Opioid Dose, Pain, and Recovery following Abdominal Surgery: A Retrospective Cohort Study
Abstract
1. Introduction
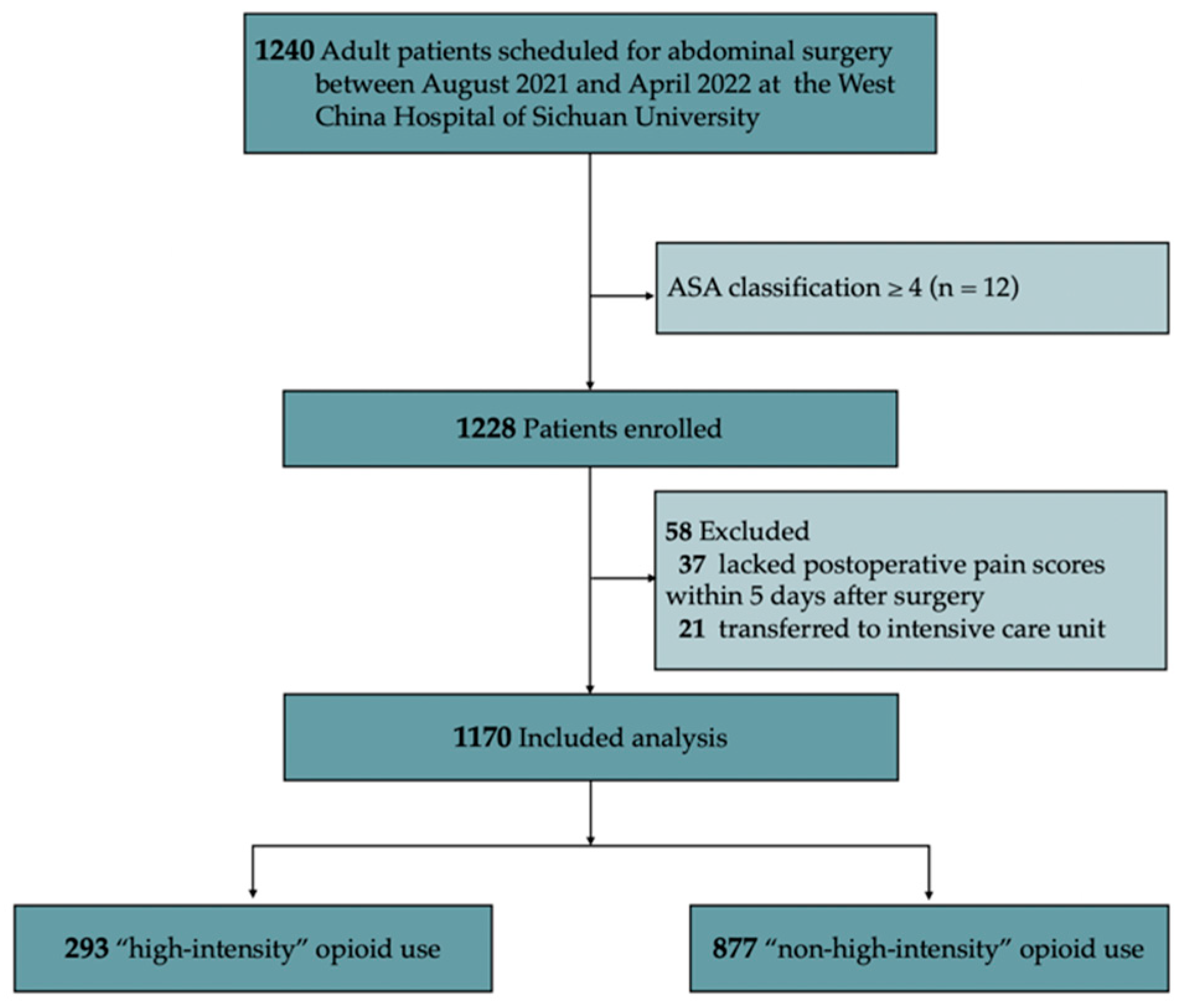
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. OME Calculation
2.4. Primary and Secondary Outcomes
2.5. Statistical Analysis
3. Results
3.1. Primary Outcome
3.2. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

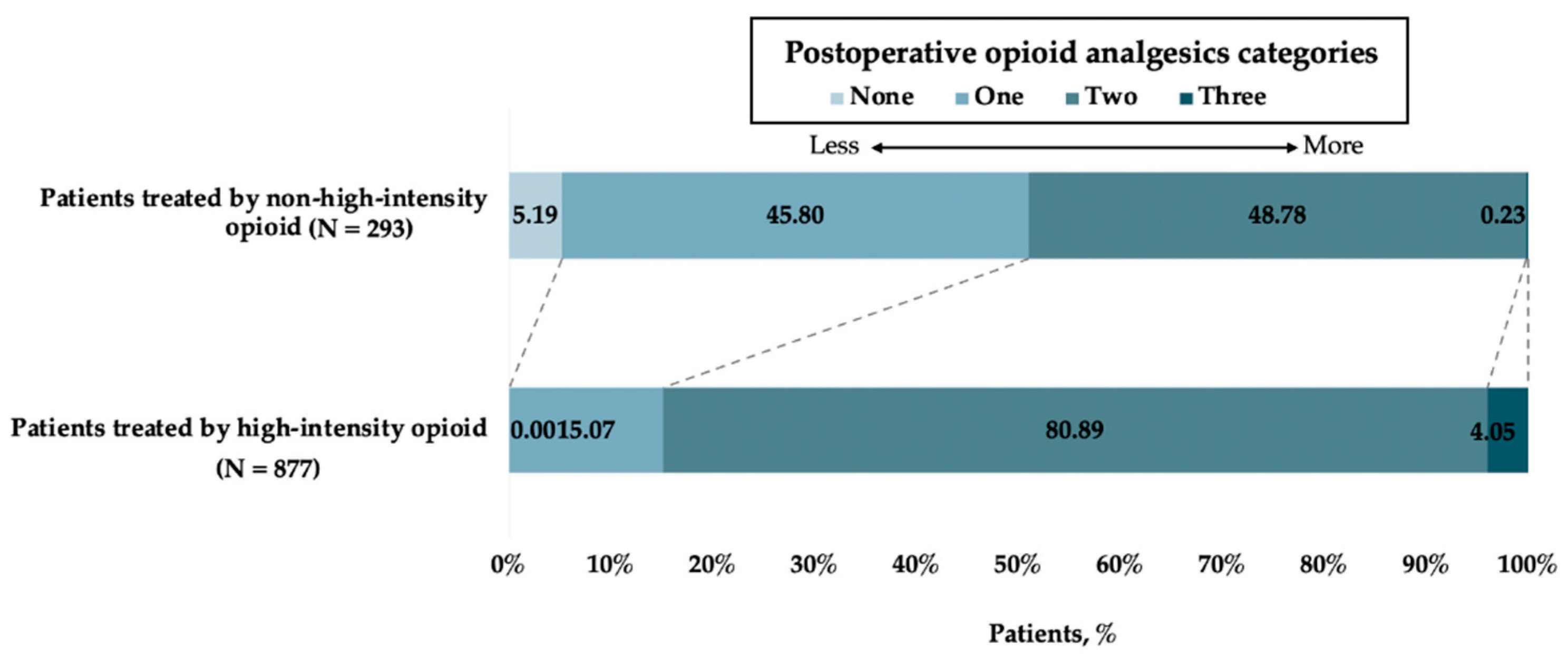
| Outcome | Patients Treated by High-Intensity Opioid n = 293 | Patients Treated by Non-High-Intensity Opioid n = 877 | Crude OR (95% CI) | Adjusted OR # (95% CI) | p |
|---|---|---|---|---|---|
| At rest, n (%) | |||||
| Day 1 | 51 (17.41) | 84 (9.58) | 1.99 (1.36, 2.89) | 1.67 (1.08, 2.55) | 0.019 |
| Day 2 | 30 (10.24) | 36 (4.10) | 2.66 (1.60, 4.41) | 1.98 (1.15, 3.42) | 0.014 |
| Day 3 | 17 (5.80) | 20 (2.28) | 2.64 (1.35, 5.11) | 2.21 (1.08, 4.52) | 0.028 |
| Day 5 | 13 (4.44) | 10 (1.14) | 2.35 (0.99, 5.40) | 1.76 (0.70, 4.37) | 0.220 |
| During movement, n (%) | |||||
| Day 1 | 184 (62.80) | 419 (47.78) | 1.85 (1.41, 2.43) | 1.53 (1.14, 2.05) | 0.004 |
| Day 2 | 175 (59.73) | 359 (40.94) | 2.14 (1.64, 2.81) | 1.64 (1.23, 2.19) | 0.001 |
| Day 3 | 143 (48.81) | 241 (27.48) | 2.52 (1.92, 3.31) | 1.88 (1.41, 2.52) | <0.001 |
| Day 5 | 112 (38.23) | 173 (19.73) | 2.52 (1.89, 3.36) | 2.25 (1.65, 3.07) | <0.001 |
References
- Gerbershagen, H.J.; Aduckathil, S.; van Wijck, A.J.M.; Peelen, L.M.; Kalkman, C.J.; Meissner, W. Pain intensity on the first day after surgery. Anesthesiology 2013, 118, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.D. Techniques to ensure adequate portal flow in the presence of splenorenal shunts. Liver Transplant. 2007, 13, 767–768. [Google Scholar] [CrossRef]
- Behman, R.; Cleary, S.; McHardy, P.; Kiss, A.; Sawyer, J.; Ladak, S.S.J.; McCluskey, S.A.; Srinivas, C.; Katz, J.; Coburn, N.; et al. Predictors of post-operative pain and opioid consumption in patients undergoing liver surgery. World J. Surg. 2019, 43, 2579–2586. [Google Scholar] [CrossRef]
