Association of Red Blood Cell Life Span with Abnormal Changes in Cardiac Structure and Function in Non-Dialysis Patients with Chronic Kidney Disease Stages 3–5
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
- We excluded patients with a follow-up time of fewer than six months;
- Oral roxadustat (FG-4592) for more than one month;
- Other causes of anemia (based on clinical presentation, past medical history, family history of disease, and serological data);
- A clear sign of acute infection;
- Unable to cooperate with the test due to a combination of chronic lung disease or cancer;
- Congenital heart disease and a previous diagnosis of coronary artery disease or atrial fibrillation.
2.2. Indicators of Anemia
2.3. RBCLS
2.4. Cardiac Color Doppler Index
2.5. Definition of Heart Failure
2.6. Definition of Calcified Heart Valves
2.7. Follow-Up Time and Indicators
- Whether or not they survived;
- Whether or not they entered renal replacement therapy (hemodialysis, peritoneal dialysis, renal transplantation), and the time to enter renal replacement therapy;
- According to a 2020 Chinese Hypertension Survey [12] on heart failure and left ventricular insufficiency in elderly patients with chronic kidney disease, we investigated 4 typical symptoms, including:
- Reduced ability to exercise;
- Shortness of breath (dyspnea) with exertion;
- Swelling (edema) in the legs, ankles, and feet;
- Shortness of breath (dyspnea) when lying down.
2.8. Statistical Methods
3. Results
3.1. Baseline Characteristics of the Study Cohort
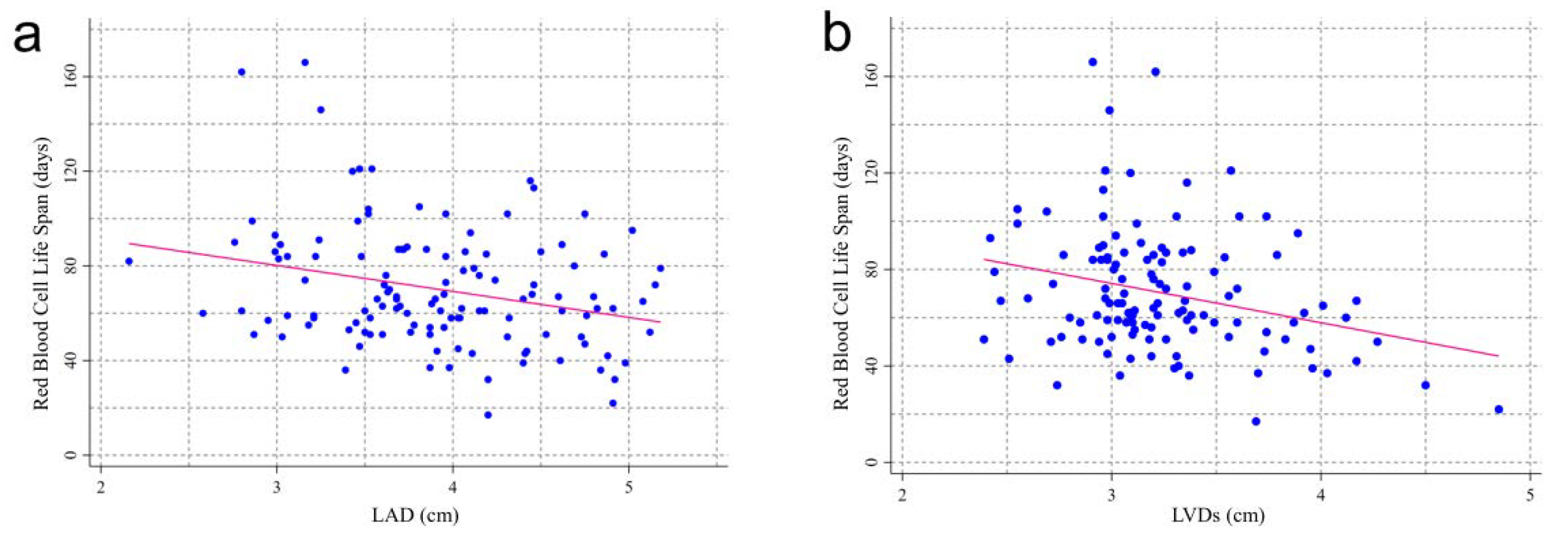
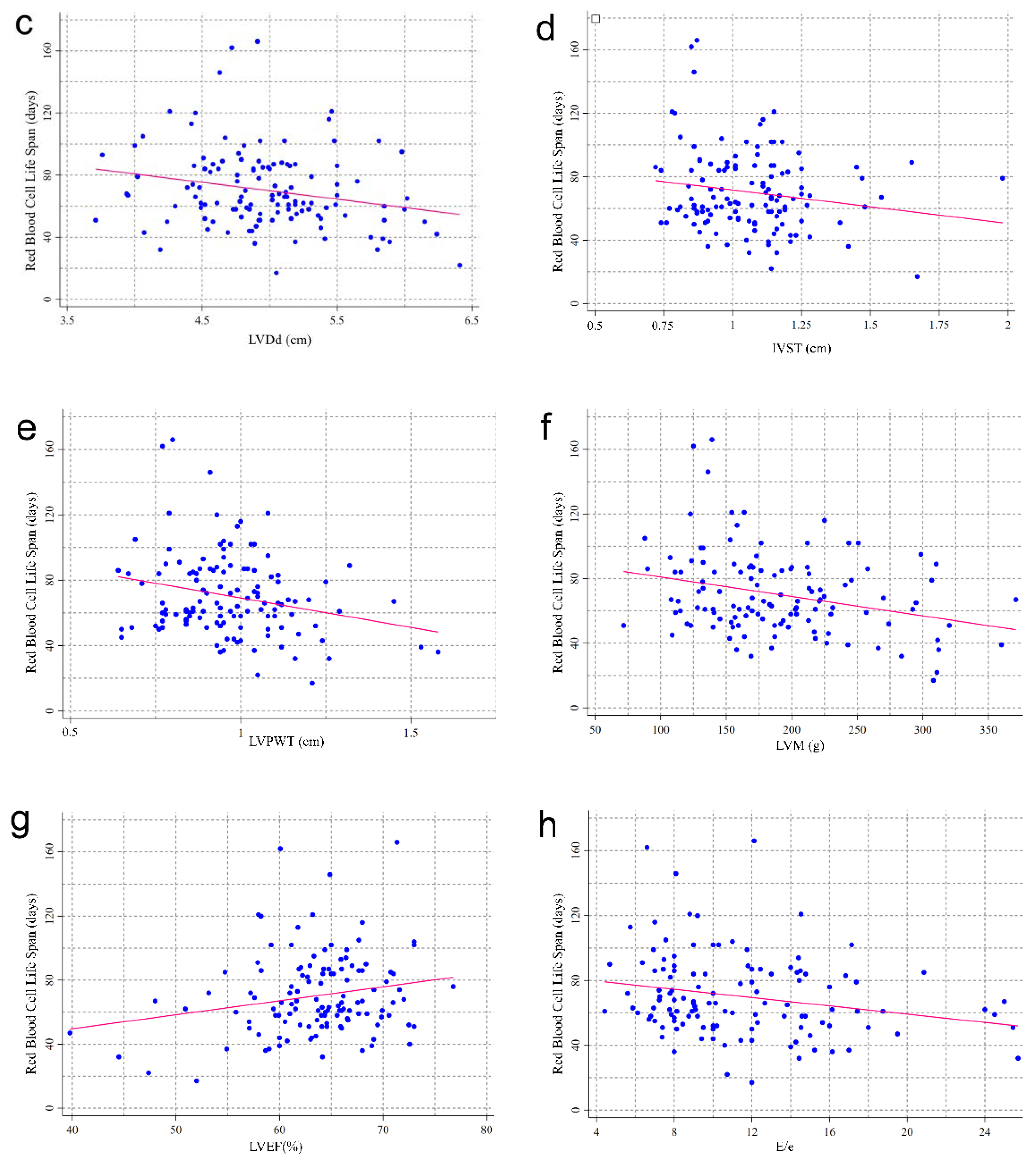
3.2. RBCLS and Baseline Echocardiographic Index
3.3. Association between RBCLS and Heart Failure
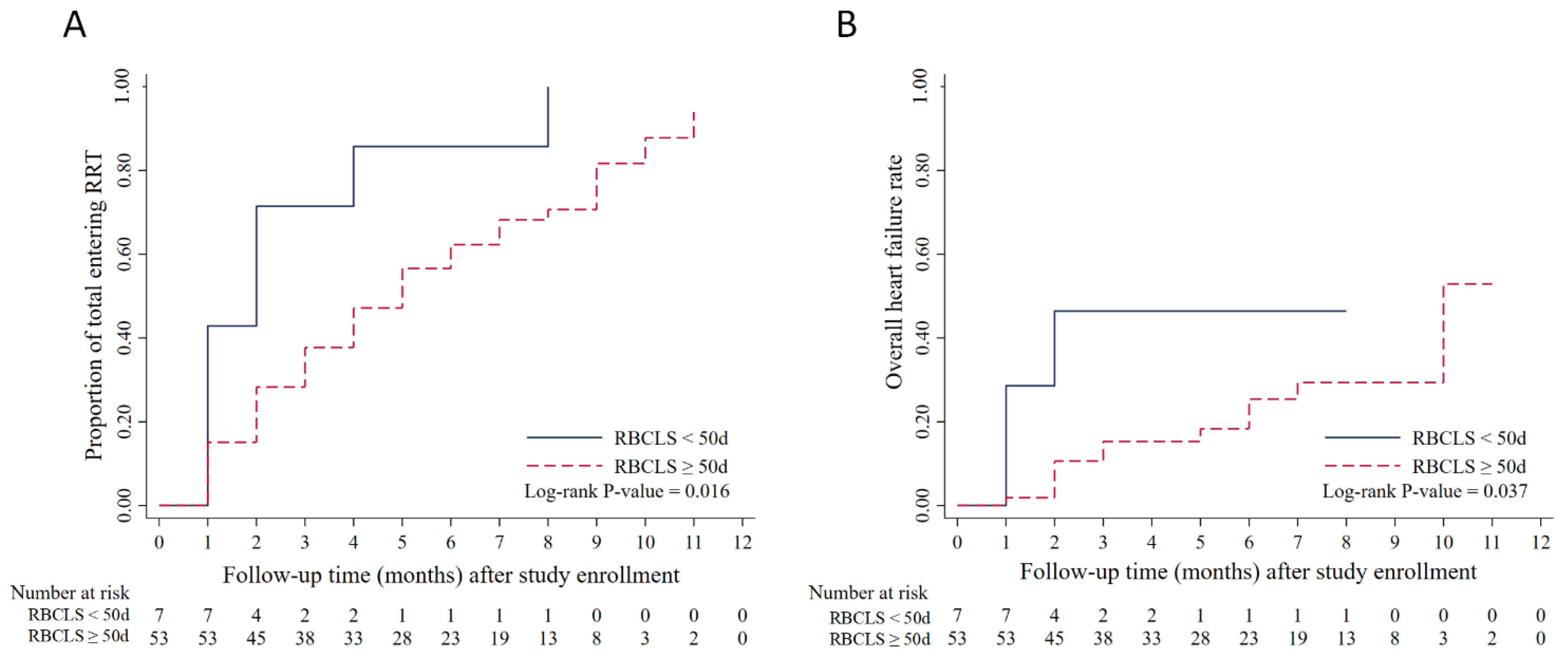
3.4. RBCLS and Time to Renal Replacement
3.5. RBCLS and the Time at Which Heart Failure Occurs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonelli, M.; Muntner, P.; Lloyd, A.; Manns, B.J.; Klarenbach, S.; Pannu, N.; James, M.T.; Hemmelgarn, B.R. Alberta Kidney Disease N. