerts, A.; Verhelst, X.; Van Vlierberghe, H.; Degroote, H.; Devisscher, L.; Vandenbroucke, R.E.; Van Steenkiste, C. The neurogliovascular unit in hepatic encephalopathy. JHEP Rep. 2021, 3, 100352. [Google Scholar] [CrossRef]
- Görg, B.; Qvartskhava, N.; Keitel, V.; Bidmon, H.J.; Selbach, O.; Schliess, F.; Häussinger, D. Ammonia induces RNA oxidation in cultured astrocytes and brain in vivo. Hepatology 2008, 48, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Yin, X.Y.; Duan, Z.J.; Guo, S.B.; Sun, X.Y. Role of the heme oxygenase/carbon monoxide pathway in the pathogenesis and prevention of hepatic encephalopathy. Mol. Med. Rep. 2013, 8, 67–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baldassarre, M.; Naldi, M.; Zaccherini, G.; Bartoletti, M.; Antognoli, A.; Laggetta, M.; Gagliardi, M.; Tufoni, M.; Domenicali, M.; Waterstradt, K.; et al. Determination of effective albumin in patients with decompensated cirrhosis: Clinical and prognostic implications. Hepatology 2021, 74, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Naldi, M.; Baldassarre, M.; Domenicali, M.; Giannone, F.A.; Bossi, M.; Montomoli, J.; Sandahl, T.D.; Glavind, E.; Vilstrup, H.; Caraceni, P.; et al. Mass spectrometry characterization of circulating human serum albumin microheterogeneity in patients with alcoholic hepatitis. J. Pharm. Biomed Anal. 2016, 122, 141–147. [Google Scholar] [CrossRef]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Miwa, Y.; Shiraki, M.; Gomi, I.; Toda, K.; Kuriyama, S.; Nakamura, H.; Wakahara, T.; Era, S.; Moriwaki, H. Oral branched-chain amino acid supplementation improves the oxidized/reduced albumin ratio in patients with liver cirrhosis. Hepatol. Res. 2007, 37, 765–770. [Google Scholar] [CrossRef]
- Hanai, T.; Nishimura, K.; Miwa, T.; Maeda, T.; Ogiso, Y.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M. Usefulness of nutritional therapy recommended in the Japanese Society of Gastroenterology/Japan Society of Hepatology evidence-based clinical practice guidelines for liver cirrhosis 2020. J. Gastroenterol. 2021, 56, 928–937. [Google Scholar] [CrossRef]
- Caraceni, P.; Vargas, V.; Solà, E.; Alessandria, C.; de Wit, K.; Trebicka, J.; Angeli, P.; Mookerjee, R.P.; Durand, F.; Pose, E.; et al. The use of rifaximin in patients with cirrhosis. Hepatology 2021, 74, 1660–1673. [Google Scholar] [CrossRef]
- Suzuki, K.; Endo, R.; Takikawa, Y.; Moriyasu, F.; Aoyagi, Y.; Moriwaki, H.; Terai, S.; Sakaida, I.; Sakai, Y.; Nishiguchi, S.; et al. Efficacy and safety of rifaximin in Japanese patients with hepatic encephalopathy: A phase II/III, multicenter, randomized, evaluator-blinded, active-controlled trial and a phase III, multicenter, open trial. Hepatol. Res. 2018, 48, 411–423. [Google Scholar] [CrossRef]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef]
- Yoshiji, H.; Nagoshi, S.; Akahane, T.; Asaoka, Y.; Ueno, Y.; Ogawa, K.; Kawaguchi, T.; Kurosaki, M.; Sakaida, I.; Shimizu, M.; et al. Evidence-based clinical practice guidelines for liver cirrhosis 2020. Hepatol. Res. 2021, 51, 725–749. [Google Scholar] [CrossRef]
- Yoshiji, H.; Nagoshi, S.; Akahane, T.; Asaoka, Y.; Ueno, Y.; Ogawa, K.; Kawaguchi, T.; Kurosaki, M.; Sakaida, I.; Shimizu, M.; et al. Evidence-based clinical practice guidelines for liver cirrhosis 2020. J. Gastroenterol. 2021, 56, 593–619. [Google Scholar] [CrossRef]
- Alcaraz-Quiles, J.; Casulleras, M.; Oettl, K.; Titos, E.; Flores-Costa, R.; Duran-Güell, M.; López-Vicario, C.; Pavesi, M.; Stauber, R.E.; Arroyo, V.; et al. Oxidized albumin triggers a cytokine storm in leukocytes through P38 mitogen-activated protein kinase: Role in systemic inflammation in decompensated cirrhosis. Hepatology 2018, 68, 1937–1952. [Google Scholar] [CrossRef] [PubMed]