- Pirie, K.; Traer, E.; Finniss, D.; Myles, P.S.; Riedel, B. Current approaches to acute postoperative pain management after major abdominal surgery: A narrative review and future directions. Br. J. Anaesth. 2022, 129, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Frieden TR, H.D. Reducing the risks of relief-The CDC opioid-prescribing guideline. N. Engl. J. Med. 2016, 374, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Olotu, B.; Mathew, A.; Waitman, L.R.; Rasu, R. Lumbar spine surgeries and medication usage during hospital stay: One-center perspective. Hosp. Pharm. 2017, 52, 774–780. [Google Scholar] [CrossRef]
- Chu, L.F.; Angst, M.S.; Clark, D. Opioid-induced hyperalgesia in humans: Molecular mechanisms and clinical considerations. Clin. J. Pain 2008, 24, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Kleeman-Forsthuber, L.; Pollet, A.; Johnson, R.M.; Boyle, J.; Jennings, J.M.; Dennis, D.A. Evaluation of low-dose versus high-dose opioid pathway in opioid-naïve patients after total knee arthroplasty. Arthroplast. Today 2022, 14, 81–85. [Google Scholar] [CrossRef]
- Cooper, D.W.; Lindsay, S.L.; Ryall, D.M.; Kokri, M.S.; Eldabe, S.S.; Lear, G.A. Does intrathecal fentanyl produce acute cross-tolerance to i.v. morphine? Br. J. Anaesth. 1997, 78, 311–313. [Google Scholar] [CrossRef]
- Aoyagi, K.; He, J.; Simpson, M.; Melton, B.L.; Chandaka, S.; Waitman, L.R.; Sharma, N.K. Association between opioid dose, acute post-operative pain and walking distance following lumbar spine surgery. J. Clin. Pharm. Ther. 2020, 45, 169–178. [Google Scholar] [CrossRef]
- Cozowicz, C.; Olson, A.; Poeran, J.; Mörwald, E.E.; Zubizarreta, N.; Girardi, F.P.; Hughes, A.P.; Mazumdar, M.; Memtsoudis, S.G. Opioid prescription levels and postoperative outcomes in orthopedic surgery. Pain 2017, 158, 2422–2430. [Google Scholar] [CrossRef]
- Kinjo, S.; Sands, L.P.; Lim, E.; Paul, S.; Leung, J.M. Prediction of postoperative pain using path analysis in older patients. J Anesth 2012, 26, 1–8. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; Von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, 1628–1654. [Google Scholar] [CrossRef]
- Nafziger, A.N.; Barkin, R.L. Opioid therapy in acute and chronic pain. J. Clin. Pharmacol. 2018, 58, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.-S.; Zhang, J.; Zuo, Y. Validation of the Chinese version of the quality of recovery-15 score and its comparison with the post-operative quality recovery scale. Patient 2016, 9, 251–259. [Google Scholar] [CrossRef]
