A Smart Glove Digital System Promotes Restoration of Upper Limb Motor Function and Enhances Cortical Hemodynamic Changes in Subacute Stroke Patients with Mild to Moderate Weakness: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. RAPAEL® Smart Glove Digital System
2.4. Intervention Protocols
2.5. Outcome Measures
2.5.1. Primary Outcome: Motor Function
2.5.2. Secondary Outcome: Cortical Activation Changes in the Motor Cortical Regions
2.6. Statistical Analysis
3. Results
3.1. Participants Characteristics
3.2. Primary Outcome Results
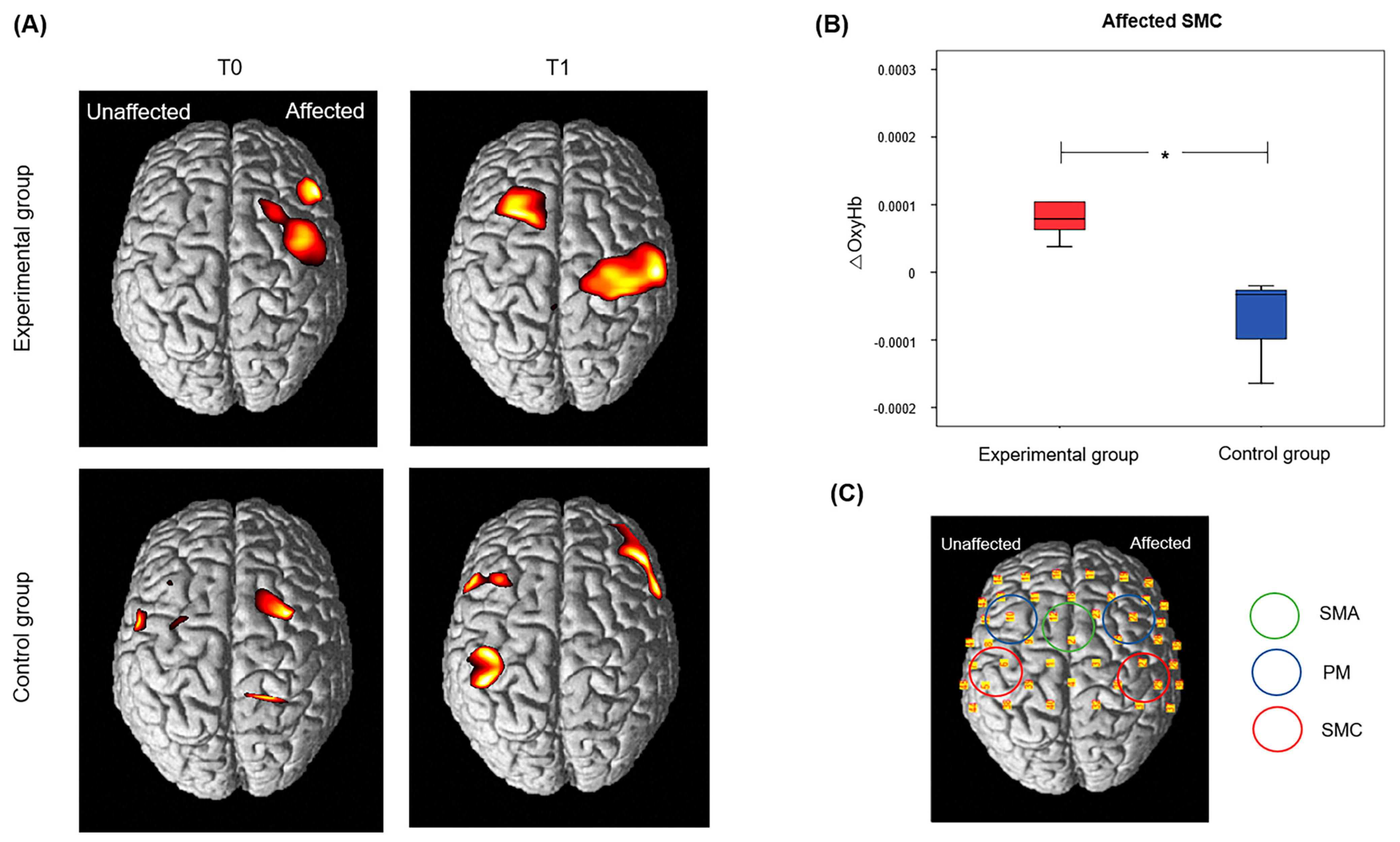
3.3. Secondary Outcome Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kernan, W.N.; Ovbiagele, B.; Black, H.R.; Bravata, D.M.; Chimowitz, M.I.; Ezekowitz, M.D.; Fang, M.C.; Fisher, M.; Furie, K.L.; Heck, D.V.; et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2160–2236. [Google Scholar] [CrossRef] [PubMed]
- Colomer, C.; Baldovi, A.; Torrome, S.; Navarro, M.D.; Moliner, B.; Ferri, J.; Noe, E. Efficacy of Armeo(R) Spring during the chronic phase of stroke. Study in mild to moderate cases of hemiparesis. Neurologia 2013, 28, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Sale, P.; Lombardi, V.; Franceschini, M. Hand robotics rehabilitation: Feasibility and preliminary results of a robotic treatment in patients with hemiparesis. Stroke Res.Treat. 2012, 2012, 820931. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, S.R.; Krakauer, J.W. The interaction between training and plasticity in the poststroke brain. Curr. Opin. Neurol. 2013, 26, 609–616. [Google Scholar] [CrossRef]
- Bernhardt, J.; Godecke, E.; Johnson, L.; Langhorne, P. Early rehabilitation after stroke. Curr. Opin. Neurol. 2017, 30, 48–54. [Google Scholar] [CrossRef]
- Coleman, E.R.; Moudgal, R.; Lang, K.; Hyacinth, H.I.; Awosika, O.O.; Kissela, B.M.; Feng, W. Early Rehabilitation After Stroke: A Narrative Review. Curr. Atheroscler. Rep. 2017, 19, 59. [Google Scholar] [CrossRef]
- Nudo, R.J. Neural bases of recovery after brain injury. J. Commun. Disord. 2011, 44, 515–520. [Google Scholar] [CrossRef]