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: A population-level cohort study. Lancet 2012, 380, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Coresh, J.; Sang, Y.; Chalmers, J.; Fox, C.; Guallar, E.; Jafar, T.; Jassal, S.K.; Landman, G.W.D.; Muntner, P.; et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015, 3, 514–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Major, R.W.; Cheng, M.R.I.; Grant, R.A.; Shantikumar, S.; Xu, G.; Oozeerally, I.; Brunskill, N.J.; Gray, L.J. Cardiovascular disease risk factors in chronic kidney disease: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0192895. [Google Scholar] [CrossRef] [PubMed]
- Kim-Mitsuyama, S.; Soejima, H.; Yasuda, O.; Node, K.; Jinnouchi, H.; Yamamoto, E.; Sekigami, T.; Ogawa, H.; Matsui, K. Anemia is an independent risk factor for cardiovascular and renal events in hypertensive outpatients with well-controlled blood pressure: A subgroup analysis of the ATTEMPT-CVD randomized trial. Hypertens. Res. 2019, 42, 883–891. [Google Scholar] [CrossRef]
- Roestenberg, M.; Hoogerwerf, M.-A.; Ferreira, D.M.; Mordmüller, B.; Yazdanbakhsh, M. Experimental infection of human volunteers. Lancet Infect. Dis. 2018, 18, e312–e322. [Google Scholar] [CrossRef]
- Zhang, H.-D.; Ma, Y.-J.; Liu, Q.-F.; Ye, T.-Z.; Meng, F.-Y.; Zhou, Y.-W.; Yu, G.-P.; Yang, J.-P.; Jiang, H.; Wang, Q.-S.; et al. Human erythrocyte lifespan measured by Levitt’s CO breath test with newly developed automatic instrument. J. Breath Res. 2018, 12, 036003. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [CrossRef]
- Chawla, L.S.; Herzog, C.A.; Costanzo, M.R.; Tumlin, J.; Kellum, J.A.; McCullough, P.A.; Ronco, C.; Workgroup, A.X. Proposal for a Functional Classification System of Heart Failure in Patients With End-Stage Renal Disease: Proceedings of the acute dialysis quality initiative (ADQI) XI workgroup. J. Am. Coll. Cardiol. 2014, 63, 1246–1252. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of, Cardiovascular Imaging. Eur. Hear. J.-Cardiovasc. Imaging 2016, 17, 412. [Google Scholar] [CrossRef]