- Zeremski, M.; Zavala, R.; Dimova, R.B.; Chen, Y.; Kritz, S.; Sylvester, C.; Brown, L.S.; Talal, A.H. Improvements in HCV-related knowledge among substance users on opioid agonist therapy after an educational intervention. J. Addict. Med. 2016, 10, 104–109. [Google Scholar] [CrossRef]
- Cen, X.; Jena, A.B.; Mackey, S.; Sun, E.C. Surgeon variation in perioperative opioid prescribing and medium- or long-term opioid utilization after total knee arthroplasty: A cross-sectional analysis. Anesthesiology 2022, 137, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Myles, D.B.; Galagher, W.; Chew, C.; Macdonald, N.; Dennis, A. Minimal clinically important difference for three quality of recovery scales. Anesthesiology 2016, 125, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, B.; Gutenbrunner, C.; Barke, A.; Karst, M.; Schiller, J.; Schäfer, P.; Falter, S.; Korwisi, B.; Rief, W.; Treede, R.D. The IASP classification of chronic pain for ICD-11: Functiofing properties of chronic pain. Pain 2019, 160, 88–94. [Google Scholar] [CrossRef]
- Claxton, A.R.; McGuire, G.; Chung, F.; Cruise, C. Evaluation of morphine versus fentanyl for postoperative analgesia after ambulatory surgical procedures. Anesth. Analg. 1997, 84, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L.; Abdi, S.; Atluri, S.; Balog, C.C.; Benyamin, R.M.; Boswell, M.V.; Brown, K.R.; Bruel, B.M.; Bryce, D.A.; Burks, P.A.; et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part I--evidence assessment. Pain Physician 2012, 15, 67–116. [Google Scholar] [CrossRef]
- Rivas, E.; Cohen, B.; Pu, X.; Xiang, L.; Saasouh, W.; Mao, G.; Minko, P.; Mosteller, L.; Volio, A.; Maheshwari, K.; et al. Pain and opioid consumption and mobilization after surgery: Post hoc analysis of two randomized trials. Anesthesiology 2022, 136, 115–126. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Nwosu, K.; Jiang, W.; Yau, A.L.; Chaudhary, M.A.; Scully, R.E.; Koehlmoos, T.; Kang, J.D.; Haider, A.H. Risk factors for prolonged opioid use following spine surgery, and the association with surgical intensity, among opioid-naive patients. J. Bone Jt. Surg.