- Song, G.B. The effects of task-oriented versus repetitive bilateral arm training on upper limb function and activities of daily living in stroke patients. J. Phys. Ther. Sci. 2015, 27, 1353–1355. [Google Scholar] [CrossRef]
- Giuliani, C. Constraint-induced movement therapy early after stroke improves rate of upper limb motor recovery but not long-term motor function. J. Physiother. 2015, 61, 95. [Google Scholar] [CrossRef][Green Version]
- Kim, J.; Yim, J. Effects of High-Frequency Repetitive Transcranial Magnetic Stimulation Combined with Task-Oriented Mirror Therapy Training on Hand Rehabilitation of Acute Stroke Patients. Med. Sci. Monit. 2018, 24, 743–750. [Google Scholar] [CrossRef]
- Choi, Y.H.; Paik, N.J. Mobile Game-based Virtual Reality Program for Upper Extremity Stroke Rehabilitation. J. Vis. Exp. 2018, 133, e56241. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Lee, K.J.; Song, C.H. Game-Based Virtual Reality Canoe Paddling Training to Improve Postural Balance and Upper Extremity Function: A Preliminary Randomized Controlled Study of 30 Patients with Subacute Stroke. Med. Sci. Monit. 2018, 24, 2590–2598. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; Lee, B.H. Effect of Virtual Reality-based Bilateral Upper Extremity Training on Upper Extremity Function after Stroke: A Randomized Controlled Clinical Trial. Occup. Ther. Int. 2016, 23, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.R.; Wang, P.; Xing, L.; Mei, L.P.; Zhao, J.; Zhang, T. Leap Motion-based virtual reality training for improving motor functional recovery of upper limbs and neural reorganization in subacute stroke patients. Neural Regen. Res. 2017, 12, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Yao, Z.; Harp, K.; Gwon, D.Y.; Chen, Z.; Siu, K.C. Effects of virtual reality in the early-stage stroke rehabilitation: A systematic review and meta-analysis of randomized controlled trials. Physiother. Theory Pract. 