Prognostic Value of Serum Transferrin Analysis in Patients with Ovarian Cancer and Cancer-Related Functional Iron Deficiency: A Retrospective Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants Selection
2.3. Iron Status Assessment
2.4. Genotyping
2.5. Immunoaffinity Fractionation of Holo-Tf and Apo-Tf Using Single-Domain Antibodies for Holo-Transferrin (aTf1) and Apo-Transferrin (aTf2)
2.6. Data and Statistical Analyses
3. Results
3.1. Clinical Indicators of the Status of Iron Metabolism in the Group of Patients with Ovarian Cancer and in Women without Malignant Diseases
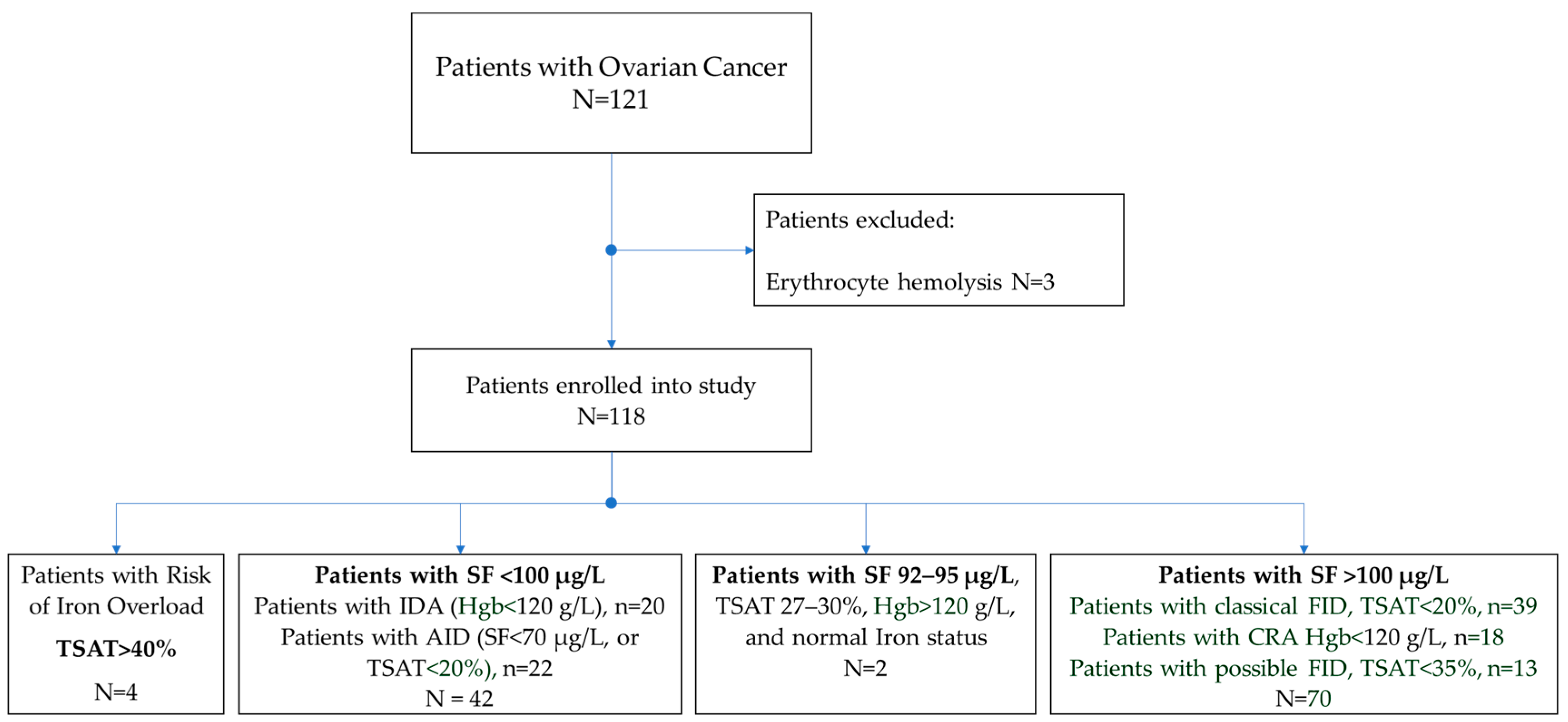
3.2. Identification of Patients with Associated Disorders of Iron Metabolism among Women with Ovarian Cancer
- SF < 100 μg/L. Iron-deficiency anemia (IDA): Hgb < 120 g/L, N = 20, and absolute iron-deficiency (AID): SF < 70 μg/L or TSAT < 20%, N = 22, total 42 (36%);
- SF > 100 μg/L. Cancer-related anemia (CRA): Hgb < 120 g/L, N = 18, and patients with classical functional iron deficiency (FID): SF > 100 μg/L and TSAT < 20%, N = 39; possible FID: SF > 100 μg/L, TSAT < 35%, N = 13, total = 70 (59%);
- Risk of iron overload: TSAT > 40%, N = 4 (3%);
- Normal iron status: SF 92–95 μg/L, TSAT 27–30%, Hgb > 120 g/L. N = 2 (2%).
