Bone Modeling after Orthodontic Extrusion: A Histomorphometric Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
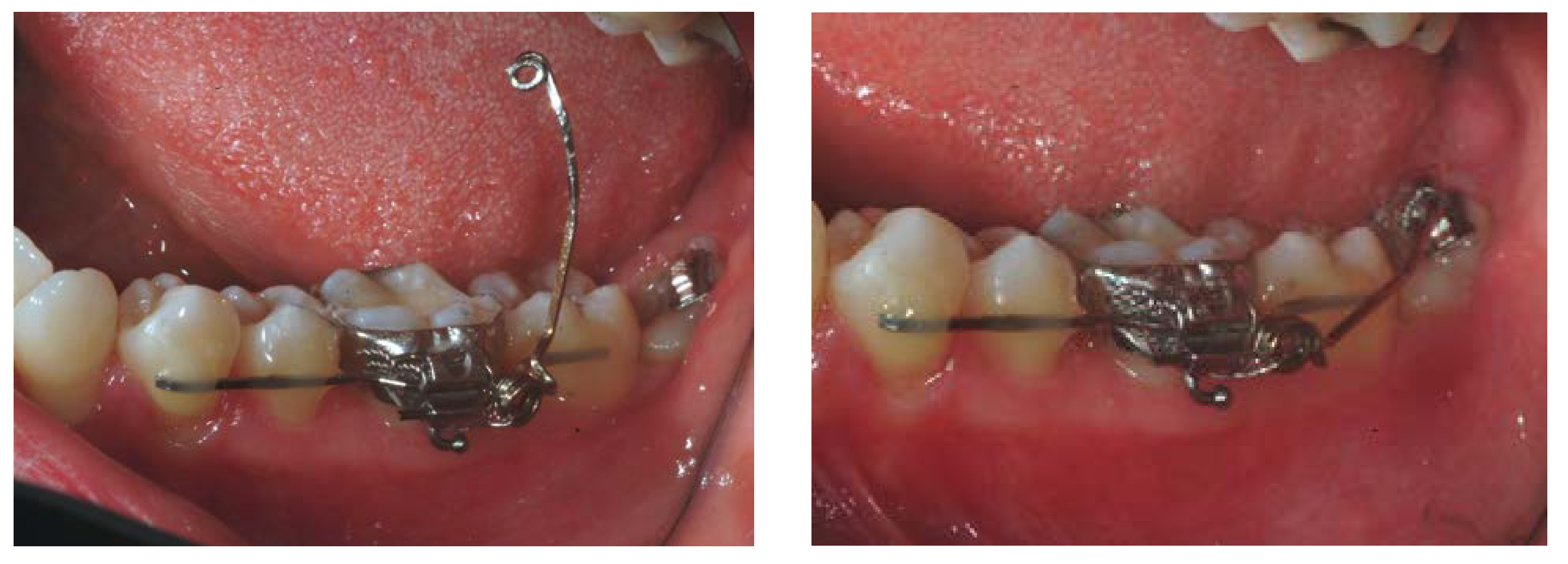
2.1. Clinical Procedures
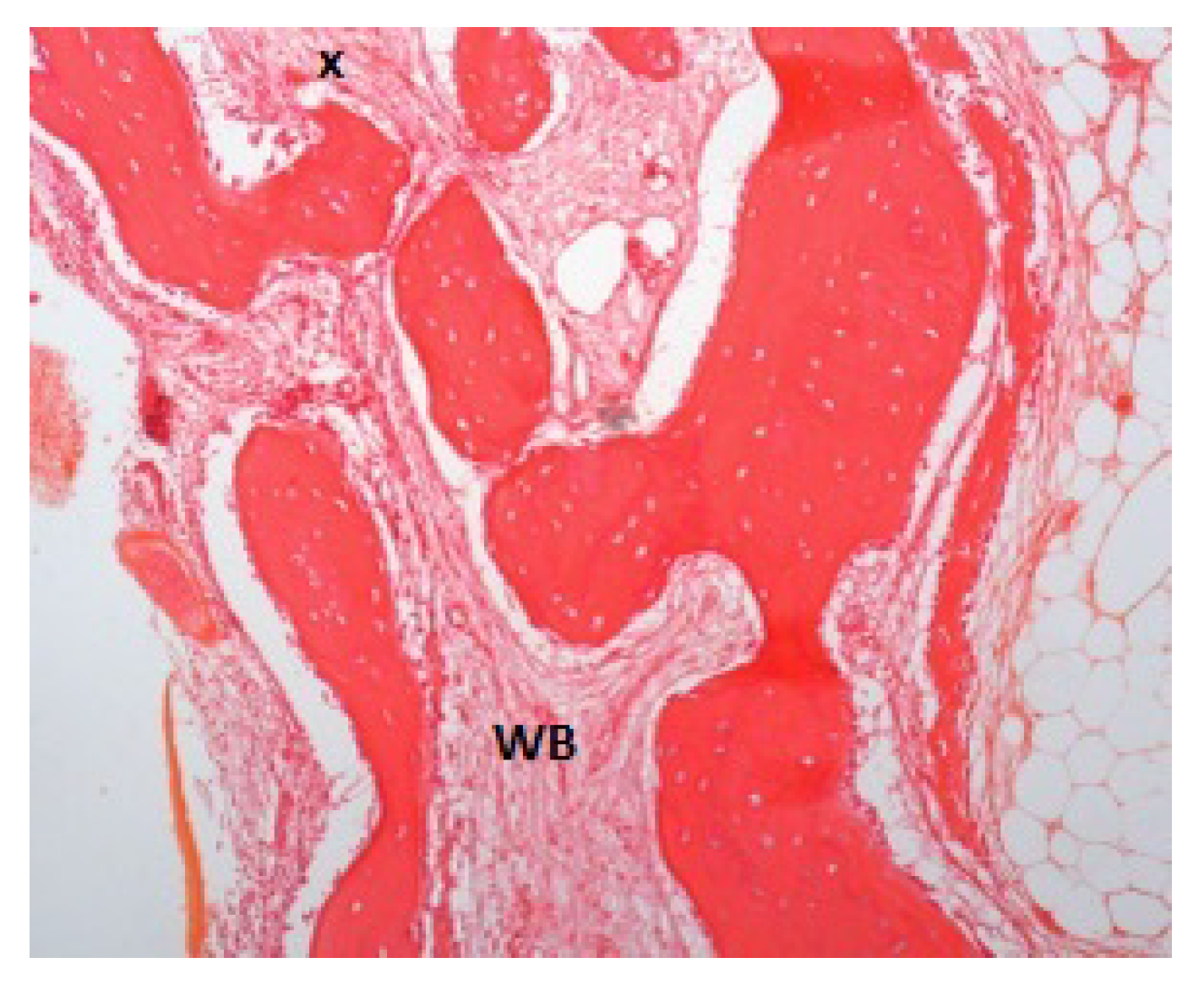
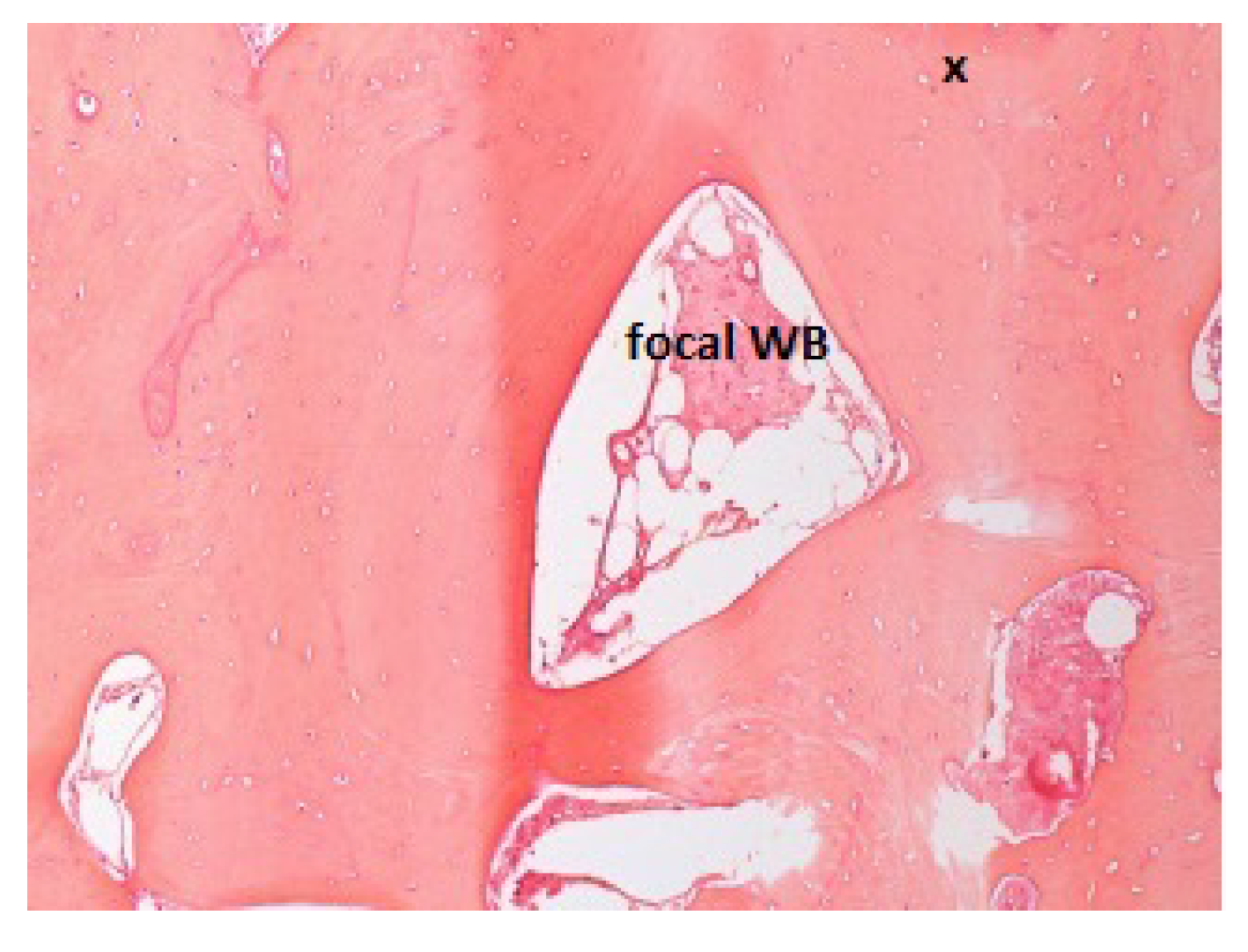
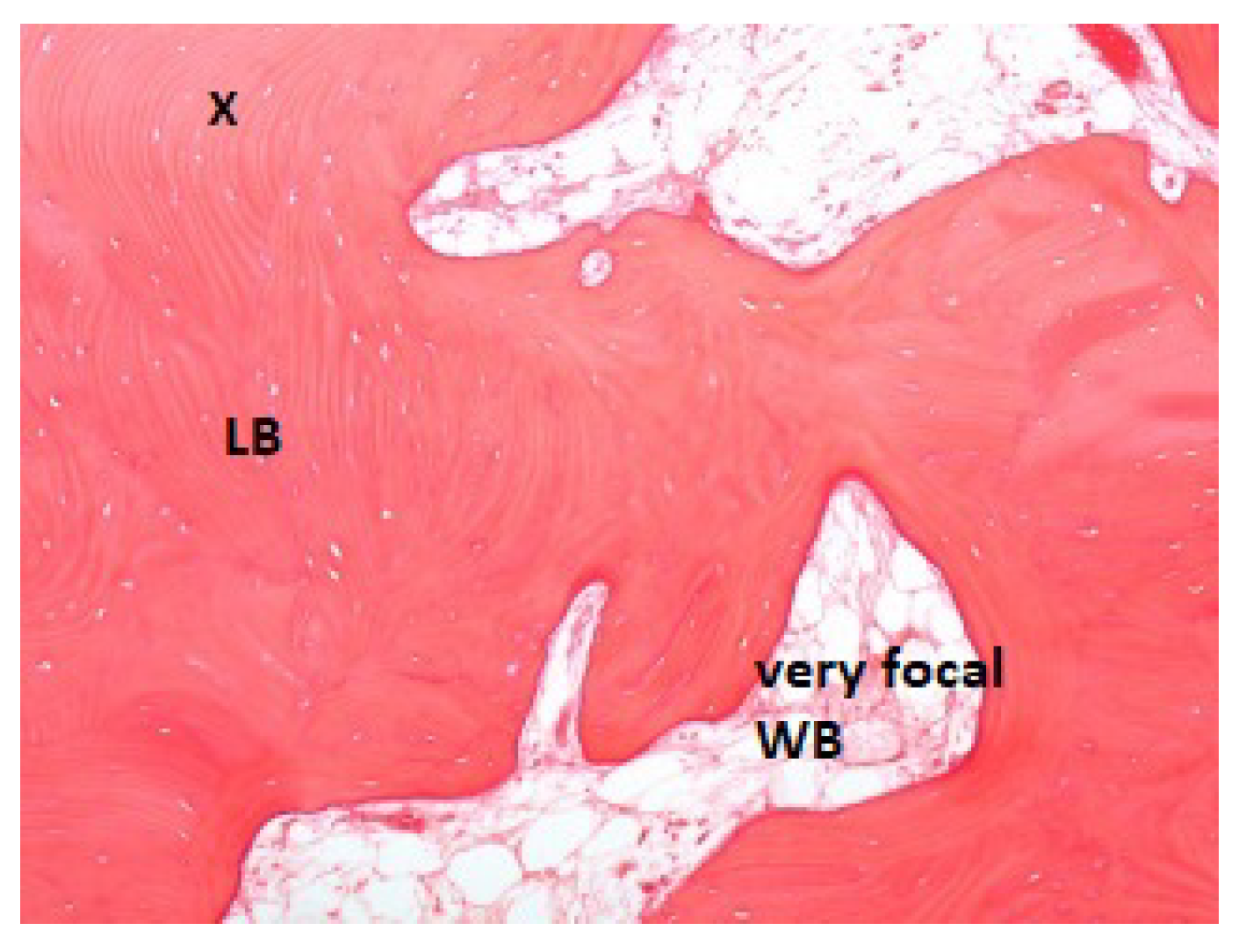
2.2. Histologic Procedures
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Min, Q.; Li, X.; Liu, L.; Lv, Y.; Xu, W.; Liu, X.; Wang, H. Immune System Acts on Orthodontic Tooth Movement: Cellular and Molecular Mechanisms. Biomed. Res. Int. 2022, 2022, 9668610. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhan, Q.; Bao, M.; Yi, J.; Li, Y. Biomechanical and biological responses of periodontium in orthodontic tooth movement: Up-date in a new decade. Int. J. Oral Sci. 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, G.; Cordasco, G.; Perillo, L.; Ramaglia, L. Mechanobiology of the tooth movement during the orthodontic treatment: A literature review. Minerva Stomatol. 2016, 65, 299–327. [Google Scholar] [PubMed]
- Borggaard, X.G.; Nielsen, M.H.; Delaisse, J.M.; Andreasen, C.M.; Andersen, T.L. Spatial Organization of Osteoclastic Coupling Factors and Their Receptors at Human Bone Remodeling Sites. Front. Mol. BioSci. 2022, 9, 896841. [Google Scholar] [CrossRef]
- Baloul, S.S. Osteoclastogenesis and Osteogenesis during Tooth Movement. Front. Oral Biol. 2016, 18, 75–79. [Google Scholar] [CrossRef]