-Am. Vol. 2017, 99, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Hill, S.; Carty, S.; Rockett, M.; Bastable, R.; Knaggs, R.; Lambert, D.; Levy, N.; Hughes, J.; Wilkinson, P. Surgery and opioids: Evidence-based expert consensus guidelines on the perioperative use of opioids in the United Kingdom. Br. J. Anaesth. 2021, 126, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Kharasch, E.D.; Clark, J.D.; Adams, J.M. Opioids and public health: The prescription opioid ecosystem and need for improved management. Anesthesiology 2022, 136, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Myles, D.B.; Galagher, W.; Boyd, D.; Chew, C.; MacDonald, N.; Dennis, A. Measuring acute postoperative pain using the visual analog scale: The minimal clinically important difference and patient acceptable symptom state. Br. J. Anaesth. 2017, 118, 424–429. [Google Scholar] [CrossRef]
- Edwards, D.A.; Hedrick, T.L.; Jayaram, J.; Argoff, C.; Gulur, P.; Holubar, S.D.; Gan, T.J.; Mythen, M.G.; Miller, T.E.; Shaw, A.D.; et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on perioperative management of patients on preoperative opioid therapy. Anesth. Analg. 2019, 129, 553–566. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Li, C.; Chen, D.; Yang, L.; Li, Q. Effect of standardized multimodal analgesia on opioid consumption after major upper abdominal surgery under enhanced recovery after surgery pathway. Chin. J. Bases Clin. Gen. Surg. 2020, 29, 475–480. [Google Scholar] [CrossRef]
- Neckebroek, M.; Ghita, M.; Ghita, M.; Copot, D.; Ionescu, C.M. Pain detection with bioimpedance methodology from 3-dimensional exploration of nociception in a postoperative observational trial. J. Clin. Med. 2020, 9, 684. [Google Scholar] [CrossRef]
- Gélinas, C.; Shahiri, S.; Richard-Lalonde, M.; Laporta, D.; Morin, J.F.; Boitor, M.; Ferland, C.E.; Bourgault, P.; Richebé, P. Exploration of a multi-parameter technology for pain assessment in postoperative patients after cardiac surgery in the intensive care unit: The nociception level index (NOL)TM. J. Pain Res. 2021, 14, 3723–3731. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total n = 1170 | Patients Treated by High-Intensity Opioid n = 293 | Patients Treated by Non-High-Intensity Opioid n = 877 | p |