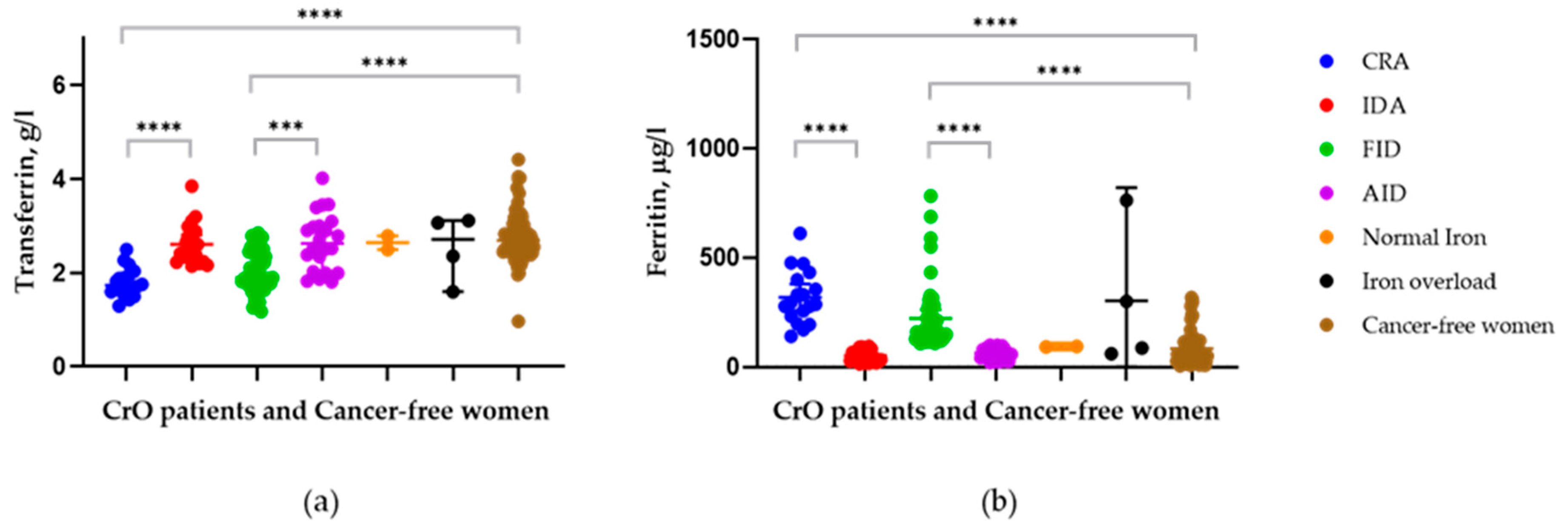
3.3. Comparison of Transferrin Levels between Patients with Different Iron Statuses and the Control Group
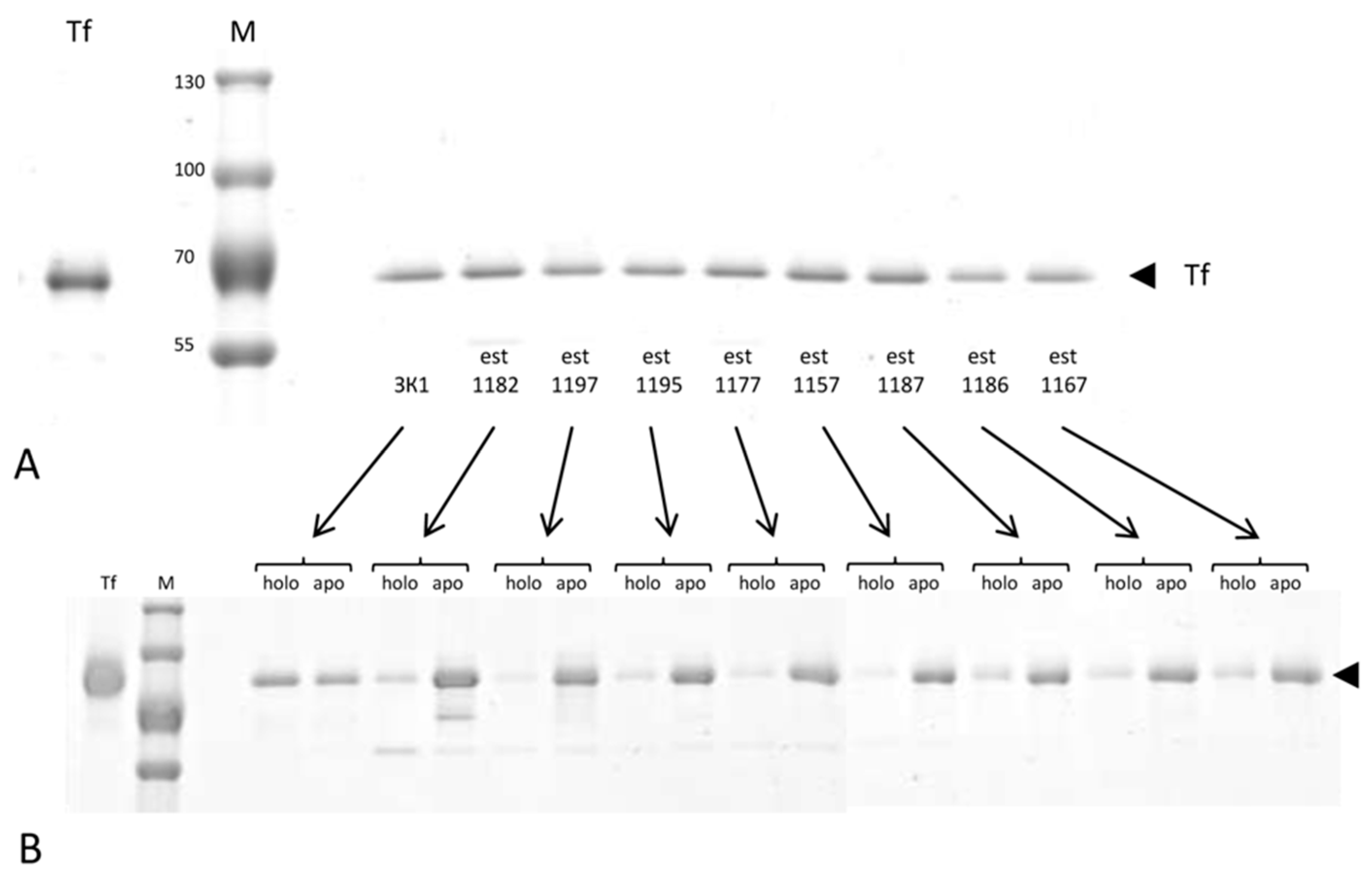
3.4. Analysis of the Ratio of Holo- and Apo- Forms of Transferrin in Cancer Patients and the Control
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Chan, W.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E., III; Xu, W.; Zheng, Z.-J.; Elcarte, E.; Withers, M.; et al. Worldwide Burden, Risk Factors, and Temporal Trends of Ovarian Cancer: A Global Study. Cancers 2022, 14, 2230. [Google Scholar] [CrossRef]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. 1), 61–85. [Google Scholar] [CrossRef] [PubMed]
- Shegai, P.V.; Shatalov, P.A.; Zabolotneva, A.A.; Falaleeva, N.A.; Ivanov, S.A.; Kaprin, A.D. Challenges Faced by Clinicians in the Personalized Treatment Planning: A Literature Review and the First Results of the Russian National Cancer Program. Crit. Care Res. Pract. 2021, 2021, 6649771. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.F.; Lu, Z.B.; Fu, L.Q.; Tong, Y.; Wang, Z.; Li, W.F.; Mou, X.Z. The role of iron homeostasis and iron-mediated ROS in cancer. Am. J. Cancer Res. 2021, 11, 1895–1912. [Google Scholar]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Stockwe. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Cheng, K.; Guo, Q.; Shen, Z.; Yang, W.; Zhou, Y.; Sun, Z.; Yao, X.; Wu, H. Frontiers of ferroptosis research: An analysis from the top 100 most influential articles in the field. Front. Oncol. 2022, 12, 948389. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.C.; Knuiman, M.W.; Trinder, D.; Divitini, M.L.; Olynyk, J.K. Higher concentrations of serum iron and transferrin saturation but not serum ferritin are associated with cancer outcomes. Am. J. Clin. Nutr. 2016, 104, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.V.; Torti, F.M. Iron and Cancer: 2020 Vision. Cancer Res. 2020, 80, 5435–5448. [Google Scholar] [CrossRef] [PubMed]
- Cymbaluk-Płoska, A.; Chudecka, K.; Chudecka-Głaz, A.; Piotrowska, K.; Kwiatkowski, S.; Tarnowski, M. Importance of Potential New Biomarkers in Patient with Serouse Ovarian Cancer. Diagnostics 2021, 11, 1026. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, N. Ferroptosis, necroptosis, and pyroptosis in the occurrence and development of ovarian cancer. Front. Immunol. 2022, 13, 920059. [Google Scholar] [CrossRef]
- Huang, X. Does iron have a role in breast cancer? Lancet Oncol. 2008, 9, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.U.; McSorley, S.T.; Patel, P.; Talwar, D. Interpreting iron studies. BMJ 2017, 357, j2513. [Google Scholar] [CrossRef] [PubMed]
- Natalucci, V.; Virgili, E.; Calcagnoli, F.; Valli, G.; Agostini, D.; Zeppa, S.D.; Barbieri, E.; Emili, R. Cancer Related Anemia: An Integrated Multitarget Approach and Lifestyle Interventions. Nutrients 2021, 13, 482. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.; Battaglia, A.M.; Botta, C.; Aversa, I.; Mancuso, S.; Costanzo, F.; Biamonte, F. Iron Metabolism in the Tumor Microenvironment—Implications for Anti-Cancer Immune Response. Cells 2021, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Gilreath, J.A.; Stenehjem, D.D.; Rodgers, G.M. Diagnosis and treatment of cancer-related anemia. Am. J. Hematol. 2014, 89, 203–212. [Google Scholar] [CrossRef]