- Shapiro, F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur. Cell Mater. 2008, 15, 53–76. [Google Scholar] [CrossRef]
- Chen, J.; Hendriks, M.; Chatzis, A.; Ramasamy, S.K.; Kusumbe, A.P. Bone Vasculature and Bone Marrow Vascular Niches in Health and Disease. J. Bone Min. Res. 2020, 35, 2103–2120. [Google Scholar] [CrossRef]
- Meeran, N.A. Cellular response within the periodontal ligament on application of orthodontic forces. J. Indian Soc. Periodontol. 2013, 17, 16–20. [Google Scholar] [CrossRef]
- Schröder, A.; Bauer, K.; Spanier, G.; Proff, P.; Wolf, M.; Kirschneck, C. Expression kinetics of human periodontal ligament fibroblasts in the early phases of orthodontic tooth movement. J. Orofac. Orthop. 2018, 79, 337–351. [Google Scholar] [CrossRef]
- Isola, G.; Nucera, R.; Damonte, S.; Ugolini, A.; De Mari, A.; Migliorati, M. Implant Site Changes in Three Different Clinical Approaches: Orthodontic Extrusion, Regenerative Surgery and Spontaneous Healing after Extraction: A Systematic Review. J. Clin. Med. 2022, 11, 6347. [Google Scholar] [CrossRef]
- Alsahhaf, A.; Att, W. Orthodontic extrusion for pre-implant site enhancement: Principles and clinical guidelines. J. Prosthodont Res. 2016, 60, 145–155. [Google Scholar] [CrossRef]
- Checchi, L.; Alessandri Bonetti, G.; Pelliccioni, G.A. Removing high-risk impacted mandibular third molars: A surgical–orthodontic approach. J. Am. Dent. Assoc. 1996, 127, 1214–1217. [Google Scholar] [PubMed]
