Clinicopathological Features of Intrathoracic Liposarcoma—A Systematic Review with an Illustrative Case
Abstract
:1. Introduction
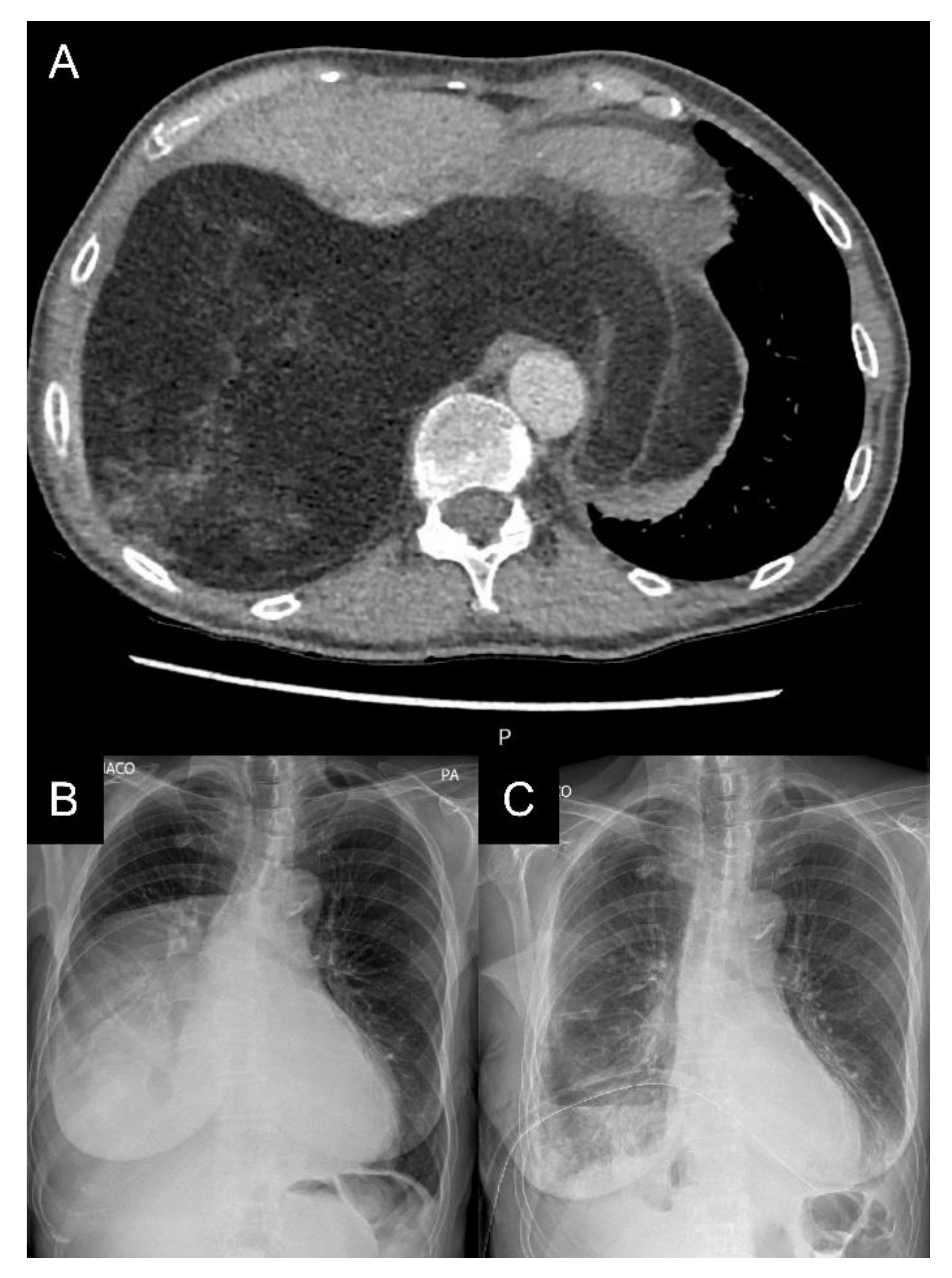
2. Case Report
3. Materials and Methods
4. Results
5. Discussion
5.1. Clinical Course
5.2. Radiological Imaging
5.3. Treatment
5.4. Pathology
| Reference | Histology | S100 | Vimentin | Desmin | SMA | CD31 | CD34 | CD36 | MDM2 | CDK4 | p16 | HHF-35 | EMA | CK | BCL2 | CD99 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [47] | WDLPS | 1 | 1 | 1 | ||||||||||||
| [48] | WDLPS | 1 | 1 | |||||||||||||
| [49] | WDLPS | 1 | ||||||||||||||
| [49] | WDLPS | 0 | 0 | 1 | ||||||||||||
| [49] | WDLPS | 1 | 1 | 1 | ||||||||||||
| [50] | WDLPS | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | ||||||
| [51] | WDLPS | 1 | 1 | 0 | 1 | 1 | ||||||||||
| [52] | WDLPS | 0 | 0 | |||||||||||||
| [53] | WDLPS | 1 | 1 | 1 | ||||||||||||
| [21] | WDLPS | 0 | 1 | 0 | 0 | 1 | ||||||||||
| [54] | WDLPS | 1 | 1 | 1 | ||||||||||||
| [55] | WDLPS | 1 | ||||||||||||||
| [56] | WDLPS | 1 | ||||||||||||||
| [57] | WDLPS | 1 | 1 | |||||||||||||
| [58] | WDLPS | 1 | 1 | |||||||||||||
| [59] | WDLPS | 1 | 1 | 1 | ||||||||||||
| [60] | WDLPS | 1 | ||||||||||||||
| [61] | WDLPS | 1 | 1 | 1 | ||||||||||||
| [61] | WDLPS | 1 | 1 | |||||||||||||
| [62] | WDLPS | 1 | ||||||||||||||
| [63] | DDLPS | 1 | 1 | 1 | 1 | |||||||||||
| [64] | DDLPS | 1 | 0 | 0 | 0 | 1 | 1 | 0 | ||||||||
| [65] | DDLPS | 0 | 0 | 0 | ||||||||||||
| [66] | DDLPS | 1 | 1 | |||||||||||||
| [67] | DDLPS | 0 | 0 | 1 | 0 | 0 | 0 | 0 | ||||||||
| [68] | DDLPS | 1 | 1 | |||||||||||||
| [49] | DDLPS | 0 | 0 | 0 | 1 | |||||||||||
| [49] | DDLPS | 0 | 1 | 1 | 1 | |||||||||||
| [69] | DDLPS | 1 | 1 | |||||||||||||
| [70] | DDLPS | 1 | 1 | |||||||||||||
| [71] | DDLPS | 1 | 1 | 1 | 1 | |||||||||||
| [6] | DDLPS | 1 | 1 | 0 | ||||||||||||
| [72] | DDLPS | 1 | 1 | |||||||||||||
| [73] | DDLPS | 1 | 1 | 1 | ||||||||||||
| [74] | DDLPS | 1/0 | 1 | 0 | 0 | 1 | 0 | |||||||||
| [75] | DDLPS | 1 | ||||||||||||||
| [76] | DDLPS | 1 | ||||||||||||||
| [77] | DDLPS | 1 | 1 | 1 | 0 | 1 | ||||||||||
| [78] | DDLPS | 0 | 1 | 1 | 1 | 1 | 1 | |||||||||
| [79] | MLPS | 1 | 1 | 0 | 0 | |||||||||||
| [80] | MLPS | 1 | 1 | 0 | 0 | |||||||||||
| [81] | MLPS | 1 | 1 | 0 | 0 | 1 | ||||||||||
| [82] | MLPS | 1 | 0 | |||||||||||||
| [82] | MLPS | 1 | 0 | |||||||||||||
| [49] | MLPS | 1 | 0 | 0 | ||||||||||||
| [49] | MLPS | 1 | 0 | 0 | ||||||||||||
| [49] | MLPS | 1 | 0 | 0 | ||||||||||||
| [83] | MLPS | 0 | 1 | 0 | 1 | 0 | 0 | 0 | ||||||||
| [84] | PLPS | 1 | 0 | 0 | 0 | 0 | 0 | |||||||||
| [85] | PLPS | 1 | 1 | 1 | 0 |
5.5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thway, K. Well-Differentiated Liposarcoma and Dedifferentiated Liposarcoma: An Updated Review. Semin. Diagn. Pathol. 2019, 36, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Thway, K.; Jordan, S.; Fisher, C.; Nicholson, A.G. Updates in the Approach to Intrathoracic Sarcomas. Histopathology 2015, 67, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. The CARE Guidelines: Consensus-Based Clinical Case Reporting Guideline Development. Glob. Adv. Health Med. 2013, 2, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Jahangirifard, A.; Ahmadi, Z.H.; Kakhaki, A.D.; Farzanegan, B.; Sheikhy, K. ECMO-Assisted Resection of Huge Thoracic Mass. J. Cardiovasc. Thorac. Res. 2018, 10, 174–176. [Google Scholar] [CrossRef]