|---|---|---|---|---|
| Age, years, median (IQR) | 55.00 (47.00, 62.00) | 56.000 (49.00, 64.00) | 55.00 (47.00, 62.00) | 0.018 a |
| Age group, n (%) | 0.092 b | |||
| <65 years | 955 (81.62) | 229 (78.16) | 726 (82.78) | |
| ≥65 years | 215 (18.38) | 64 (21.84) | 151 (17.22) | |
| Sex, n (%) | 0.979 b | |||
| Male | 740 (63.25) | 186 (63.48) | 554 (63.17) | |
| Female | 430 (36.75) | 107 (36.52) | 323 (36.83) | |
| BMI, kg/m2, median (IQR) | 22.60 (20.55, 24.81) | 22.94 (20.76, 25.09) | 22.49 (20.52, 24.80) | 0.288 a |
| BMI group, n (%) | 0.934 b | |||
| <24 | 771 (65.90) | 192 (65.53) | 579 (66.02) | |
| ≥24 | 399 (34.10) | 101 (34.47) | 298 (33.98) | |
| Preoperative comorbidities, n (%) | ||||
| Hypertension | 164 (14.02) | 41 (13.99) | 123 (14.03) | >0.999 b |
| Diabetes mellitus | 104 (8.89) | 23 (7.85) | 81 (9.24) | 0.546 b |
| Cardiovascular disease | 8 (0.68) | 2 (0.68) | 6 (0.68) | >0.999 c |
| COPD | 44 (3.76) | 20 (6.83) | 24 (2.74) | 0.003 b |
| Location of surgery, n (%) | <0.001 b | |||
| Lower abdominal | 651 (55.64) | 208 (70.99) | 443 (50.51) | |
| Upper abdominal | 519 (44.36) | 85 (29.01) | 434 (49.49) | |
| Type of surgery, n (%) | <0.001 b | |||
| Open | 834 (71.28) | 235 (80.21) | 599 (68.30) | |
| Laparoscopic | 336 (28.72) | 58 (19.79) | 278 (31.70) | |
| Anesthesia method, n (%) | 0.180 b | |||
| Inhalation or total intravenous anesthesia | 821 (70.17) | 196 (66.89) | 625 (71.27) | |
| Inhalation and intravenous anesthesia | 349 (29.83) | 97 (33.11) | 252 (28.73) | |
| ASA class, n (%) | 0.906 b | |||
| I~II | 967 (82.65) | 241 (82.25) | 726 (82.78) | |
| III | 203 (17.35) | 52 (17.75) | 151 (17.22) | |
| NSAIDs use at preoperative day 1, n (%) | 214 (18.29) | 4 (1.37) | 210 (23.95) | <0.001 c |
| Opioid use at preoperative day 1, n (%) | 14 (1.20) | 5 (1.71) | 9 (1.03) | 0.358 c |
| Intraoperative sufentanil, μg, median (IQR) | 32.50 (27.50, 40.00) | 35.00 (27.50, 40.00) | 32.50 (27.50, 38.00) | 0.098 b |
| Intraoperative remifentanil, μg, median (IQR) | 1751.95 (1248.23, 2323.75) | 1825.00 (1428.00, 2259.00) | 1723.00 (1226.00, 2328.00) | 0.020 b |