- Alessandri Bonetti, G.; Bendandi, M.; Laino, L.; Checchi, V.; Checchi, L. Orthodontic extraction: Riskless extraction of impacted lower third molars close to the mandibular canal. J. Oral Maxillofac. Surg. 2007, 65, 2580–2586. [Google Scholar] [CrossRef] [PubMed]
- Montevecchi, M.; Incerti Parenti, S.; Checchi, V.; Palumbo, B.; Checchi, L.; Alessandri Bonetti, G. Periodontal healing after ‘orthodontic extraction’ of mandibular third molars: A retrospective cohort study. Int. J. Oral Maxillofac. Surg. 2014, 43, 1137–1141. [Google Scholar] [PubMed]
- Chiapasco, M.; Lang, N.P. Quality and quantity of bone following alveolar distraction osteogenesis in the human mandible. Clin. Oral Impl. Res. 2006, 17, 394–402. [Google Scholar] [CrossRef]
- Zaffe, D.; Bertoldi, C.; Palumbo, C. Morphofunctional and clinical study on mandibular alveolar distraction osteogenesis. Clin. Oral Impl. Res. 2002, 13, 550–557. [Google Scholar] [CrossRef]
- Van Venrooy, J.R.; Yukna, R.A. Orthodontic extrusion of single-rooted teeth affected with advanced periodontal disease. Am. J. Orthod. 1985, 87, 67–74. [Google Scholar] [CrossRef]
- Berglundh, T.; Marinello, C.P.; Lindhe, J.; Thilander, B.; Liljenberg, B. Periodontal tissue reactions to orthodontic extrusion. An experimental study in dog. J. Clin. Periodontol. 1991, 18, 330–336. [Google Scholar] [CrossRef]
- Ingber, J.S. Forced eruption. II. A method of treating non restorable teeth –periodontal and restorative considerations. J. Periodontol. 1976, 47, 203–216. [Google Scholar] [CrossRef]