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascón, P.; Glaspy, J.; Hofmann, A.; Link, H.; Littlewood, T.; Ludwig, H.; et al. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29 (Suppl. S4), iv96–iv110. [Google Scholar] [CrossRef]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/handle/10665/85839 (accessed on 14 September 2022).
- Busti, F.; Marchi, G.; Ugolini, S.; Castagna, A.; Girelli, D. Anemia and Iron Deficiency in Cancer Patients: Role of Iron Replacement Therapy. Pharmaceuticals 2018, 11, 94. [Google Scholar] [CrossRef]
- Madeddu, C.; Gramignano, G.; Astara, G.; Demontis, R.; Sanna, E.; Atzeni, V.; Macciò, A. Pathogenesis and Treatment Options of Cancer Related Anemia: Perspective for a Targeted Mechanism-Based Approach. Front. Physiol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Schwartz, R.N. Anemia in patients with cancer: Incidence, causes, impact, management, and use of treatment guidelines and protocols. Am. J. Health Syst. Pharm. 2007, 64 (Suppl. 2), S5–S13. [Google Scholar] [CrossRef]
- Macciò, A.; Madeddu, C.; Gramignano, G.; Mulas, C.; Tanca, L.; Cherchi, M.C.; Floris, C.; Omoto, I.; Barracca, A.; Ganz, T. The role of inflammation, iron, and nutritional status in cancer-related anemia: Results of a large, prospective, observational study. Haematologica 2015, 100, 124–132. [Google Scholar] [CrossRef]
- Kanuri, G.; Sawhney, R.; Varghese, J.; Britto, M.; Shet, A. Iron deficiency anemia coexists with cancer related anemia and adversely impacts quality of life. PLoS ONE 2016, 11, e0163817. [Google Scholar] [CrossRef][Green Version]
- National Cancer Institute. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 29 October 2022).