| Duration of surgery, min, median (IQR) | 210.00 (160.00, 271.00) | 214.00 (175.00, 269.00) | 207.00 (152.00, 272.00) | 0.029 a |
| Duration of surgery group, n (%) | 0.487 b | |||
| <2 h | 82 (7.01) | 16 (5.46) | 66 (7.52) | |
| 2~4 h | 670 (57.26) | 171 (58.36) | 499 (56.90) | |
| ≥4 h | 418 (35.73) | 106 (36.18) | 312 (35.58) | |
| Local infiltration, n (%) | 441 (37.69) | 94 (32.08) | 347 (39.57) | 0.026 b |
| Peripheral nerve block, n (%) | 752 (64.27) | 199 (67.92) | 553 (63.06) | 0.152 b |
| PCIA, n (%) | 992 (84.79) | 244 (83.28) | 748 (85.29) | 0.461 b |
| NSAIDs use on postoperative day, n (%) | ||||
| 1 | 261 (22.31) | 30 (10.24) | 231 (26.34) | <0.001 b |
| 2 | 261 (22.31) | 33 (11.26) | 228 (26.00) | <0.001 b |
| 3 | 228 (19.49) | 32 (10.92) | 196 (22.35) | <0.001 b |
| 4 | 211 (18.03) | 31 (10.58) | 180 (20.53) | <0.001 b |
| 5 | 187 (15.98) | 29 (9.90) | 158 (18.02) | <0.001 b |
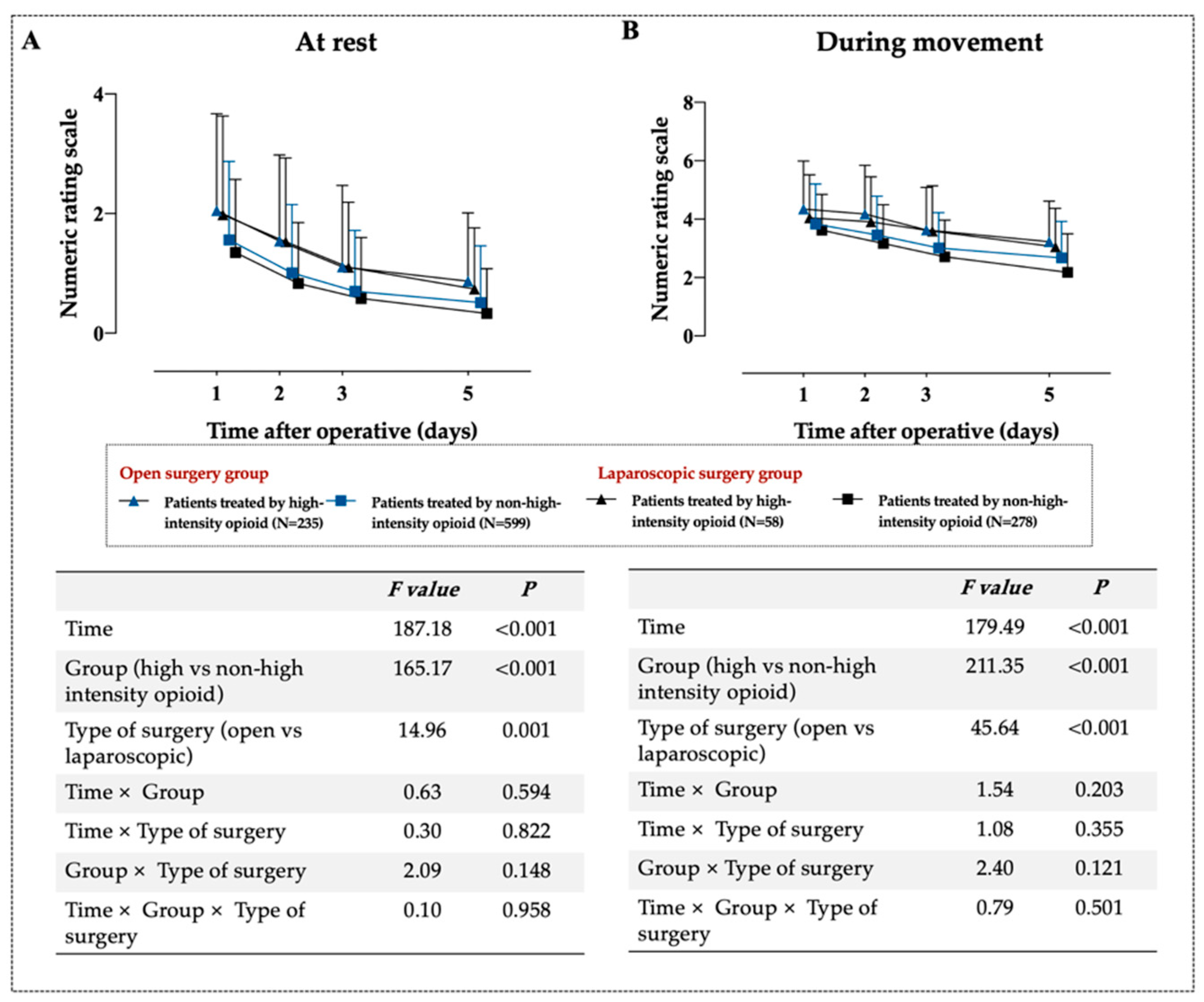
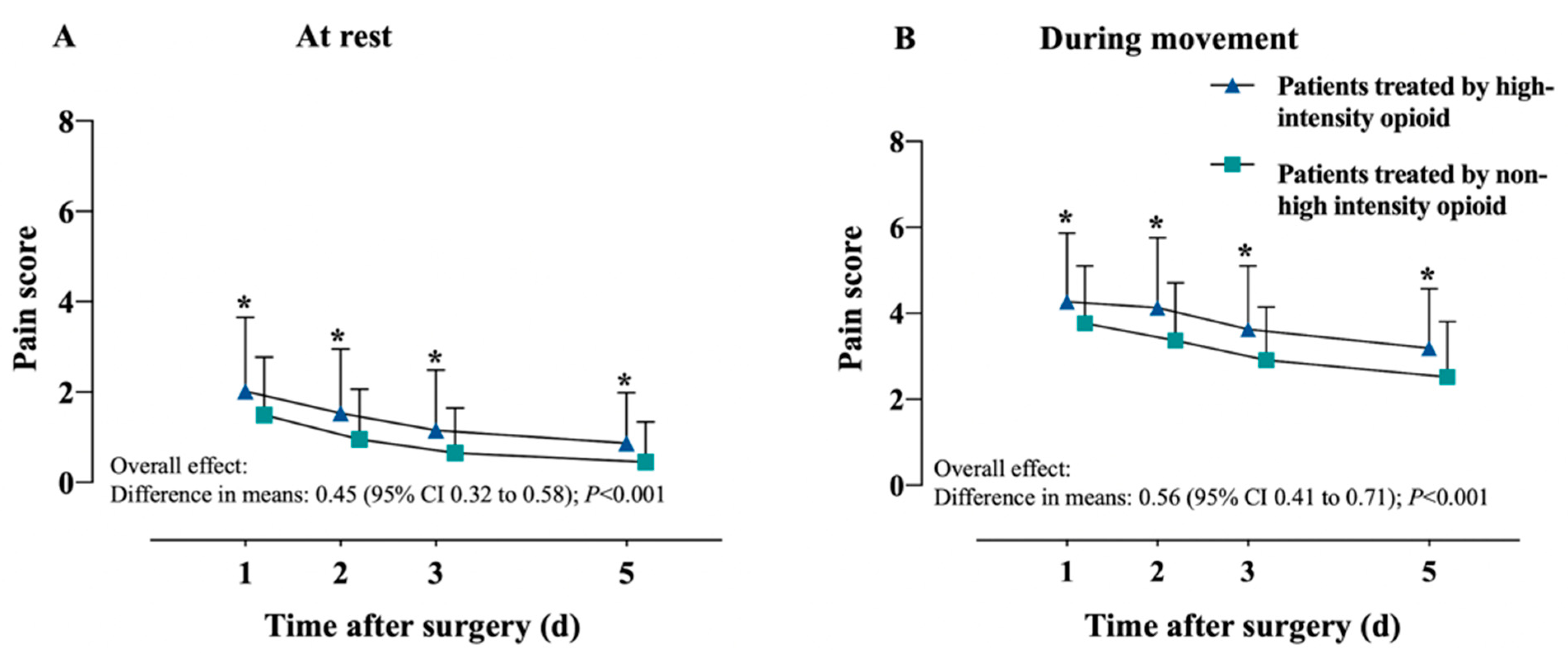
| Pain Scores | Patients Treated by High-Intensity Opioid n = 293 | Patients Treated by Non-High-Intensity Opioid n = 877 | Unadjusted Differences (95% CI) | p | Adjusted Differences (95% CI) ** | p |
|---|---|---|---|---|---|---|
| At rest | ||||||
| Model effect * | ||||||
| Overall effect, marginal mean | 1.77 (1.36) | 1.25 (1.44) | 0.51 (0.42, 0.59) | <0.001 | 0.45 (0.32, 0.58) | <0.001 |
| Time | <0.001 | <0.001 | ||||
| Time × exposure interaction † | 0.410 | 0.514 | ||||
| 1 day, mean ± SD | 2.02 (1.63) | 1.49 (1.28) | 0.53 (0.32, 0.73) | <0.001 | 0.47 (0.26, 0.67) | <0.001 |
| 2 days, mean ± SD | 1.53 (1.42) | 0.95 (1.11) | 0.57 (0.40, 0.75) | <0.001 | 0.51 (0.33, 0.69) | <0.001 |
| 3 days, mean ± SD | 1.15 (1.34) | 0.65 (1.00) | 0.50 (0.33, 0.66) | <0.001 | 0.37 (0.22, 0.51) | <0.001 |
| 5 days, mean ± SD | 0.87 (1.12) | 0.45 (0.89) | 0.42 (0.28, 0.56) | <0.001 | 0.44 (0.27, 0.61) | <0.001 |
| During movement | ||||||
| Model effect * | ||||||
| Overall effect, marginal mean | 3.43 (1.36) | 2.77 (1.23) | 0.66 (0.57, 0.76) | <0.001 | 0.56 (0.41, 0.71) | <0.001 |
| Time | <0.001 | <0.001 | ||||
| Time × exposure interaction † | 0.310 | 0.060 | ||||