- Felippe, L.A.; Monteiro, J.S.; Vieira, L.C.; Araujo, E. Reestablishing biologic width with forced eruption. Quintessence Int. 2003, 34, 733–738. [Google Scholar]
- Montevecchi, M.; Checchi, V.; Alessandri Bonetti, G. Management of a deeply impacted mandibular third molar and associated large dentigerous cyst to avoid nerve injury and improve periodontal healing: Case report. J. Can. Dent. Assoc. 2012, 78, c59. [Google Scholar] [PubMed]
- Huang, G.; Yang, M.; Qali, M.; Wang, T.J.; Li, C.; Chang, Y.C. Clinical Considerations in Orthodontically Forced Eruption for Restorative Purposes. J. Clin. Med. 2021, 10, 5950. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Corinaldesi, G.; Pieri, F.; Degidi, M.; Piattelli, A. Alveolar distraction osteogenesis for bone augmentation of severely atrophic ridges in 10 consecutive cases: A histologic and histomorphometric study. J. Periodontol. 2007, 78, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Cardaropoli, G.; Araujo, M.; Lindhe, J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J. Clin. Periodontol. 2003, 30, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Checchi, V.; Savarino, L.; Montevecchi, M.; Felice, P.; Checchi, L. Clinical radiographic and histological evaluation of two hydroxyapatites in human extraction sockets: A pilot study. Int. J. Oral Maxillofac. Surg. 2011, 40, 526–532. [Google Scholar] [CrossRef]