- Soeroso, N.N.; Pradana, A.; Djaka, M.; Ayudika, M.; Ngadimin, S.; Soeroso, L. An Unusual Case of Recurrent Huge Primary Mediastinal Dedifferentiated Liposarcoma. Int. J. Surg. Case Rep. 2018, 50, 140–143. [Google Scholar] [CrossRef]
- Ashraf, U.; Dudekula, R.A.; Roy, S.; Burack, J.; Malik, S.; Khaja, M. Recurrent Intrathoracic Dedifferentiated Liposarcoma: A Case Report and Literature Review. Respir. Med. Case Rep. 2019, 26, 281–284. [Google Scholar] [CrossRef]
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and Perspectives. Pathologica 2020, 113, 70–84. [Google Scholar] [CrossRef]
- Kallen, M.E.; Hornick, J.L. The 2020 WHO Classification: What’s New in Soft Tissue Tumor Pathology? Am. J. Surg. Pathol. 2020, 45, e1–e23. [Google Scholar] [CrossRef]
- Vos, M.; Grünhagen, D.J.; Koseła-Paterczyk, H.; Rutkowski, P.; Sleijfer, S.; Verhoef, C. Natural History of Well-Differentiated Liposarcoma of the Extremity Compared to Patients Treated with Surgery. Surg. Oncol. 2019, 29, 84–89. [Google Scholar] [CrossRef]
- Dillman, J.R.; Pernicano, P.G.; McHugh, J.B.; Attili, A.K.; Mourany, B.; Pinsky, R.W.; Strouse, P.J.; Kazerooni, E.A. Cross-Sectional Imaging of Primary Thoracic Sarcomas with Histopathologic Correlation: A Review for the Radiologist. Curr. Probl. Diagn. Radiol. 2010, 39, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Roeser, A.; Chauveau, S.; De Montpreville, V.T.; Sadoun, D.; Pradere, P.; Brillet, P.Y.; Nunes, H.; Jebri, S.; Uzunhan, Y. Pulmonary Artery Sarcoma: A Differential Diagnosis of Persistent Pulmonary Embolism. Respir. Med. Res. 2021, 80, 100842. [Google Scholar] [CrossRef] [PubMed]
- Baheti, A.D.; Sewatkar, R.; Hornick, J.L.; Saboo, S.S.; Jagannathan, J.P.; Ramaiya, N.H.; Tirumani, S.H. Imaging Features of Primary and Recurrent Intrathoracic Synovial Sarcoma: A Single-Institute Experience. Clin. Imaging 2015, 39, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, J.; Chen, M.; Liu, W.; He, X. Diagnosis and Prognosis of Retroperitoneal Liposarcoma: A Single Asian Center Cohort of 57 Cases. J. Oncol. 2021, 2021, 7594027. [Google Scholar] [CrossRef] [PubMed]
- Hallifax, R.J.; Talwar, A.; Wrightson, J.M.; Edey, A.; Gleeson, F.V. State-of-the-Art: Radiological Investigation of Pleural Disease. Respir. Med. 2017, 124, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, M.; Liu, W.; Yuan, Y.; Yu, F. Successful Removal of Giant Mediastinal Lipoma and Liposarcoma Involving Both Chest Cavities. Medicine 2018, 97, e11806. [Google Scholar] [CrossRef]
- Koseła-Paterczyk, H.; Wągrodzki, M. Liposarcoma—Spectrum of Disease. Oncol. Clin. Pract. 2019, 14, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Yimin, N.; He, Z.; Chen, X. Giant Mediastinal Liposarcoma Resected by Median Sternotomy: A Case Report. Transl. Cancer Res. 2020, 9, 6522–6527. [Google Scholar] [CrossRef]