2022, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Nef, T.; Mihelj, M.; Riener, R. ARMin: A robot for patient-cooperative arm therapy. Med. Biol. Eng. Comput. 2007, 45, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Masiero, S.; Celia, A.; Rosati, G.; Armani, M. Robotic-assisted rehabilitation of the upper limb after acute stroke. Arch. Phys. Med. Rehabil. 2007, 88, 142–149. [Google Scholar] [CrossRef]
- Yap, H.K.; Lim, J.H.; Nasrallah, F.; Yeow, C.H. Design and Preliminary Feasibility Study of a Soft Robotic Glove for Hand Function Assistance in Stroke Survivors. Front. Neurosci. 2017, 11, 547. [Google Scholar] [CrossRef]
- Biggar, S.; Yao, W. Design and Evaluation of a Soft and Wearable Robotic Glove for Hand Rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. A Publ. IEEE Eng. Med. Biol. Soc. 2016, 24, 1071–1080. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, M.Y.; Lee, J.Y.; Jeon, Y.J.; Kim, S.; Lee, S.; Seo, B.; Choi, Y. Effects of virtual reality-based rehabilitation on distal upper extremity function and health-related quality of life: A single-blinded, randomized controlled trial. J. Neuroeng. Rehabil. 2016, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Caeiro-Rodríguez, M.; Otero-González, I.; Mikic-Fonte, F.A.; Llamas-Nistal, M. A Systematic Review of Commercial Smart Gloves: Current Status and Applications. Sensors 2021, 21, 2667. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lim, J.H.; Jeon, B.H.; Song, C.S. Non-immersive Virtual Reality Rehabilitation Applied to a Task-oriented Approach for Stroke Patients: A Randomized Controlled Trial. Restor. Neurol. Neurosci. 2020, 38, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.J.; Ku, K.H.; Park, Y.S.; Park, J.G.; Cho, E.S.; Seo, J.S.; Kim, C.W.; O, S.H. Effects of Virtual Reality-Based Rehabilitation on Upper Extremity Function among Children with Cerebral Palsy. Healthcare 2020, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Leff, D.R.; Orihuela-Espina, F.; Elwell, C.E.; Athanasiou, T.; Delpy, D.T.; Darzi, A.W.; Yang, G.Z. Assessment of the cerebral cortex during motor task behaviours in adults: A systematic review of functional near infrared spectroscopy (fNIRS) studies. NeuroImage 2011, 54, 2922–2936. [Google Scholar] [CrossRef]