- Fletcher, A.; Singh, T.; Syed, M.; Dweck, M. Imaging aortic valve calcification: Significance, approach and implications. Clin. Radiol. 2020, 76, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hao, G.; Chen, L.; Zhang, L.F.; Chen, Z.; Kang, Y.T.; Yang, Y.; Zheng, C.Y.; Zhou, H.Q.; Wang, Z.W.; et al. Heart failure and left ventricular dysfunction in older patients with chronic kidney disease: The China Hypertension Survey (2012–2015). J. Geriatr. Cardiol. 2020, 17, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Novoradovskaya, N.; Lee, J.; Yu, Z.X.; Ferrans, V.J.; Brantly, M. Inhibition of intracellular degradation increases secretion of a mutant form of alpha1-antitrypsin associated with profound deficiency. J. Clin. Investig. 1998, 101, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.M.; Franco, R.S.; Khera, P.K.; Smith, E.P.; Lindsell, C.J.; Ciraolo, P.J.; Palascak, M.B.; Joiner, C.H. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 2008, 112, 4284–4291. [Google Scholar] [CrossRef] [Green Version]
- Mock, D.M.; Matthews, N.I.; Zhu, S.; Strauss, R.G.; Schmidt, R.L.; Nalbant, D.; Cress, G.A.; Widness, J. Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities. Transfusion 2010, 51, 1047–1057. [Google Scholar] [CrossRef] [Green Version]
- Farah, C.; Michel, L.Y.M.; Balligand, J.-L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef]
- Kuhn, V.; Diederich, L.; Keller, T.C.S.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef] [Green Version]
- Salgado, M.T.; Cao, Z.; Nagababu, E.; Mohanty, J.G.; Rifkind, J.M. Red Blood Cell Membrane-Facilitated Release of Nitrite-Derived Nitric Oxide Bioactivity. Biochemistry 2015, 54, 6712–6723. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Rifkind, J.M.; Mohanty, J.G.; Nagababu, E.; Salgado, M.T.; Cao, Z. Potential Modulation of Vascular Function by Nitric Oxide and Reactive Oxygen Species Released from Erythrocytes. Front. Physiol. 2018, 9, 690. [Google Scholar] [CrossRef]
- Zhou, Z.; Mahdi, A.; Tratsiakovich, Y.; Zahorán, S.; Kövamees, O.; Nordin, F.; Uribe Gonzalez, A.E.; Alvarsson, M.; Östenson, C.-G.; Andersson, D.C.; et al. Erythrocytes from Patients with Type 2 Diabetes Induce Endothelial Dysfunction Via Arginase I. J. Am. Coll. Cardiol. 2018, 72, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, X.; Mahdi, A.; Zhou, Z.; Tratsiakovich, Y.; Jiao, T.; Kiss, A.; Kövamees, O.; Alvarsson, M.; Catrina, S.