- Park, J.W.; Jeong, W.G.; Lee, J.E.; Lee, H.; Ki, S.Y.; Lee, B.C.; Kim, H.O.; Kim, S.K.; Heo, S.H.; Lim, H.S.; et al. Pictorial Review of Mediastinal Masses with an Emphasis on Magnetic Resonance Imaging. Korean J. Radiol. 2021, 22, 139–154. [Google Scholar] [CrossRef]
- Gaikwad, N.M.; Srikrishna, S.V.; Srikanth, K. A Rare Case of a Giant Anterior Mediastinal Liposarcoma. Indian J. Thorac. Cardiovasc. Surg. 2019, 36, 148–150. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Bai, C.-Y.; Li, Z.-C.; Li, W.-J.; Li, Y. Giant Primary Liposarcoma of the Anterior Mediastinum. Medicine 2018, 97, e12873. [Google Scholar] [CrossRef] [PubMed]
- Taki, K.; Watanabe, M.; Iwagami, S.; Nagai, Y.; Iwatsuki, M.; Ishimoto, T.; Baba, Y.; Miyamoto, Y.; Baba, H. Giant Liposarcoma of the Posterior Mediastinum and Retroperitoneum. BMJ Case Rep. 2011, 2011, bcr0620114341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homsy, P.; Heiskanen, I.; Sampo, M.; Rönty, M.; Tukiainen, E.; Blomqvist, C. Single Centre 30-year Experience in Treating Retroperitoneal Liposarcomas. J. Surg. Oncol. 2020, 122, 1163–1172. [Google Scholar] [CrossRef]
- Lee, H.S.; Yu, J.I.; Lim, D.H.; Kim, S.J. Retroperitoneal Liposarcoma: The Role of Adjuvant Radiation Therapy and the Prognostic Factors. Radiat. Oncol. J. 2016, 34, 216–222. [Google Scholar] [CrossRef]
- Bonvalot, S.; Gronchi, A.; Le Péchoux, C.; Swallow, C.J.; Strauss, D.; Meeus, P.; van Coevorden, F.; Stoldt, S.; Stoeckle, E.; Rutkowski, P.; et al. Preoperative Radiotherapy plus Surgery versus Surgery Alone for Patients with Primary Retroperitoneal Sarcoma (EORTC-62092: STRASS): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2020, 21, 1366–1377. [Google Scholar] [CrossRef]
- Chung, P.W.M.; Deheshi, B.M.; Ferguson, P.C.; Wunder, J.S.; Griffin, A.M.; Catton, C.N.; Bell, R.S.; White, L.M.; Kandel, R.A.; O’Sullivan, B. Radiosensitivity Translates into Excellent Local Control in Extremity Myxoid Liposarcoma. Cancer 2009, 115, 3254–3261. [Google Scholar] [CrossRef]
- Li, H.; Wozniak, A.; Sciot, R.; Cornillie, J.; Wellens, J.; Van Looy, T.; Vanleeuw, U.; Stas, M.; Hompes, D.; Debiec-Rychter, M.; et al. Pazopanib, a Receptor Tyrosine Kinase Inhibitor, Suppresses Tumor Growth through Angiogenesis in Dedifferentiated Liposarcoma Xenograft Models. Transl. Oncol. 2014, 7, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Saponara, M.; Stacchiotti, S.; Gronchi, A. Pharmacological Therapies for Liposarcoma. Expert Rev. Clin. Pharmacol. 2017, 10, 361–377. [Google Scholar] [CrossRef]
- Schöffski, P. Established and Experimental Systemic Treatment Options for Advanced Liposarcoma. Oncol. Res. Treat. 2022, 45, 525–543. [Google Scholar] [CrossRef]
- Setola, E.; Noujaim, J.; Benson, C.; Chawla, S.; Palmerini, E.; Jones, R.L. Eribulin in Advanced Liposarcoma and Leiomyosarcoma. Expert Rev. Anticancer Ther. 2017, 17, 717–723. [Google Scholar] [CrossRef]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus Dacarbazine in Previously Treated Patients with Advanced Liposarcoma or Leiomyosarcoma: A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesne, A.L.; Cresta, S.; Maki, R.G.; Blay, J.Y.; Verweij, J.; Poveda, A.; Casali, P.G.; Balaña, C.; Schöffski, P.; Grosso, F.; et al. A Retrospective Analysis of Antitumour Activity with Trabectedin in Translocation-Related Sarcomas. Eur. J. Cancer 2012, 48, 3036–3044. [Google Scholar] [CrossRef] [PubMed]