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/331505 (accessed on 14 September 2022).
- WHO. Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations; Vitamin and Mineral Nutrition Information System: Geneva, Switzerland, 2011; Available online: http://www.who.int/vmnis/indicators/serum_ferritin_ru.pdf (accessed on 14 September 2022).
- Serum Ferritin Concentrations for Assessing Iron Status in Populations: A Technical Brief; World Health Organization: Geneva, Switzerland, 2020.
- Orr, W.S.; Malkas, L.H.; Robert, J.; Hickey, R.J.; Sandoval, J.A. Acute Phase Proteins as Cancer Biomarkers. In Acute Phase Proteins as Early Non-Specific Biomarkers of Human and Veterinary Diseases; Veas, F., Ed.; IntechOpen: London, UK, 2011; 422p, Available online: https://www.intechopen.com/books/534 (accessed on 10 October 2022). [CrossRef]
- Wu, S.J.; Zhang, Z.Z.; Cheng, N.S.; Xiong, X.Z.; Yang, L. Preoperative serum ferritin is an independent prognostic factor for liver cancer after hepatectomy. Surg. Oncol. 2019, 29, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Carmona, W.; Díaz-Fabregat, B.; Yuri Yoshigae, A.; Musa de Aquino, A.; Scarano, W.R.; de Souza Castilho, A.C.; Avansini Marsicano, J.; Leal do Prado, R.; Pessan, J.P.; de Oliveira Mendes, L. Are Serum Ferritin Levels a Reliable Cancer Biomarker? A Systematic Review and Meta-Analysis. Nutr. Cancer 2022, 74, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Szőke, D.; Panteghini, M. Diagnostic value of transferrin. Clin. Chim. Acta 2012, 413, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.E.; Sharif, M.U.; Stack, A.G. Transferrin Saturation: A Body Iron Biomarker. Adv. Clin. Chem. 2016, 75, 71–97. [Google Scholar] [CrossRef]
- Claise, C.; Saleh, J.; Rezek, M.; Vaulont, S.; Peyssonnaux, C.; Edeas, M. Low transferrin levels predict heightened inflammation in patients with COVID-19: New insights. Int. J. Infect. Dis. 2022, 116, 74–79. [Google Scholar] [CrossRef]
- Shigeta, S.; Toyoshima, M.; Kitatani, K.; Ishibashi, M.; Usui, T.; Yaegashi, N. Transferrin facilitates the formation of DNA double-strand breaks via transferrin receptor 1: The possible involvement of transferrin in carcinogenesis of high-grade serous ovarian cancer. Oncogene 2016, 35, 3577–3586. [Google Scholar] [CrossRef]
- Ahmed, N.; Oliva, K.T.; Barker, G.; Hoffmann, P.; Reeve, S.; Smith, I.A.; Quinn, M.A.; Rice, G.E. Proteomic tracking of serum protein isoforms as screening biomarkers of ovarian cancer. Proteomics 2005, 5, 4625–4636. [Google Scholar] [CrossRef]
- Kumari, S. Serum Biomarker Based Algorithms in Diagnosis of Ovarian Cancer: A Review. Indian J. Clin. Biochem. 2018, 33, 382–386. [Google Scholar] [CrossRef]
- Levina, A.; Chetcuti, A.R.M.; Lay, P.A. Controversial Role of Transferrin in the Transport of Ruthenium Anticancer Drugs. Biomolecules 2022, 12, 1319. [Google Scholar] [CrossRef]
- Guo, W.; Zheng, W.; Luo, Q.; Li, X.; Zhao, Y.; Xiong, S.; Wang, F. Transferrin serves as a mediator to deliver organometallic ruthenium(II) anticancer complexes into cells. Inorg. Chem. 2013, 52, 5328–5338. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, H.; Xu, X.; Lai, T.-P.; Zhou, Y.; Hao, Q.; Li, H.; Sun, H. Binding of ruthenium and osmium at non-iron sites of transferrin accounts for their iron-independent cellular uptake. J. Inorg. Biochem. 2022, 234, 111885. [Google Scholar] [CrossRef] [PubMed]
- Tillib, S.V.; Goryainova, O.S.; Sachko, A.M.; Ivanova, T.I. High-Affinity Single-Domain Antibodies for Analyzing Human Apo- and Holo-Transferrin. Acta Nat. 2022, 14, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Prat, J.; FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef]
- Mutch, D.G.; Prat, J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol. Oncol. 2014, 133, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Beckman Coulter, Transferrin, OSR6152 (2012-01). Available online: https://www.beckmancoulter.com/wsrportal/techdocs?docname=/cis/BAOSR6x52/%25%25/EN_ (accessed on 14 September 2022).