-B.; et al. Red Blood Cells in Type 2 Diabetes Impair Cardiac Post-Ischemic Recovery through an Arginase-Dependent Modulation of Nitric Oxide Synthase and Reactive Oxygen Species. JACC: Basic Transl. Sci. 2018, 3, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Wautier, J.-L.; Wautier, M.-P. Molecular basis of erythrocyte adhesion to endothelial cells in diseases. Clin. Hemorheol. Microcirc. 2013, 53, 11–21. [Google Scholar] [CrossRef]
- Pinlaor, S.; Onsurathum, S.; Boonmars, T.; Pinlaor, P.; Hongsrichan, N.; Chaidee, A.; Haonon, O.; Limviroj, W.; Tesana, S.; Kaewkes, S.; et al. Distribution and Abundance of Opisthorchis viverrini Metacercariae in Cyprinid Fish in Northeastern Thailand. Korean J. Parasitol. 2013, 51, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.I.; Hwang, S.-J.; Larson, M.; Meigs, J.B.; Levy, D.; Fox, C.S. Cardiovascular Disease Risk Factors in Chronic Kidney Disease: Overall burden and rates of treatment and control. Arch. Intern. Med. 2006, 166, 1884–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manjunath, G.; Tighiouart, H.; Ibrahim, H.; MacLeod, B.; Salem, D.N.; Griffith, J.L.; Coresh, J.; Levey, A.S.; Sarnak, M.J. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J. Am. Coll. Cardiol. 2002, 41, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Hickson, L.J.; Negrotto, S.M.; Onuigbo, M.; Scott, C.G.; Rule, A.D.; Norby, S.M.; Albright, R.C.; Casey, E.T.; Dillon, J.J.; Pellikka, P.A.; et al. Echocardiography Criteria for Structural Heart Disease in Patients with End-Stage Renal Disease Initiating Hemodialysis. J. Am. Coll. Cardiol. 2016, 67, 1173–1182. [Google Scholar] [CrossRef]
- Pluta, A.; Stróżecki, P.; Krintus, M.; Odrowąż-Sypniewska, G.; Manitius, J. Left ventricular remodeling and arterial remodeling in patients with chronic kidney disease stage 1–3. Ren. Fail. 2015, 37, 1105–1110. [Google Scholar] [CrossRef]
- Pernow, J.; Jung, C. Arginase as a potential target in the treatment of cardiovascular disease: Reversal of arginine steal? Cardiovasc. Res. 2013, 98, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Kövamees, O.; Shemyakin, A.; Checa, A.; Wheelock, C.E.; O Lundberg, J.; Östenson, C.-G.; Pernow, J. Arginase inhibition improves microvascular endothelial function in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2016, 101, 3952–3958. [Google Scholar] [CrossRef]