- Jalalvandi, M.; Riyahi Alam, N.; Sharini, H.; Hashemi, H.; Nadimi, M. Brain Cortical Activation during Imagining of the Wrist Movement Using Functional Near-Infrared Spectroscopy (fNIRS). J. Biomed. Phys. Eng. 2021, 11, 583–594. [Google Scholar] [CrossRef]
- Cui, X.; Bray, S.; Bryant, D.M.; Glover, G.H.; Reiss, A.L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. NeuroImage 2011, 54, 2808–2821. [Google Scholar] [CrossRef]
- Lloyd-Fox, S.; Blasi, A.; Elwell, C.E. Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010, 34, 269–284. [Google Scholar] [CrossRef]
- Lundquist, C.B.; Maribo, T. The Fugl-Meyer assessment of the upper extremity: Reliability, responsiveness and validity of the Danish version. Disabil. Rehabil. 2017, 39, 934–939. [Google Scholar] [CrossRef]
- Allgower, K.; Hermsdorfer, J. Fine motor skills predict performance in the Jebsen Taylor Hand Function Test after stroke. Clin. Neurophysiol. 2017, 128, 1858–1871. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, I.S.; Han, T.R. New Scoring System for Jebsen Hand Function Test. J. Korean Acad. Rehabil. Med. 2007, 31, 623–629. [Google Scholar]
- Ye, J.C.; Tak, S.; Jang, K.E.; Jung, J.; Jang, J. NIRS-SPM: Statistical parametric mapping for near-infrared spectroscopy. NeuroImage 2009, 44, 428–447. [Google Scholar] [CrossRef] [PubMed]
- Cope, M.; Delpy, D.T. System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination. Med. Biol. Eng. Comput. 1988, 26, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Hensel, L.; Tscherpel, C.; Freytag, J.; Ritter, S.; Rehme, A.K.; Volz, L.J.; Eickhoff, S.B.; Fink, G.R.; Grefkes, C. Connectivity-Related Roles of Contralesional Brain Regions for Motor Performance Early after Stroke. Cereb. Cortex 2021, 31, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.; Markert, J.; Sauseng, P.; Hoppe, J.; Plewnia, C.; Gerloff, C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J. Neurosci. 2006, 26, 6096–6102. [Google Scholar] [CrossRef]