- Ivanova, T.I.; Krikunova, L.I.; Ryabchenko, N.I.; Mkrtchyan, L.S.; Khorokhorina, V.A.; Salnikova, L.E. Association of the apolipoprotein E 2 allele with concurrent occurrence of endometrial hyperplasia and endometrial carcinoma. Oxidative Med. Cell. Longev. 2015, 2015, 593658. [Google Scholar] [CrossRef]
- Fitzsimons, E.J.; Cullis, J.O.; Thomas, D.W.; Tsochatzis, E.; Griffiths, W.J.H.; British Society for Haematology. Diagnosis and therapy of genetic haemochromatosis (review and 2017 update). Br. J. Haematol. 2018, 181, 293–303. [Google Scholar] [CrossRef]
- Cullis, J.O.; Fitzsimons, E.J.; Griffiths, W.J.; Tsochatzis, E.; Thomas, D.W.; British Society for Haematology. Investigation and management of a raised serum ferritin. Br. J. Haematol. 2018, 181, 331–340. [Google Scholar] [CrossRef]
- Pietrangelo, A.; Deugnier, Y.; Dooley, J.; Erhardt, A.; Zoller, H.; Safadi, R. EASL clinical practice guidelines for HFE hemochromatosis. J. Hepatol. 2010, 53, 3–22. [Google Scholar] [CrossRef]
- Royal College of Pathologists of Australasia. In Iron Studies Standardised Reporting Protocol; RCPA: Sydney, Australia, 2021; Available online: https://www.rcpa.edu.au/getattachment/554ba672-4d34-4e7c-b812-5741359bca78/Iron-Studies-Standardised-Reporting-Protocol.aspx (accessed on 14 September 2022).
- Khalil, R.H.; Al-Humadi, N. Types of acute phase reactants and their importance in vaccination. Biomed. Rep. 2020, 12, 143–152. [Google Scholar] [CrossRef]
- Cacoub, P.; Choukroun, G.; Cohen-Solal, A.; Luporsi, E.; Peyrin-Biroulet, L.; Peoc’h, K.; Andrieu, V.; Lasocki, S.; Puy, H.; Trochu, J.-N. Towards a Common Definition for the Diagnosis of Iron Deficiency in Chronic Inflammatory Diseases. Nutrients 2022, 14, 1039. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Macuks, R.; Baidekalna, I.; Gritcina, J.; Avdejeva, A.; Donina, S. Apolipoprotein A1 and transferrin as biomarker in ovarian cancer diagnostics. Acta Chir. Latv. 2010, 10, 16–20. [Google Scholar] [CrossRef]
- Worwood, M.; May, A.M.; Bain, B.J. Iron Deficiency Anaemia and Iron Overload. In Dacie and Lewis Practical Haematology, 12th ed.; Bain, B., Bates, I., Laffan, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 165–186. [Google Scholar] [CrossRef]
- Babitt, J.L.; Eisenga, M.F.; Haase, V.H.; Kshirsagar, A.V.; Levin, A.; Locatelli, F.; Małyszko, J.; Swinkels, D.W.; Tarng, D.C.; Cheung, M.; et al. Controversies in optimal anemia management: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2021, 99, 1280–1295. [Google Scholar] [CrossRef] [PubMed]
- Lorcerie, B.; Audia, S.; Samson, M.; Millière, A.; Falvo, N.; Leguy-Seguin, V.; Berthier, S.; Bonnotte, B. Diagnosis of hyperferritinemia in routine clinical practice. Presse Med. 2017, 46 Pt 2, e329–e338. [Google Scholar] [CrossRef]