- Yang, J.; Gonon, A.T.; Sjöquist, P.-O.; Lundberg, J.O.; Pernow, J. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proc. Natl. Acad. Sci. USA 2013, 110, 15049–15054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottgen, A.; Russell, S.D.; Loehr, L.; Crainiceanu, C.M.; Rosamond, W.D.; Chang, P.P.; Chambless, L.E.; Coresh, J. Reduced Kidney Function as a Risk Factor for Incident Heart Failure: The Atherosclerosis Risk in Communities (ARIC) Study. J. Am. Soc. Nephrol. 2007, 18, 1307–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronksley, P.E.; Tonelli, M.; Manns, B.J.; Weaver, R.G.; Thomas, C.M.; MacRae, J.; Ravani, P.; Quinn, R.R.; James, M.T.; Lewanczuk, R.; et al. Emergency Department Use among Patients with CKD: A Population-Based Analysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Porter, A.C.; Lash, J.P.; Xie, D.; Pan, Q.; DeLuca, J.; Kanthety, R.; Kusek, J.W.; Lora, C.M.; Nessel, L.; Ricardo, A.C.; et al. Predictors and Outcomes of Health–Related Quality of Life in Adults with CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afsar, B.; Rossignol, P.; van Heerebeek, L.; Paulus, W.J.; Damman, K.; Heymans, S.; van Empel, V.; Sag, A.; Maisel, A.; Kanbay, M. Heart failure with preserved ejection fraction: A nephrologist-directed primer. Hear. Fail. Rev. 2017, 22, 765–773. [Google Scholar] [CrossRef]
- Diaz-Ricart, M.; Torramadé-Moix, S.; Pascual, G.; Palomo, M.; Moreno-Castaño, A.B.; Martinez-Sanchez, J.; Vera, M.; Cases, A.; Escolar, G. Endothelial Damage, Inflammation and Immunity in Chronic Kidney Disease. Toxins 2020, 12, 361. [Google Scholar] [CrossRef]
- Nohara, N.; Io, H.; Matsumoto, M.; Furukawa, M.; Okumura, K.; Nakata, J.; Shimizu, Y.; Horikoshi, S.; Tomino, Y. Predictive factors associated with increased progression to dialysis in early chronic kidney disease (stage 1–3) patients. Clin. Exp. Nephrol. 2015, 20, 740–747. [Google Scholar] [CrossRef]

| Characteristic | Red Blood Cell Life Span (Day) | ||||
|---|---|---|---|---|---|
| ≥70 | 60 to 70 | 50 to 60 | <50 | p | |