- Oujamaa, L.; Relave, I.; Froger, J.; Mottet, D.; Pelissier, J.Y. Rehabilitation of arm function after stroke. Literature review. Ann. Phys. Rehabil. Med. 2009, 52, 269–293. [Google Scholar] [CrossRef]
- Bergfeldt, U.; Jonsson, T.; Bergfeldt, L.; Julin, P. Cortical activation changes and improved motor function in stroke patients after focal spasticity therapy--an interventional study applying repeated fMRI. BMC Neurol. 2015, 15, 52. [Google Scholar] [CrossRef]
- Kaelin-Lang, A.; Luft, A.R.; Sawaki, L.; Burstein, A.H.; Sohn, Y.H.; Cohen, L.G. Modulation of human corticomotor excitability by somatosensory input. J. Physiol. 2002, 540, 623–633. [Google Scholar] [CrossRef]
- Merians, A.S.; Tunik, E.; Adamovich, S.V. Virtual reality to maximize function for hand and arm rehabilitation: Exploration of neural mechanisms. Stud. Health Technol. Inform. 2009, 145, 109–125. [Google Scholar]
- Forlim, C.G.; Bittner, L.; Mostajeran, F.; Steinicke, F.; Gallinat, J.; Kuhn, S. Stereoscopic Rendering via Goggles Elicits Higher Functional Connectivity During Virtual Reality Gaming. Front. Hum. Neurosci. 2019, 13, 365. [Google Scholar] [CrossRef]
- Kim, W.S.; Cho, S.; Ku, J.; Kim, Y.; Lee, K.; Hwang, H.J.; Paik, N.J. Clinical Application of Virtual Reality for Upper Limb Motor Rehabilitation in Stroke: Review of Technologies and Clinical Evidence. J. Clin. Med. 2020, 9, 3369. [Google Scholar] [CrossRef] [PubMed]
- Everard, G.; Declerck, L.; Detrembleur, C.; Leonard, S.; Bower, G.; Dehem, S.; Lejeune, T. New technologies promoting active upper limb rehabilitation after stroke. An overview and network meta-analysis. Eur. J. Phys. Rehabil. Med. 2022, 58, 530–548. [Google Scholar] [CrossRef] [PubMed]