- Grosso, F.; Jones, R.L.; Demetri, G.D.; Judson, I.R.; Blay, J.-Y.; Le Cesne, A.; Sanfilippo, R.; Casieri, P.; Collini, P.; Dileo, P.; et al. Efficacy of Trabectedin (Ecteinascidin-743) in Advanced Pretreated Myxoid Liposarcomas: A Retrospective Study. Lancet Oncol. 2007, 8, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.; Kim, J.E.; Kim, W.; Lee, J.-S.; Song, S.Y.; Lee, M.H.; Chung, H.W.; Cho, K.-J.; Song, J.S.; Ahn, J.-H. Clinicopathologic Characteristics and Clinical Outcome of Localized Liposarcoma: A Single-Center Experience over 25 Years and Evaluation of PD-L1 Expression. Cancer Res. Treat. 2022, 54, 579–589. [Google Scholar] [CrossRef]
- Resag, A.; Toffanin, G.; Benešová, I.; Müller, L.; Potkrajcic, V.; Ozaniak, A.; Lischke, R.; Bartunkova, J.; Rosato, A.; Jöhrens, K.; et al. The Immune Contexture of Liposarcoma and Its Clinical Implications. Cancers 2022, 14, 4578. [Google Scholar] [CrossRef]
- Haddox, C.L.; Riedel, R.F. Recent Advances in the Understanding and Management of Liposarcoma. Fac. Rev. 2021, 10, 1. [Google Scholar] [CrossRef]
- Sugiyama, K.; Washimi, K.; Sato, S.; Hiruma, T.; Sakai, M.; Okubo, Y.; Miyagi, Y.; Yokose, T. Differential Diagnosis of Lipoma and Atypical Lipomatous Tumor/Well-differentiated Liposarcoma by Cytological Analysis. Diagn. Cytopathol. 2022, 50, 112–122. [Google Scholar] [CrossRef]
- Cornillie, J.; Wozniak, A.; Li, H.; Gebreyohannes, Y.K.; Wellens, J.; Hompes, D.; Debiec-Rychter, M.; Sciot, R.; Schöffski, P. Anti-Tumor Activity of the MDM2-TP53 Inhibitor BI-907828 in Dedifferentiated Liposarcoma Patient-Derived Xenograft Models Harboring MDM2 Amplification. Clin. Transl. Oncol. 2019, 22, 546–554. [Google Scholar] [CrossRef]
- Nishio, J.; Nakayama, S.; Nabeshima, K.; Yamamoto, T. Biology and Management of Dedifferentiated Liposarcoma: State of the Art and Perspectives. J. Clin. Med. 2021, 10, 3230. [Google Scholar] [CrossRef]
- Altun, E.; Yuksel, S.; Kaygusuz, G.; Yildiz, H.Y. Diagnostic Importance of Clinicopathologic Features and P16, CD34, MDM2 Expression in Differential Diagnosis of Adipocytic Tumors. Acta Orthop. Traumatol. Turc. 2020, 54, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Scapa, J.V.; Cloutier, J.M.; Raghavan, S.S.; Peters-Schulze, G.; Varma, S.; Charville, G.W. DDIT3 Immunohistochemistry Is a Useful Tool for the Diagnosis of Myxoid Liposarcoma. Am. J. Surg. Pathol. 2020, 45, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.S.; Bass, D.; Chen, I.Y.; Thomas, R.; Velez, M.J.; Hobbs, S.K. Imaging and Clinical Findings in a Series of Six Cases of Rare Primary Mediastinal Liposarcoma. Radiol. Cardiothorac. Imaging 2022, 4. [Google Scholar] [CrossRef]
- Lee, A.T.J.; Thway, K.; Huang, P.H.; Jones, R.L. Clinical and Molecular Spectrum of Liposarcoma. J. Clin. Oncol. 2018, 36, 151–159. [Google Scholar] [CrossRef]