| (n = 54) | (n = 28) | (n = 31) | (n = 20) | ||
| Demographics | |||||
| Age (mean (SD)) | 60.6 (16.0) | 53.9 (16.3) | 55.7 (13.4) | 61.0 (17.0) | 0.195 |
| Male gender (n (%)) | 32 (59.3) | 18 (64.3) | 12 (38.7) | 16 (80.0) c | 0.027 |
| Cardiovascular risk factors | |||||
| Smoking (n (%)) | 6 (11.1) | 5 (17.9) | 3 (9.7) | 5 (25.0) | 0.353 |
| BMI (mean (SD)) | 23.5 (3.1) | 22.5 (3.0) | 23.8 (3.6) | 23.3 (2.6) | 0.385 |
| Diabetes (n (%)) | 23 (42.6) | 8 (28.6) | 11 (35.5) | 9 (45.0) | 0.564 |
| Hypertension (n (%)) | 44 (81.5) | 25 (89.3) | 27 (87.1) | 20 (100) | 0.186 |
| Anemia (n (%)) | 49 (91.8) | 26 (92.3) | 31 (100) | 20 (100) | 0.203 |
| SBP (mmHg; mean (SD)) | 135.6 (22.4) | 138.7 (27.4) | 141.6 (27.8) | 155.9 (24.1) a | 0.023 |
| DBP (mmHg; mean (SD)) | 75.0 (14.0) | 79.8 (17.0) | 85.0 (16.7) a | 84.1 (20.1) | 0.031 |
| MAP (mmHg; mean (SD)) | 95.2 (14.0) | 99.4 (18.5) | 103.8 (18.9) | 108.0 (18.1) a | 0.017 |
| LDL cholesterol (mmol/L; mean (1/4, 3/4)) | 2.4 (1.8, 3.0) | 2.8 (2.4, 3.3) | 2.3 (2.0, 2.9) | 2.6 (2.4, 3.6) | 0.067 |
| Triglycerides (mmol/L; mean (1/4, 3/4)) | 1.5 (0.9, 2.2) | 1.4 (1.1, 2.0) | 1.6 (1.1, 2.0) | 1.4 (1.0, 2.0) | 0.948 |
| C-reactive protein (mg/L; mean (1/4, 3/4)) | 5.0 (5.0, 6.0) | 5.0 (5.0, 9.5) | 5.0 (5.0, 8.6) | 9.4 abc (5.0, 25.5) | 0.027 |
| BNP (ng/L; mean (1/4, 3/4)) | 48.0 (14.0, 224.6) | 80.0 (33.0, 244.0) | 65.5 (30.6, 252.3) | 330.7 abc (166.0, 518.7) | 0.003 |
| eGFR (mL/min per 1.73 m2; mean (1/4, 3/4)) | 12.8 (9.5, 34.5) | 17.6 (9.2, 35.6) | 10.5 (5.8, 18.7) | 16.4 (7.1, 33.6) | 0.221 |
| Other serological indicators | |||||
| Hemoglobin (g/L; mean (SD)) | 92.0 (19.3) | 96.0 (21.7) | 83.6 (11.7) ab | 79.4 (19.7) ab | 0.004 |
| Hematocrit (L/L; mean (SD)) | 0.28 (0.06) | 0.29 (0.06) | 0.25 (0.04) ab | 0.24 (0.06) ab | 0.005 |
| RDW (%; mean (1/4, 3/4)) | 15.4 (13.7, 15.4) | 14.5 (13.5, 16.0) | 14.5 ab (13.3, 15.6) | 15.8 ab (15.0, 17.0) | 0.032 |
| Serum albumin (g/L; mean (1/4, 3/4)) | 37.0 (32.2, 29.9) | 36.4 (31.7, 39.4) | 32.7 a (29.4, 38.3) | 32.9 ab (28.5, 35.2) | 0.020 |
| Transferrin saturation (%; mean (1/4, 3/4)) | 22.6 (13.23, 32.77) | 20.1 (14.96, 30.99) | 31.33 (19.63, 46.06) | 20.95 (13.58, 29.37) | 0.1154 |