- Morone, G.; Cocchi, I.; Paolucci, S.; Iosa, M. Robot-assisted therapy for arm recovery for stroke patients: State of the art and clinical implication. Expert Rev. Med. Devices 2020, 17, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Maclean, N.; Pound, P.; Wolfe, C.; Rudd, A. Qualitative analysis of stroke patients’ motivation for rehabilitation. BMJ 2000, 321, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Lansberg, M.G.; Legault, C.; MacLellan, A.; Parikh, A.; Muccini, J.; Mlynash, M.; Kemp, S.; Buckwalter, M.S.; Flavin, K. Home-based virtual reality therapy for hand recovery after stroke. PM&R 2022, 14, 320–328. [Google Scholar] [CrossRef]
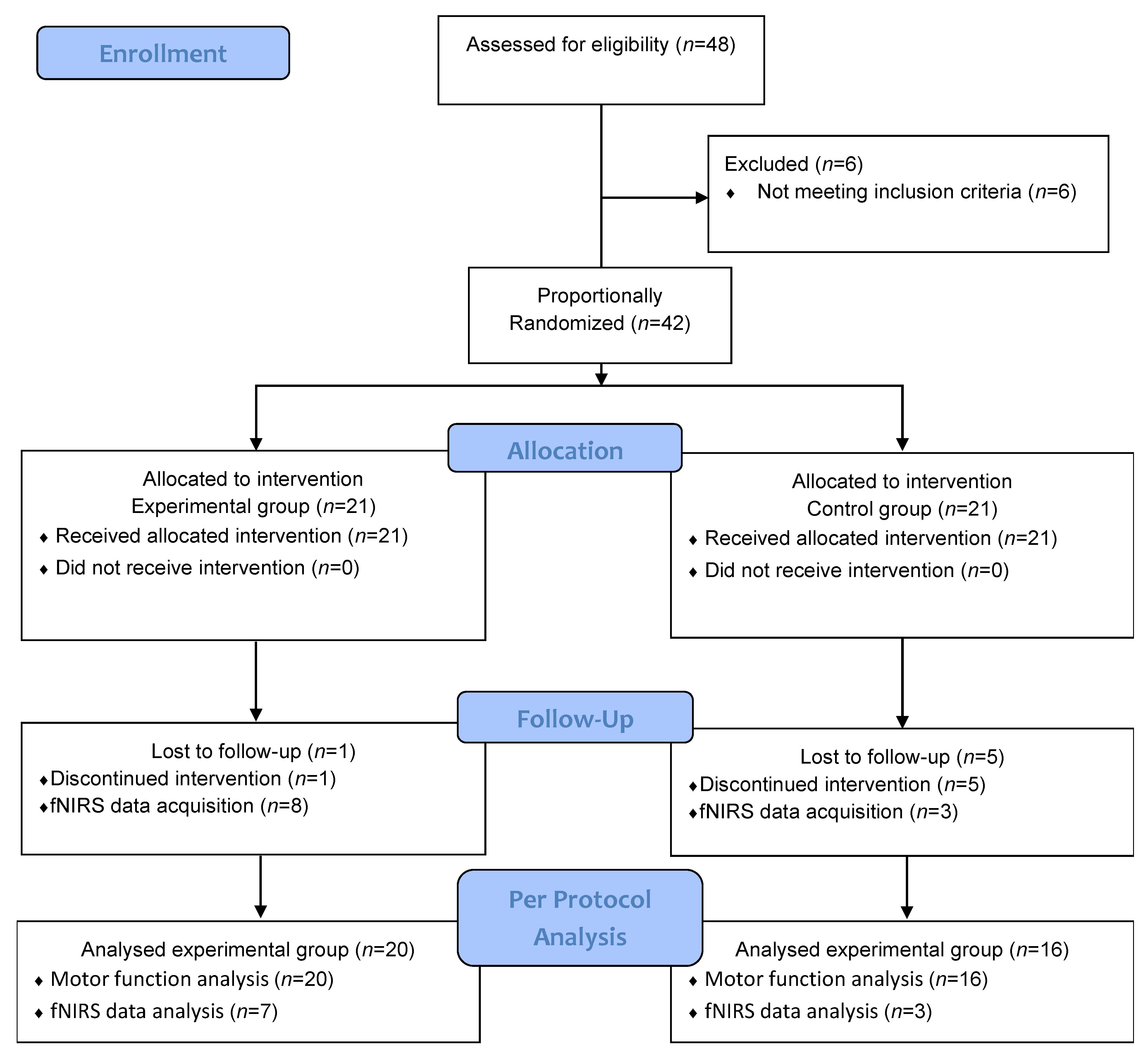

| Characteristic | Experimental Group (n = 20) | Control Group (n = 16) | p-Value |
|---|---|---|---|
| Sex (male: female) | 10:10 | 7:9 | 0.709 b |
| Age (years) | 57.00 (12.78) | 63.69 (8.58) | 0.070 a |
| Stroke onset duration (days) | 24.70 (16.26) | 34.00 (25.49) | 0.336 c |
| Stroke type | |||
| Ischemic: Hemorrhagic | 11:09 | 13:03 | 0.097 b |
| Side of stroke | |||
| Right: left | 13:07 | 9:07 | 0.593 b |
| Upper extremity function | |||
| UFMA, total | 41.30 (8.90) | 37.13 (12.84) | 0.258 a |
| JTT, total | 12.00 (15.29) | 8.25 (11.93) | 0.290 c |
| Spasticity of upper extremity | |||
| MAS 0: MAS 1 | 18:02 | 15:01 | 0.585 b |
| K-MMSE | 24.95 (3.97) | 25.31 (3.57) | 0.778 a |
| Variables | Experimental Group (n = 20) | Control Group (n = 16) | Time × Group Interaction | ||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | p-Value | |
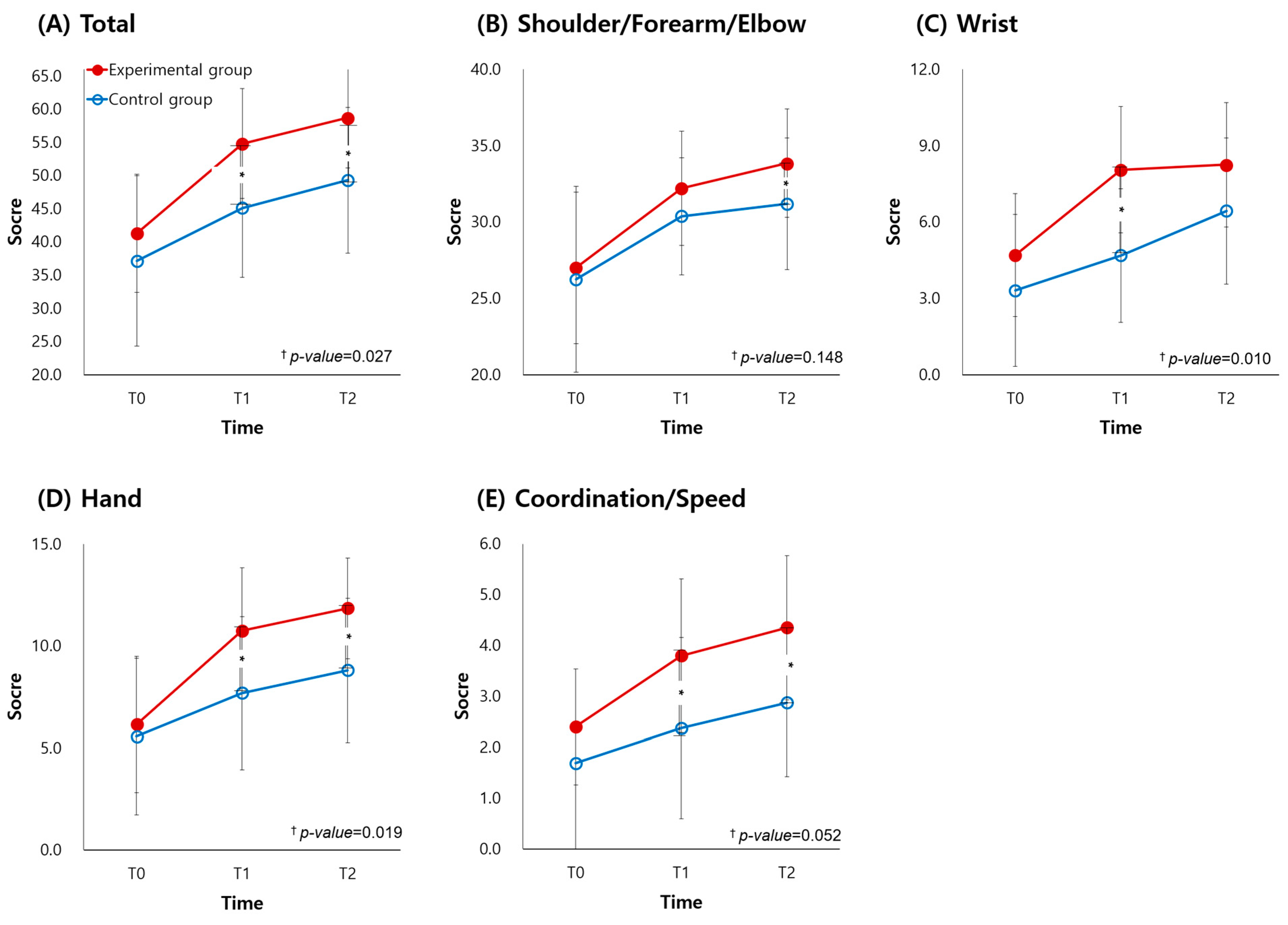
| UFMA, total | 41.30 (8.90) | 54.80 (8.27) a | 58.70 (7.53) b,c | 37.13 (12.84) | 45.13(10.44) a | 49.32 (10.98) b,c | 0.027 * |
| UFMA, subscore | |||||||
| Shoulder/Elbow/Forearm | 27.00 (4.95) | 32.20 (3.73) a | 33.85 (3.54) b,c | 26.25 (6.10) | 30.38 (3.85) a | 31.19 (4.31) b | 0.148 |
| Wrist | 4.70 (2.41) | 8.05 (2.48) a | 8.25 (2.45) b | 3.31 (2.98) | 4.69 (2.63) a | 6.44 (2.87) b,c | 0.010 * |
| Hand | 6.15 (3.34) | 10.75 (3.09) a | 11.85 (2.48) b,c | 5.56 (3.85) | 7.69(3.75) a | 8.81 (3.54) b,c | 0.019 * |
| Coordination/Speed | 2.40 (1.14) | 3.80 (1.51) a | 4.35 (1.42) b,c | 1.69 (1.85) | 2.38 (1.78) a | 2.88 (1.45) b | 0.052 |