- Luongo, R.; Tallarico, M.; Canciani, E.; Graziano, D.; Dellavia, C.; Gargari, M.; Ceruso, F.M.; Melodia, D.; Canullo, L. Histomorphometry of Bone after Intentionally Exposed Non-Resorbable d-PTFE Membrane or Guided Bone Regeneration for the Treatment of Post-Extractive Alveolar Bone Defects with Implant-Supported Restorations: A Pilot Randomized Controlled Trial. Materials 2022, 15, 5838. [Google Scholar] [CrossRef]
- Zhuang, L.; Bai, Y.; Meng, X. Three-dimensional morphology of root and alveolar bone during tooth movement using micro-computed tomography. Angle Orthod. 2011, 81, 420–425. [Google Scholar]
- Irie, M.S.; Rabelo, G.D.; Spin-Neto, R.; Dechichi, P.; Borges, J.S.; Soares, P.B.F. Use of Micro-Computed Tomography for Bone Evaluation in Dentistry. Braz Dent. J. 2018, 29, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Hassumi, J.S.; Mulinari-Santos, G.; Fabris, A.L.D.S.; Jacob, R.G.M.; Gonçalves, A.; Rossi, A.C.; Freire, A.R.; Faverani, L.P.; Okamoto, R. Alveolar bone healing in rats: Micro-CT, immunohistochemical and molecular analysis. J. Appl. Oral Sci. 2018, 26, e20170326. [Google Scholar] [CrossRef]
- Okata, H.; Nakamura, M.; Henmi, A.; Yamaguchi, S.; Mikami, Y.; Shimauchi, H.; Sasano, Y. Calcification during bone healing in a standardised rat calvarial defect assessed by micro-CT and SEM-EDX. Oral Dis. 2015, 21, 74–82. [Google Scholar]
- Proffit, W.R.; Fields, H.W.; Sarver, D.M. Contemporary Orthodontics, 5th ed.; Elsevier: Philadelphia, PA, USA, 2013. [Google Scholar]