- Hassan, I.; Park, S.Z.; Donohue, J.H.; Nagorney, D.M.; Kay, P.A.; Nasciemento, A.G.; Schleck, C.D.; Ilstrup, D.M. Operative Management of Primary Retroperitoneal Sarcomas. Ann. Surg. 2004, 239, 244–250. [Google Scholar] [CrossRef]
- Vos, M.; Boeve, W.C.; van Ginhoven, T.M.; Sleijfer, S.; Verhoef, C.; Grünhagen, D.J. Impact of Primary Tumor Location on Outcome of Liposarcoma Patients, a Retrospective Cohort Study. Eur. J. Surg. Oncol. 2019, 45, 2437–2442. [Google Scholar] [CrossRef]
- Chaput-Dugas, M.-E.; Chughtai, T.; Liberman, M.; Duranceau, A.; Martin, J.; Barkat, F.; Ferraro, P. Successful Resection of a Large Incidentally Found Primary Mediastinal Liposarcoma. J. Surg. Case Rep. 2010, 2010, 1. [Google Scholar] [CrossRef] [Green Version]
- Billè, A.; Garofalo, G.; Leo, F.; Pastorino, U. Giant Liposarcoma Elongating Mediastinal Vessels with Intrathoracic Inferior Vena Cava Replacement. Eur. J. Cardiothorac. Surg. 2013, 44, 570–572. [Google Scholar] [CrossRef]
- Ortega, P.; Suster, D.; Falconieri, G.; Zambrano, E.; Moran, C.A.; Morrison, C.; Suster, S. Liposarcomas of the Posterior Mediastinum: Clinicopathologic Study of 18 Cases. Mod. Pathol. 2014, 28, 721–731. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Zhang, H.-M.; Zhang, L.-W.; Zheng, M.-W.; Yu, M. Primary Mediastinal Giant Liposarcoma with Smooth Muscle and Neural Differentiation: A Case Report. Oncol. Lett. 2015, 9, 2667–2669. [Google Scholar] [CrossRef]
- Mani, V.R.; Ofikwu, G.; Safavi, A. Surgical Resection of a Giant Primary Liposarcoma of the Anterior Mediastinum. J. Surg. Case Rep. 2015, 2015, rjv126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiura, Y.; Hashizume, T.; Fujimoto, H.; Nemoto, E. A Giant Mediastinal Liposarcoma Weighing 3500 g Resected with Clam Shell Approach, a Case Report with Review of Literature. Int. J. Surg. Case Rep. 2017, 41, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Edagawa, M.; Haratake, N.; Shimamatsu, S.; Toyozawa, R.; Nosaki, K.; Hirai, F.; Yamaguchi, M.; Taguchi, K.; Kaneko, K.; Seto, T.; et al. Surgical Resection of a Well-Differentiated Inflammatory Liposarcoma of the Middle Mediastinum: A Case Report. J. Thorac. Dis. 2017, 9, E689–E693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, X.; Li, M.; Xia, Y. A Huge Mediastinal, Well-Differentiated Liposarcoma with Heterogenous Smooth Muscle Differentiation: A Case Report. Int. J. Clin. Exp. Pathol. 2019, 12, 2763–2766. [Google Scholar] [PubMed]
- Stolten, M.; Sahasrabudhe, D.; Constine, L. Liposarcoma of the Left Hemithorax and Implications of MDM2. J. Radiother. Pract. 2019, 18, 397–399. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S.; Shi, H.; Li, W.; Wei, Z. Resection of a Huge Mediastinal Well-Differentiated Liposarcoma Involving Left Thoracic Cavity. J. Cardiothorac. Surg. 2019, 14, 148. [Google Scholar] [CrossRef]
- Kang, L.H.; Hwang, C.S.; Yoon, S.H. Primary Pleural Liposarcoma Combined Spindle Cell Lipoma of the Lung. Thorac. Cancer 2020, 11, 2059–2062. [Google Scholar] [CrossRef]
- Iwamoto, N.; Matsuura, Y.; Ninomiya, H.; Ichinose, J.; Nakao, M.; Ishikawa, Y.; Okumura, S.; Mun, M. An Extremely Rare Case of Rapidly Growing Mediastinal Well-Differentiated Liposarcoma with a Sclerosing Variant: A Case Report. Surg. Case Rep. 2020, 6, 158. [Google Scholar] [CrossRef]