- Adams, P.C.; McLaren, C.E.; Speechley, M.; McLaren, G.D.; Barton, J.C.; Eckfeldt, J.H. HFE mutations in Caucasian participants of the Hemochromatosis and Iron Overload Screening study with serum ferritin level <1000 µg/L. Can. J. Gastroenterol. 2013, 27, 390–392. [Google Scholar] [CrossRef]
- Garcia-Casal, M.N.; Pasricha, S.-R.; Martinez, R.X.; Lopez-Perez, L.; Pena-Rosas, J.P. Are current serum and plasma ferritin cut-offs for iron deficiency and overload accurate and reflecting iron status? A systematic review. Arch. Med. Res. 2018, 49, 405–417. [Google Scholar] [CrossRef]
- Tillib, S.V. Prospective Applications of Single-Domain Antibodies in Biomedicine. Mol. Biol. 2020, 54, 362–373. (In Russian) [Google Scholar] [CrossRef]
- Goryainova, O.S.; Ivanova, T.I.; Rutovskaya, M.V.; Tillib, S.V. A Method for the Parallel and Sequential Generation of Single-Domain Antibodies for the Proteomic Analysis of Human Blood Plasma. Mol. Biol. 2017, 51, 985–996. [Google Scholar] [CrossRef]
- Tillib, S.V.; Ivanova, T.I.; Lyssuk, E.Y.; Larin, S.S.; Kibardin, A.V.; Korobko, E.V.; Vikhreva, P.N.; Gnuchev, N.V.; Georgiev, G.P.; Korobko, I.V. Nanoantibodies for detection and blocking of bioactivity of human vascular endothelial growth factor A(165). Biochemistry 2012, 77, 659–665. [Google Scholar] [CrossRef]
- Tillib, S.V.; Privezentseva, M.E.; Ivanova, T.I.; Vasilev, L.F.; Efimov, G.A.; Gursky, Y.G.; Georgiev, G.P.; Goldman, I.L.; Sadchikova, E.R. Single-domain antibody-based ligands for immunoaffinity separation of recombinant human lactoferrin from the goat lactoferrin of transgenic goat milk. J. Chromatogr. B 2014, 949, 48–57. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Control N = 69 |
CrO N = 118 | p Value |
Patients with Ovarian Cancer Stages I–II N = 27 |
Patients with Ovarian Cancer Stages III–IV N = 91 | p Value |
|---|---|---|---|---|---|---|
|
Age, years Mean (SD) | 53(10.5) | 54(11.8) | 0.619 | 48(15.2) | 55.5(10.1) | 0.09 |
|
Iron, μmol/L Mean (SD) | 16.8(6.1) | 8.58(5.6) | <0.0001 | 9.72(5.0) ** | 8.24(5.8) * | 0.01 |
|
TSAT, % Mean (SD) | 28.0(10.9) | 16.4(9.0) | <0.0001 | 17(8.0) | 16.2(9.3) * | 0.31 |
|
Transferrin, g/L Mean (SD) | 2.79(0.6) | 2.24(0.6) | <0.0001 | 2.51(0.6) ** | 2.16(0.5) * | 0.0002 |
| Ferritin, μkg/L Mean (SD) | 84.1(80.00) | 179(155) | <0.0001 | 122(154) * | 196(153) * | 0.003 |
| Hemoglobin, g/L Mean (SD) | 135(10.1) | 123(14.0) | <0.0001 | 127(14.7) | 122(13.8) * | 0.09 |
| N/A, N | 0 | 11 | 0.0008 ‡ | 5 | 6 | 0.123 ‡ |
| Parameter | AID + IDA N = 16 | FID + CRA N = 10 | p | AID + IDA N = 26 | FID + CRA N = 60 | p | AID + IDA N = 42 | FID + CRA N = 70 | p | Norm + Iron Over-Load N = 6 |
|---|---|---|---|---|---|---|---|---|---|---|
| I + II Stage | III + IV Stage | Total | ||||||||