| 1 day, mean ± SD | 4.27 (1.60) | 3.77 (1.33) | 0.50 (0.30, 0.71) | <0.001 | 0.40 (0.20, 0.61) | <0.001 |
| 2 days, mean ± SD | 4.13 (1.63) | 3.37 (1.34) | 0.77 (0.56, 0.97) | <0.001 | 0.66 (0.45, 0.87) | <0.001 |
| 3 days, mean ± SD | 3.63 (1.47) | 2.91 (1.24) | 0.71 (0.53, 0.90) | <0.001 | 0.61 (0.42, 0.79) | <0.001 |
| 5 days, mean ± SD | 3.19 (1.38) | 2.52 (1.29) | 0.67 (0.49, 0.85) | <0.001 | 0.57 (0.39, 0.75) | <0.001 |
| Variables | Multivariate Mode | Final Multivariate Model | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age (years) | 1.01 (1.00, 1.02) | 0.142 | ||
| COPD(yes vs. no) | 1.51 (0.75, 2.97) | 0.240 | ||
| Location of surgery | ||||
| Lower abdominal | ref | |||
| Upper abdominal | 0.77 (0.55, 1.08) | 0.134 | ||
| Type of surgery | ||||
| Open | ref | ref | ||
| Laparoscopic | 0.61 (0.42, 0.87) | 0.007 | 0.58 (0.41, 0.81) | 0.002 |
| NSAIDs use at preoperative day 1 (yes vs. no) | 0.07 (0.02, 0.17) | <0.001 | 0.05 (0.02, 0.12) | <0.001 |
| Intraoperative remifentanil | 1.00 (1.00, 1.00) | 0.219 | ||
| Duration of surgery | 1.00 (0.99, 1.00) | 0.264 | ||
| Local infiltration (yes vs. no) | 0.92 (0.66, 1.28) | 0.611 | ||
| NSAIDs use on postoperative day (yes vs. no) | ||||
| 1 | 0.61 (0.22, 1.63) | 0.337 | ||
| 2 | 0.87 (0.30, 2.45) | 0.789 | ||
| 3 | 1.00 (0.09, 7.55) | 0.997 | ||
| 4 | 0.96 (0.20, 7.11) | 0.964 | ||
| 5 | 1.53 (0.48, 6.03) | 0.501 | ||
| Postoperative moderate-to-severe pain * at rest (yes vs. no) | ||||
| Day 1 | 1.42 (0.91, 2.19) | 0.119 | ||
| Day 2 | 1.25 (0.68, 2.29) | 0.473 | ||
| Day 3 | 1.06 (0.48, 2.37) | 0.880 | ||
| Day 5 | 0.80 (0.32, 2.00) | 0.641 | ||
| Postoperative moderate-to-severe pain * during movement (yes vs. no) | ||||
| Day 1 | 1.40 (0.98, 2.00) | 0.061 | 1.44 (1.02, 2.03) | 0.036 |
| Day 2 | 1.78 (1.23, 2.58) | 0.002 | 1.89 (1.32, 2.73) | 0.001 |
| Day 3 | 1.49 (1.04, 2.12) | 0.028 | 1.60 (1.14, 2.26) | 0.007 |
| Day 5 | 2.00 (1.42, 2.81) | <0.001 | 2.07 (1.49, 2.86) | <0.001 |
| Outcome and Postoperative Day | Patients Treated by High-Intensity Opioid n = 293 | Patients Treated by Non-High-Intensity Opioid n = 877 | Crude OR/Median Difference (95% CI) | Adjusted OR/Coefficient # (95% CI) | p |
|---|---|---|---|---|---|