- Furlan, K.; Miller, I.; Rohra, P.; Mir, F.; Ocampo Gonzalez, F.A.; Gattuso, P. Well-Differentiated Liposarcoma Primary from Thymic Stroma: Case Report and Literature Review. Exp. Mol. Pathol. 2020, 116, 104517. [Google Scholar] [CrossRef]
- Siblani, D.; Kanj, M.; Ghorra, C.; Haddad, Y.; Jomaa, M.; Mansour, Z. A Liposarcoma Arising in a Pulmonary Hamartoma, Coexisting with Benign Metastasizing Leiomyoma. Ann. Thorac. Surg. 2022, 113, e203–e205. [Google Scholar] [CrossRef]
- Weissferdt, A.; Moran, C.A. Lipomatous Tumors of the Anterior Mediastinum with Muscle Differentiation: A Clinicopathological and Immunohistochemical Study of Three Cases. Virchows Arch. 2014, 464, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Ouadnouni, Y.; Serraj, M.; Ghalimi, J.; Lakranbi, M.; Smahi, M. Primary Mediastinal Liposarcoma. Ann. Afr. Surg. 2015, 12, 59–61. [Google Scholar]
- Takanami, I.; Imamura, T. Dedifferentiated Liposarcoma of the Pleura: Report of a Case. Surg. Today 2005, 35, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Benchetritt, M.; Hofman, V.; Vénissac, N.; Brennetot, C.; Italiano, A.; Aurias, A.; Padovani, B.; Pedeutour, F.; Hofman, P. Dedifferentiated Liposarcoma of the Pleura Mimicking a Malignant Solitary Fibrous Tumor and Associated with Dedifferentiated Liposarcoma of the Mediastinum: Usefulness of Cytogenetic and Molecular Genetic Analyses. Cancer Genet. Cytogenet. 2007, 179, 150–155. [Google Scholar] [CrossRef]
- Fukai, R.; Fukumura, Y.; Suzuki, K. A Dedifferentiated Liposarcoma of the Anterior Mediastinum. Int. J. Clin. Oncol. 2009, 14, 174–177. [Google Scholar] [CrossRef]
- Coulibaly, B.; Bouvier, C.; Jose Payan, M.; Thomas, P. Recurrent Dedifferentiated Liposarcoma of Mediastinum Involving Lung and Pleura. Interact. Cardiovasc. Thorac. Surg. 2009, 9, 741–742. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, T.; Takamori, S.; Hayabuchi, N.; Fumihiko, M.; Kashihara, M.; Yoshiyama, K.; Nishi, T.; Murakami, D.; Shirouzu, K. Giant Liposarcoma Occupying Most of The Hemi-Thorax and Resected in the Supine Position: Report of a Rare Case. Kurume Med. J. 2011, 58, 63–65. [Google Scholar] [CrossRef]
- Asaka, S.; Hashizume, M.; Ito, K.; Yoshida, K. A Mediastinal Liposarcoma Resected Using a Double Approach with a Thoracoscope. Thorac. Cardiovasc. Surg. Rep. 2013, 2, 46–49. [Google Scholar] [CrossRef]
- Harth, S.; Litzlbauer, H.; Behrens, C.; Roller, F.; Gamerdinger, U.; Burchert, D.; Krombach, G. Dedifferentiated Liposarcoma of the Anterior Mediastinum: A Rare Case. RoFo 2015, 188, 95–97. [Google Scholar] [CrossRef]
- Hamanaka, K.; Ohashi, M.; Nakamura, T. Primary Mediastinal Dedifferentiated Liposarcoma Resected by Lateral Thoracotomy with Video-Assisted Thoracoscopic Surgery. J. Surg. Case Rep. 2016, 2016, rjv163. [Google Scholar] [CrossRef] [Green Version]
- Sbrana, F.; Ugolini, C.; Taddei, C.; Alì, G.; Pasanisi, E.M. Mediastinal Dedifferentiated Liposarcoma. Acta Cardiol. 2017, 72, 499–500. [Google Scholar] [CrossRef]