| JTT, total | 12.00 (15.29) | 33.10 (21.12) a | 40.90 (23.52) b,c | 8.25 (11.93) a | 13.88 (14.62) | 18.00 (15.41) b,c | 0.004 ** |
| JTT, subscore | |||||||
| Writing | 3.05 (4.32) | 7.50 (5.24) a | 8.85 (5.13) b,c | 1.81 (3.43) | 4..38 (4.02) a | 5.38 (4.47) b,c | 0.180 |
| Simulated page turning | 0.45 (0.76) | 1.90 (1.78) a | 2.95 (2.06) b,c | 0.63 (1.54) | 1.01 (1.77) a | 1.38 (1.89) b | 0.003 ** |
| Picking up small objects | 0.75 (1.77) | 3.55 (3.19) a | 4.75 (3.70) b,c | 0.69 (1.30) | 1.19 (1.80) | 1.69 (2.12) b | 0.004 ** |
| Simulated feeding | 1.90 (2.97) | 5.90 (4.25) a | 7.40 (4.80) b,c | 1.50 (2.81) | 2.13 (3.61) | 2.69 (3.57) b | 0.001 ** |
| Stacking checkers | 1.90 (2.59) | 5.75 (3.88) a | 6.75 (3.93) b | 1.63 (2.96) | 2.25 (3.59) | 2.88 (3.67) b | 0.002 ** |
| Picking up large light objects | 1.90 (3.11) | 4.45 (3.49) a | 5.35 (4.17) b,c | 0.94 (1.48) | 1.38 (1.71) a | 1.88 (2.00) b | 0.008 ** |
| Picking up large heavy objects | 2.05 (3.10) | 4.05 (3.44) a | 4.85 (3.83) b | 1.06 (1.53) | 1.50 (1.79) a | 2.13 (1.89) b | 0.038 * |
| Variables | T1-T0 | T2-T1 | ||||
|---|---|---|---|---|---|---|
| Experimental | Control | p-Value | Experimental | Control | p-Value | |
| UFMA, total | 13.50 (7.49) | 8.00 (4.44) | 0.014 * | 3.90 (2.55) | 4.19 (3.71) | 0.488 |
| UFMA, subscore | ||||||
| Shoulder/Elbow/Forearm | 5.20 (3.22) | 4.13 (2.75) | 0.297 | 1.65 (1.79) | 0.81 (1.91) | 0.362 |
| Wrist | 3.35 (2.25) | 1.38 (1.36) | 0.024 * | 0.20 (1.15) | 1.75 (1.44) | 0.001 ** |
| Hand | 4.60 (3.68) | 2.13 (1.86) | 0.043 * | 1.10 (1.33) | 1.13 (1.09) | 0.716 |
| Coordination/Speed | 1.40 (1.14) | 0.69 (0.79) | 0.048 * | 0.55 (0.69) | 0.50 (0.89) | 0.587 |
| JTT, total | 21.10 (20.84) | 5.63 (5.06) | 0.012 * | 7.80 (6.21) | 4.13 (3.84) | 0.073 |
| JTT, subscore | ||||||
| Writing | 4.45 (5.06) | 2.56 (3.24) | 0.374 | 1.35 (1.53) | 1.00 (1.10) | 0.519 |
| Simulated page turning | 1.45 (1.85) | 0.44 (0.51) | 0.083 | 1.05 (1.36) | 0.31 (0.60) | 0.075 |