- Adel-Khattab, D.; Afifi, N.S.; El Sadat, S.M.A.; Aboul-Fotouh, M.N.; Tarek, K.; Horowitz, R.A. Bone regeneration and graft material resorption in extraction sockets grafted with bioactive silica-calcium phosphate composite (SCPC) versus non-grafted sockets: Clinical, radiographic, and histological findings. J. Periodontal Implant. Sci. 2020, 50, 418–434. [Google Scholar] [CrossRef] [PubMed]
- Keil, C.; Gollmer, B.; Zeidler-Rentzsch, I.; Gredes, T.; Heinemann, F. Histological evaluation of extraction sites grafted with Bio-Oss Collagen: Randomized controlled trial. Ann. Anat 2021, 237, 151722. [Google Scholar] [CrossRef] [PubMed]
- Cannizzaro, G.; Felice, P.; Leone, M.; Checchi, V.; Esposito, M. Flapless versus open flap implant surgery in partially edentulous patients subject to immediate loading: 1 year results from a split-mouth randomised controlled trial. Eur. J. Oral Implant. 2011, 4, 177–188. [Google Scholar]
- Borzabadi-Farahani, A.; Zadeh, H.H. Adjunctive Orthodontic Applications in Dental Implantology. J. Oral Implant. 2015, 41, 501–508. [Google Scholar] [CrossRef]
- Amato, F.; Mirabella, A.D.; Macca, U.; Tarnow, D.P. Implant site development by orthodontic forced extraction: A preliminary study. Int. J. Oral Maxillofac. Implant. 2012, 7, 411–420. [Google Scholar]
| Patient Id n = 9 | Age | Tooth Number | Tooth Inclination | Smoke | Drugs |
|---|---|---|---|---|---|
| A | 21 | 48 | Horizontal | No | No |
| 38 | Vertical | ||||
| B | 21 | 48 | Vertical | No | Birth-control pill |
| C | 28 | 48 | Vertical | No | Birth-control pill |
| D | 30 | 48 | Horizontal | No | Levothyroxine sodium |