- Miura, K.; Hamanaka, K.; Matsuoka, S.; Takeda, T.; Agatsuma, H.; Hyogotani, A.; Ito, K.; Nishimaki, F.; Koizumi, T.; Uehara, T. Primary Mediastinal Dedifferentiated Liposarcoma: Five Case Reports and a Review. Thorac. Cancer 2018, 9, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-J.; Chou, S.-H.; Yang, S.-F.; Kao, C.-N.; Chang, P.-C.; Liu, Y.-W. Rapidly Growing Pleural Liposarcoma Masquerading as Extrapleural Hematoma. Thorac. Cancer 2019, 10, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Matsukuma, S.; Oshika, Y.; Utsumi, Y.; Obara, K.; Tanimoto, T.; Katsurada, Y.; Takeo, H. Pleural Dedifferentiated Liposarcoma: A Case Report. Mol. Clin. Oncol. 2019, 10, 132–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Froelich, J.J.; Dawson, H.; Peters, A.A.; Tappero, C.; Heverhagen, J.T. Inflammatory Calcified De-differentiated Liposarcoma of the Anterior Mediastinum. ANZ J. Surg. 2019, 89, 1326–1327. [Google Scholar] [CrossRef] [PubMed]
- Boatright, C.; Walker, C.M.; Donald, J.; Cui, W.; Nagji, A.S. Incidental Dedifferentiated Mediastinal Liposarcoma on F-18-Fluciclovine PET/CT. Clin. Imaging 2020, 59, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-G.; Zhang, K.; Wu, W.-B.; Wu, Y.-H.; Zhang, J.; Gu, L.-J.; Li, X.-J. Combining Surgery with 125I Brachytherapy for Recurrent Mediastinal Dedifferentiated Liposarcoma: A Case Report and Review of Literature. World J. Clin. Cases 2020, 8, 939–945. [Google Scholar] [CrossRef]
- Ochi, T.; Mizobuchi, T.; Hiroshima, K.; Nagato, K.; Itoh, T.; Kuroda, F.; Yamazaki, K.; Kato, I.; Hisaoka, M.; Nakatani, Y. A Successful Trimodality Therapy for Difficult-to-Diagnose Primary Mediastinal Dedifferentiated Liposarcoma, Which Originated from the Perihilar Fat and Invaded the Right Lungs. Gen. Thorac. Cardiovasc. Surg. 2021, 70, 298–302. [Google Scholar] [CrossRef]
- Caraglia, M.; Montella, L.; Addeo, R.; Costanzo, R.; Faiola, V.; Del Prete, S.; Baldi, F.; Baldi, A.; Abbruzzese, A.; Alloisio, M. Conditions Suggesting Lymphoma: Case 2. Mediastinal Liposarcoma in a Patient with Previous Testicular Cancer. J. Clin. Oncol. 2005, 23, 3844–3846. [Google Scholar] [CrossRef] [Green Version]
- Dagli, A.F.; Pehlivan, S.; Ozercan, M.R. Pleural Liposarcoma Mimicking Carcinoma in Pleural Effusion Cytology. Acta Cytol. 2010, 54, 601–604. [Google Scholar] [CrossRef]
- Liu, L.-G.; Wei, X.; Pan, T.-C. A Giant Primary Myxoid Liposarcoma of the Posterior Mediastinum. Chin. Med. J. 2010, 123, 1818–1820. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, J.; Zhu, L.; Zhou, C.; Zhao, H. Primary Intrathoracic Liposarcoma: A Clinicopathologic Study and Prognostic Analysis of 23 Cases. J. Cardiothorac. Surg. 2014, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Arrarás-Martínez, M.J.; Rieger-Reyes, C.; Panadero-Paz, C.; Landa-Oviedo, H.S.; García-Tirado, J. Giant Primary Mediastinal Liposarcoma: A Rare Cause of Atrial Flutter. Asian Cardiovasc. Thorac. Ann. 2015, 23, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Ibe, T.; Otani, Y.; Shimizu, K.; Nakano, T.; Sano, T.; Morishita, Y. Pulmonary Pleomorphic Liposarcoma. Jpn. J. Thorac. Cardiovasc. Surg. 2005, 53, 443–447. [Google Scholar] [CrossRef]