| Picking up small objects | 2.80 (3.16) | 0.50 (1.10) | 0.004 ** | 1.20 (1.15) | 0.50 (0.73) | 0.044 * |
| Simulated feeding | 4.00 (4.12) | 0.63 (1.26) | 0.003 ** | 1.50 (1.82) | 0.56 (1.21) | 0.067 |
| Stacking checkers | 3.85 (4.04) | 0.63 (1.50) | 0.001 ** | 1.00 (1.81) | 0.63 (0.96) | 0.647 |
| Picking up large light objects | 2.55 (2.72) | 0.44 (0.51) | 0.004 ** | 0.90 (1.33) | 0.50 (0.73) | 0.451 |
| Picking up large heavy objects | 2.00 (2.68) | 0.44 (0.51) | 0.041 * | 0.80 (1.32) | 0.63 (1.15) | 0.713 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Lee, H.-J.; Chang, W.H.; Ko, S.H.; Shin, Y.-I.; Kim, Y.-H. A Smart Glove Digital System Promotes Restoration of Upper Limb Motor Function and Enhances Cortical Hemodynamic Changes in Subacute Stroke Patients with Mild to Moderate Weakness: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 7343. https://doi.org/10.3390/jcm11247343
Shin S, Lee H-J, Chang WH, Ko SH, Shin Y-I, Kim Y-H. A Smart Glove Digital System Promotes Restoration of Upper Limb Motor Function and Enhances Cortical Hemodynamic Changes in Subacute Stroke Patients with Mild to Moderate Weakness: A Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(24):7343. https://doi.org/10.3390/jcm11247343
Chicago/Turabian StyleShin, Seyoung, Hwang-Jae Lee, Won Hyuk Chang, Sung Hwa Ko, Yong-Il Shin, and Yun-Hee Kim. 2022. "A Smart Glove Digital System Promotes Restoration of Upper Limb Motor Function and Enhances Cortical Hemodynamic Changes in Subacute Stroke Patients with Mild to Moderate Weakness: A Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 24: 7343. https://doi.org/10.3390/jcm11247343
APA StyleShin, S., Lee, H.-J., Chang, W. H., Ko, S. H., Shin, Y.-I., & Kim, Y.-H. (2022). A Smart Glove Digital System Promotes Restoration of Upper Limb Motor Function and Enhances Cortical Hemodynamic Changes in Subacute Stroke Patients with Mild to Moderate Weakness: A Randomized Controlled Trial. Journal of Clinical Medicine, 11(24), 7343. https://doi.org/10.3390/jcm11247343