| E | 31 | 38 | Horizontal | No | No |
| F | 35 | 38 | Vertical | No | No |
| 48 | Vertical | ||||
| G | 39 | 48 | Vertical | Yes(5 cig/die) | No |
| H | 45 | 38 | Vertical | No | No |
| 48 | Vertical | ||||
| I | 62 | 38 | Vertical | No | No |
| Bone Parameters | Median Percentage (%) | Interquartile Range (%) |
|---|---|---|
| Bone tissue (%) | 100 | 92.5–100 |
| Connective tissue (%) | 0 | 0–7.5 |
| Mineralized bone (%) | 80 | 70–90 |
| Osteoid tissue (%) | 10 | 6.25–27.5 |
| Woven bone (%) | 15 | 2.5–37, 5 |
| Lamellar bone (%) | 69.5 | 15–80 |
| Trabecular bone (%) | 0 | 0–0 |
| Bone marrow (%) | 0 | 0–0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montevecchi, M.; Marucci, G.; Pignataro, B.; Piana, G.; Alessandri-Bonetti, G.; Checchi, V. Bone Modeling after Orthodontic Extrusion: A Histomorphometric Pilot Study. J. Clin. Med. 2022, 11, 7329. https://doi.org/10.3390/jcm11247329
Montevecchi M, Marucci G, Pignataro B, Piana G, Alessandri-Bonetti G, Checchi V. Bone Modeling after Orthodontic Extrusion: A Histomorphometric Pilot Study. Journal of Clinical Medicine. 2022; 11(24):7329. https://doi.org/10.3390/jcm11247329
Chicago/Turabian StyleMontevecchi, Marco, Gianluca Marucci, Barbara Pignataro, Gabriela Piana, Giulio Alessandri-Bonetti, and Vittorio Checchi. 2022. "Bone Modeling after Orthodontic Extrusion: A Histomorphometric Pilot Study" Journal of Clinical Medicine 11, no. 24: 7329. https://doi.org/10.3390/jcm11247329