| PONV after surgery, n (%) | |||||
| Day 1 | 31 (10.58) | 168 (19.16) | 0.50 (0.33, 0.74) | 0.50 (0.31, 1.02) | 0.052 |
| Day 2 | 18 (6.14) | 75 (8.55) | 0.70 (0.40, 1.18) | 0.58 (0.31, 1.04) | 0.075 |
| Day 3 | 11 (3.75) | 42 (4.79) | 0.78 (0.38, 1.49) | 0.61 (0.27, 1.29) | 0.211 |
| Day 5 | 30 (10.24) | 41 (4.68) | 2.33(1.41, 3.80) | 1.36 (0.79, 2.33) | 0.263 |
| Pruritus after surgery, n (%) | |||||
| Day 1 | 11 (3.75) | 66 (7.53) | 0.49 (0.24, 0.90) | 0.77 (0.36, 1.54) | 0.478 |
| Day 2 | 5 (1.71) | 31 (3.54) | 0.49 (0.16, 1.16) | 0.66 (0.21, 1.76) | 0.437 |
| Day 3 | 4 (1.37) | 8 (0.91) | 1.53 (0.39, 5.01) | -& | -& |
| Day 5 | 6 (2.05) | 4 (0.46) | 4.85 (1.32 18.40) | 2.94 (0.73, 11.06) | 0.141 |
| Time to first get out of bed, h, median (IQR) | 46.33 (40.00; 63.00) | 41.00 (24.00; 50.00) | 8.83 (6.00, 12.00) | 4.31 (2.81, 6.48) * | 0.001 |
| Urine catheter retention time, h, median (IQR) | 67.12 (43.22; 90.52) | 41.00 (20.00; 64.00) | 24.88 (22.08, 27.42) | 12.26 (8.97, 16.95) * | <0.001 |
| Length of stay after surgery, d, median (IQR) | 8.50 (5.00; 8.50) | 5.10 (3.30; 7.50) | 2.00 (1.60, 2.30) | 1.17 (1.06, 2.07) * | <0.001 |
| Chronic postsurgical pain, n (%) | 113 (38.57) | 240 (27.37) | 1.67 (1.26, 2.20) | 1.54 (1.14, 2.08) | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Li, X.; Chen, Y.; Zeng, H.; Liu, J.; Li, Q. Opioid Dose, Pain, and Recovery following Abdominal Surgery: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 7320. https://doi.org/10.3390/jcm11247320
Chen D, Li X, Chen Y, Zeng H, Liu J, Li Q. Opioid Dose, Pain, and Recovery following Abdominal Surgery: A Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(24):7320. https://doi.org/10.3390/jcm11247320
Chicago/Turabian StyleChen, Dongxu, Xiaoqing Li, Yu Chen, Huolin Zeng, Jin Liu, and Qian Li. 2022. "Opioid Dose, Pain, and Recovery following Abdominal Surgery: A Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 24: 7320. https://doi.org/10.3390/jcm11247320
APA StyleChen, D., Li, X., Chen, Y., Zeng, H., Liu, J., & Li, Q. (2022). Opioid Dose, Pain, and Recovery following Abdominal Surgery: A Retrospective Cohort Study. Journal of Clinical Medicine, 11(24), 7320. https://doi.org/10.3390/jcm11247320