- Romero-Guadarrama, M.B.; Jiménez-Becerra, S.; Durán-Padilla, M.A.; Santiago-Prieto, A.C.; Cruz-Ortiz, H.; Novelo-Retana, V. Mediastinal Pleomorphic Liposarcoma Diagnosed by Fine Needle Aspiration Biopsy. Acta Cytol. 2007, 51, 440–442. [Google Scholar] [CrossRef]





| Characteristics | n (%) |
|---|---|
| Age Mean (SD) | 55 (15) |
| Age ≥ 45 | 155 (78%) |
| Age < 45 | 43 (22%) |
| Male | 120 (61%) |
| Female | 78 (39%) |
| Tumor origin: | |
| Mediastinum | 149 (75%) |
| Pleura | 35 (17%) |
| Lung | 13 (7%) |
| Supraclavicular fossa | 2 (1%) |
| Symptoms | |
| Asymptomatic | 24 (16%) |
| Chest pain | 43 (29%) |
| Dyspnea | 74 (50%) |
| Cough | 39 (26%) |
| Weight loss | 10 (7%) |
| Dysphagia | 19 (13%) |
| Fatigue | 9 (6%) |
| Features on radiological imaging | |
| Homogeneous | 7 |
| Heterogeneous | 28 |
| PET SUVmax mean (SD) | 4.3 (2.7) |
| Surgical treatment | 188 (96%) |
| Non-surgical treatment | 7 (4%) |
| Recurrent disease | 53 (37%) |
| Radiotherapy | 35 |
| Chemotherapy | 20 |
| Tumor size (max diameter) | |
| <10 cm | 35 (23%) |
| ≥10 cm and <20 cm | 64 (41%) |
| ≥20 cm and <30 cm | 33 (21%) |
| ≥30 cm | 22 (15%) |
| Well-differentiated mean (range) | 20 cm (4.5–40) |
| Dedifferentiated mean (range) | 16 cm (3.5–50) |
| Myxoid mean (range) | 14 cm (4–29.3) |
| Pleomorphic mean (range) | 14 cm (4.5–25) |
| Tumor weight | |
| <1000 g | 18 (33%) |
| ≥1000 g and <2000 g | 10 (19%) |
| ≥2000 g and <3000 g | 6 (11%) |
| ≥3000 g | 20 (37%) |
| Histological subtype | |
| Well-differentiated | 84 (44%) |
| Dedifferentiated | 41 (21%) |
| Myxoid | 37 (19%) |
| Pleomorphic | 18 (9%) |
| Immunohistochemistry/gene amplification | |
| MDM2 | 29 (34%) |
| CDK4 | 13 (15%) |
| p16 | 4 (5%) |
| 5-year survival rate | 62% |
| 5-year survival rate WDLPS | 80% |
| 5-year survival rate DDLPS | 64% |
| 5-year survival rate MLPS | 31% |
| 5-year survival rate PLPS | 41% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiełbowski, K.; Ruszel, N.; Skrzyniarz, S.A.; Wojtyś, M.E.; Becht, R.; Ptaszyński, K.; Gajić, D.; Wójcik, J. Clinicopathological Features of Intrathoracic Liposarcoma—A Systematic Review with an Illustrative Case. J. Clin. Med. 2022, 11, 7353. https://doi.org/10.3390/jcm11247353
Kiełbowski K, Ruszel N, Skrzyniarz SA, Wojtyś ME, Becht R, Ptaszyński K, Gajić D, Wójcik J. Clinicopathological Features of Intrathoracic Liposarcoma—A Systematic Review with an Illustrative Case. Journal of Clinical Medicine. 2022; 11(24):7353. https://doi.org/10.3390/jcm11247353
Chicago/Turabian StyleKiełbowski, Kajetan, Nikola Ruszel, Seweryn Adam Skrzyniarz, Małgorzata Edyta Wojtyś, Rafał Becht, Konrad Ptaszyński, Darko Gajić, and Janusz Wójcik. 2022. "Clinicopathological Features of Intrathoracic Liposarcoma—A Systematic Review with an Illustrative Case" Journal of Clinical Medicine 11, no. 24: 7353. https://doi.org/10.3390/jcm11247353
APA StyleKiełbowski, K., Ruszel, N., Skrzyniarz, S. A., Wojtyś, M. E., Becht, R., Ptaszyński, K., Gajić, D., & Wójcik, J. (2022). Clinicopathological Features of Intrathoracic Liposarcoma—A Systematic Review with an Illustrative Case. Journal of Clinical Medicine, 11(24), 7353. https://doi.org/10.3390/jcm11247353