| Corrected calcium (mmol/L; mean (1/4, 3/4)) | 2.2 (2.2, 2.3) | 2.3 (2.2, 2.4) | 2.2 (2.1, 2.3) | 2.2 (2.1, 2.3) | 0.480 |
| Phosphorus (mmol/L; mean (1/4, 3/4)) | 1.4 (1.1, 1.8) | 1.4 (1.2, 1.6) | 1.6 (1.3, 1.8) | 1.5 (1.1, 1.9) | 0.618 |
| Parathyroid hormone (pmol/L; mean (1/4, 3/4)) | 62.5 (39.0, 99.0) | 77.0 (42.5, 99.5) | 57.0 (26.0, 92.0) | 84.0 (26.5, 104.5) | 0.681 |
| Medication status | |||||
| EPO (n (%)) | 21 (38.89) | 11 (39.29) | 17 (54.84) | 9 (45.00) | 0.511 |
| Iron (n (%)) | 12 (22.22) | 3 (10.71) | 6 (19.35) | 3 (15.00) | 0.637 |
| ACEI/ARB (n (%)) | 9 (16.67) | 2 (7.14) | 2 (6.45) | 4 (20.00) | 0.332 |
| β-blockers (n (%)) | 23 (42.59) | 16 (57.14) | 15 (48.39) | 11 (55.00) | 0.589 |
| α-blockers (n (%)) | 14 (25.93) | 7 (25.00) | 11 (35.48) | 10 (50.00) | 0.194 |
| Calcium channel blocker (n (%)) | 42 (77.78) | 24 (85.71) | 27 (87.10) | 19 (95.00) | 0.299 |
| Spironolactone (n (%)) | 3 (5.56) | 4 (14.29) | 3 (9.68) | 4 (20.00) | 0.243 |
| Characteristic | Red Blood Cell Life Span (Day) | ||||
|---|---|---|---|---|---|
| ≥70 | 60 to 70 | 50 to 60 | <50 | p | |
| (n = 52) | (n = 26) | (n = 27) | (n = 19) | ||
| Cardiac ultrasound indicators | |||||
| LAD (cm; mean (SD)) | 3.78 (0.67) | 4.00 (0.61) | 3.76 (0.59) | 4.33 (0.49) ac | 0.006 |
| LVDd (cm; mean (SD)) | 4.85 (0.46) | 4.97 (0.50) | 5.04 (0.52) | 5.20 (0.65) | 0.075 |
| LVDs (cm; mean (1/4, 3/4)) | 3.11 (2.96, 3.29) | 3.16 (3.03, 3.38) | 3.16 (2.98, 3.56) | 3.37 abc (3.09, 3.96) | 0.032 |
| IVST (cm; mean (1/4, 3/4)) | 1.06 (0.89, 1.15) | 1.05 (0.93, 1.19) | 1.01 (0.87, 1.14) | 1.14 (1.05, 1.21) | 0.079 |
| LVPW (cm; mean (1/4, 3/4)) | 0.95 (0.87, 1.04) | 0.98 (0.86, 1.11) | 0.90 (0.78, 1.00) | 1.05 abc (0.96, 1.21) | 0.003 |
| LVEF (%; mean (1/4, 3/4)) | 64.57 (61.76, 67.66) | 64.54 (61.17, 66.19) | 64.58 (62.00, 67.65) | 60.00 (54.91, 64.15) | 0.058 |
| E/e (%; mean (1/4, 3/4)) | 9.43 (7.52, 12.72) | 9.06 (8.00, 12.67) | 10.00 (8.00, 14.57) | 14.00 abc (10.60, 15.23) | 0.038 |
| LVM (g; mean (1/4, 3/4)) | 169.0 (132.4, 212.6) | 180.9 (140.0, 221.0) | 174.2 (145.2, 199.3) | 226.7 abc (169.0, 308.0) | 0.018 |