- Fornai, M.; Antonioli, L.; Pellegrini, C.; Colucci, R.; Sacco, D.; Tirotta, E.; Natale, G.; Bartalucci, A.; Flaibani, M.; Renzulli, C.; et al. Small bowel protection against NSAID-injury in rats: Effect of rifaximin, a poorly absorbed, GI targeted, antibiotic. Pharmacol. Res. 2016, 104, 186–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kitagawa, R.; Kon, K.; Uchiyama, A.; Arai, K.; Yamashina, S.; Kuwahara-Arai, K.; Kirikae, T.; Ueno, T.; Ikejima, K. Rifaximin prevents ethanol-induced liver injury in obese KK-A(y) mice through modulation of small intestinal microbiota signature. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G707–G715. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Kaji, K.; Nishimura, N.; Enomoto, M.; Murata, K.; Takeda, S.; Takaya, H.; Kawaratani, H.; Moriya, K.; Namisaki, T.; et al. Dual therapy with zinc acetate and rifaximin prevents from ethanol-induced liver fibrosis by maintaining intestinal barrier integrity. World J. Gastroenterol. 2021, 27, 8323–8342. [Google Scholar] [CrossRef]
- Fujinaga, Y.; Kawaratani, H.; Kaya, D.; Tsuji, Y.; Ozutsumi, T.; Furukawa, M.; Kitagawa, K.; Sato, S.; Nishimura, N.; Sawada, Y.; et al. Effective combination therapy of angiotensin-II receptor blocker and rifaximin for hepatic fibrosis in rat model of nonalcoholic steatohepatitis. Int. J. Mol. Sci 2020, 21, 5589. [Google Scholar] [CrossRef]
- Sharma, B.C.; Sharma, P.; Lunia, M.K.; Srivastava, S.; Goyal, R.; Sarin, S.K. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am. J. Gastroenterol. 2013, 108, 1458–1463. [Google Scholar] [CrossRef]
- Xie, X.L.; He, J.T.; Wang, Z.T.; Xiao, H.Q.; Zhou, W.T.; Du, S.H.; Xue, Y.; Wang, Q. Lactulose attenuates METH-induced neurotoxicity by alleviating the impaired autophagy, stabilizing the perturbed antioxidant system and suppressing apoptosis in rat striatum. Toxicol. Lett. 2018, 289, 107–113. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Baseline | 1 Month | 2 Months | 3 Months |
|---|---|---|---|---|
| Child–Pugh score | 6 (±1) | 6 (±1) | 6 (±1) | 6 (±1) |
| MELD score | 11 (±4) | 11 (±4) | 11 (±4) | 11 (±3) |
| International normalized ratio | 1.28 (±0.43) | 1.27 (±0.45) | 1.22 (±0.26) | 1.22 (±0.29) |
| Platelets (109/L) | 76 (±27) | 74 (±26) | 77 (±33) | 75 (±37) |
| White blood cells (μL) | 3130 (±1219) | 3256 (±1456) | 3070 (±1151) | 3386 (±1126) |
| Red blood cells (104/μL) | 336 (±134) | 386 (±54) | 395 (±49) | 399 (±48) |
| Hemoglobin (g/dL) | 12.2 (±1.6) | 12.3 (±1.9) | 12.2 (±2.0) | 12.2 (±2.1) |
| Creatinine (mg/dL) | 0.74 (±0.25) | 0.77 (±0.30) | 0.78 (±0.27) | 0.71 (±0.20) |
| AST | 42 (±16) | 44 (±14) | 44 (±11) | 46 (±17) |
| ALT | 26 (±30) | 28 (±11) | 29 (±9) | 29 (±11) |
| γ-GTP | 82 (±55) | 89 (±72) | 102 (±105) | 119 (±163) |
| Albumin (g/dL) | 3.5 (±0.5) | 3.5 (±0.5) | 3.5 (±0.6) | 3.5 (±0.4) |
| Bilirubin (mg/dL) | 1.7 (±0.5) | 1.6 (±0.6) | 1.6 (±0.4) | 1.8 (±0.5) |
| Ammonia (μg/dL) | 143 (±45) | 100 (±22) | 96 (±39) | 76 (±22) * |
| HNA (%) | 41 (±7) | 39 (±9) | 40 (±7) | 36 (±5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miwa, T.; Hanai, T.; Imai, K.; Takai, K.; Shiraki, M.; Hayashi, H.; Shimizu, S.; Nishigaki, Y.; Tomita, E.; Shimizu, M. Effects of Rifaximin on Circulating Albumin Structures and Serum Ammonia Levels in Patients with Liver Cirrhosis: A Preliminary Study. J. Clin. Med. 2022, 11, 7318. https://doi.org/10.3390/jcm11247318
Miwa T, Hanai T, Imai K, Takai K, Shiraki M, Hayashi H, Shimizu S, Nishigaki Y, Tomita E, Shimizu M. Effects of Rifaximin on Circulating Albumin Structures and Serum Ammonia Levels in Patients with Liver Cirrhosis: A Preliminary Study. Journal of Clinical Medicine. 2022; 11(24):7318. https://doi.org/10.3390/jcm11247318
Chicago/Turabian StyleMiwa, Takao, Tatsunori Hanai, Kenji Imai, Koji Takai, Makoto Shiraki, Hideki Hayashi, Shogo Shimizu, Yoichi Nishigaki, Eiichi Tomita, and Masahito Shimizu. 2022. "Effects of Rifaximin on Circulating Albumin Structures and Serum Ammonia Levels in Patients with Liver Cirrhosis: A Preliminary Study" Journal of Clinical Medicine 11, no. 24: 7318. https://doi.org/10.3390/jcm11247318
APA StyleMiwa, T., Hanai, T., Imai, K., Takai, K., Shiraki, M., Hayashi, H., Shimizu, S., Nishigaki, Y., Tomita, E., & Shimizu, M. (2022). Effects of Rifaximin on Circulating Albumin Structures and Serum Ammonia Levels in Patients with Liver Cirrhosis: A Preliminary Study. Journal of Clinical Medicine, 11(24), 7318. https://doi.org/10.3390/jcm11247318