| CO (L/min; mean (1/4, 3/4)) | 5.09 (4.35, 6.00) | 5.39 (4.59, 6.56) | 5.86 (5.13, 7.02) | 5.00 (4.01, 8.00) | 0.082 |
| Calcified heart valves (n (%)) | 9 (17.31) | 6 (22.22) | 9 (33.33) | 5 (26.32) | 0.442 |
| Red Blood Cell Life Span (Days) | Prevalence of Heart Failure (%) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| PR | 95% CI | PR | 95% CI | PR | 95% CI | ||
| RBCLS ≥ 70 | 25.93 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| 60 ≤ RBCLS < 70 | 32.14 | 1.48 | 0.61, 3.59 | 1.23 | 0.52, 2.90 | 1.19 | 0.51, 2.80 |
| 50 ≤ RBCLS < 60 | 35.48 | 1.47 | 0.62, 3.49 | 1.24 | 0.56, 2.77 | 1.14 | 0.49, 2.65 |
| RBCLS < 50 | 80.00 | 3.06 | 1.41, 6.64 * | 2.68 | 1.27, 5.66 * | 2.27 | 1.02, 5.06 * |
| p | 0.005 | 0.010 | 0.045 | ||||
| Red Blood Cell Life Span (Days) | Prevalence of Heart Failure (%) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| PR | 95% CI | PR | 95% CI | PR | 95% CI | ||
| RBCLS ≥ 70 | 16.67 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| 60 ≤ RBCLS < 70 | 17.86 | 0.89 | 0.28, 2.78 | 1.04 | 0.34, 3.21 | 0.91 | 0.30, 2.82 |
| 50 ≤ RBCLS < 60 | 22.58 | 1.42 | 0.49, 4.07 | 1.19 | 0.44, 3.26 | 1.19 | 0.42, 3.42 |
| RBCLS < 50 | 30.00 | 1.29 | 0.44, 3.77 | 1.37 | 0.45, 4.17 | 1.80 | 0.57, 5.70 |
| p | 0.642 | 0.583 | 0.456 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, S.; Zhang, J.; Lin, J.; Wan, J.; Chen, Y. Association of Red Blood Cell Life Span with Abnormal Changes in Cardiac Structure and Function in Non-Dialysis Patients with Chronic Kidney Disease Stages 3–5. J. Clin. Med. 2022, 11, 7373. https://doi.org/10.3390/jcm11247373
Rao S, Zhang J, Lin J, Wan J, Chen Y. Association of Red Blood Cell Life Span with Abnormal Changes in Cardiac Structure and Function in Non-Dialysis Patients with Chronic Kidney Disease Stages 3–5. Journal of Clinical Medicine. 2022; 11(24):7373. https://doi.org/10.3390/jcm11247373
Chicago/Turabian StyleRao, Siyi, Jing Zhang, Jiaqun Lin, Jianxin Wan, and Yi Chen. 2022. "Association of Red Blood Cell Life Span with Abnormal Changes in Cardiac Structure and Function in Non-Dialysis Patients with Chronic Kidney Disease Stages 3–5" Journal of Clinical Medicine 11, no. 24: 7373. https://doi.org/10.3390/jcm11247373