| Age, years | 43.1(15.2) | 58.1(10.1) | 0.011 | 51.9(9.03) | 57.4(9.9) | 0.018 | 48.5(12.4) | 57.5(9.9) | <0.0001 | 49(14.7) |
| BMI, kg/m2 | 26.2(4.4) | 27.6(4.0) | 0.414 | 27.7(5.0) | 29.5(5.4) * | 0.151 | 27.12(4.8) | 29.2(5.3) * | 0.036 | 28.7(7.0) |
| Tf, g/L | 2.78(0.4) | 2.05(0.4) ** | 0.0003 | 2.57(0.6) | 1.95(0.4) *** | <0.0001 | 2.65(0.5) | 1.96(0.4) *** | <0.0001 | 2.57(0.6) |
| Parameter | AID+ IDA N = 16 | FID+ CRA N = 10 | p Value | AID+ IDA N = 26 | FID+ CRA N = 60 | p Value | AID+ IDA N = 42 | FID+ CRA N = 70 | p Value | Normal Iron N = 2 | Iron Overload N = 4 | Total CrO N = 118 | Control N = 69 | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I+II Stage | III+IV Stage | Total | ||||||||||||
| Tf low N (%) | 0 | 6 (60) | 0.002 | 3 (12) | 33 (55) | 0.0001 | 3 (7) | 39 (56) | <0.0001 | 0 | 1 (25) | 43 (36) | 2 (3) | <0.0001 |
| Tf > 2g/L N (%) | 16 (100) | 4 (40) | 23 (78) | 27 (45) | 39 (93) | 31 (44) | 2 (100) | 3 (75) | 75 (64) | 67 (97) | ||||
| Parameter | Iron Status in CrO Patients | Control N = 69 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CRA N = 18 | IDA N = 20 | p Value | FID N = 52 | AID N = 22 | p Value | Normal Status N = 2 | Iron Overload N = 4 | p Value | ||
| Age, mean (SD) years | 60 (10) * | 46 (11) * | 0.0002 | 57 (10) | 51 (13) | 0.045 | 49 (20) | 49 (15) | 0.96 | 53 (11) |
| BMI, mean (SD) kg/m2 | 30 (6) | 28 (6) | 0.282 | 29 (5) | 27 (4) | 0.049 | 34 (4) | 26 (7) | 0.275 | 27 (5) |
| Tf, mean (SD) g/L | 1.79 (0.3) *** | 2.64 (0.4) | 0.0001 | 2.02 (0.4) *** | 2.66 (0.6) | 0.0001 | 2.65 (0.2) | 2.54 (0.7) | 0.853 | 2.79 (0.5) |
| SF, mean (SD) μkg/L | 319 (123) *** | 51.5 (29) | 0.0001 | 222 (145) *** | 64.7 (27) | 0.0001 | 94 (7) | 303 (325) *** | 0.440 | 84.1 (80) |
| TSAT, mean (SD) % | 14 (6) *** | 12 (7) *** | 0.584 | 16 (7) *** | 17 (8) *** | 0.695 | 28 (2) | 47 (3) ** | 0.002 | 28 (11) |
| HGB, mean (SD) g/L | 105 (11) *** | 111 (6) *** | 0.05 | 132 (8) | 131 (8) | 0.708 | 127 (5) | 130 (7) | 0.560 | 135 (10) |
| Parameter | Tf | SF | TSAT | Parameter | Tf | SF | TSAT |
|---|---|---|---|---|---|---|---|
| CRA | FID | ||||||
| Control | 0.0001 | 0.0001 | 0.0001 | Control | 0.0001 | 0.0001 | 0.0001 |
| FID | 0.043 | 0.01 | 0.184 | CRA | 0.043 | 0.01 | 0.043 |
| IDA | 0.0001 | 0.0001 | 0.584 | IDA | 0.0001 | 0.0001 | 0.054 |
| AID | 0.0001 | 0.0001 | 0.159 | AID | 0.0001 | 0.0001 | 0.695 |
| Iron overload | 0.003 | 0.861 | 0.0001 | Iron overload | 0.03 | 0.336 | 0.0001 |
| Normal iron status | 0.002 | 0.021 | 0.002 | Normal iron status | 0.04 | 0.223 | 0.015 |
| Patient Code | Age, Years | Diagnosis | TNM | Ratio Holo/Apo Tf |
|---|---|---|---|---|
| 3K1 | 60 | Control | - | 55.65%/44.35% |
| Est1182 | 57 | HGSC | T3cN0M1 | 18.76%/81.24% |
| Est1197 | 63 | HGSC | T3cN0M1 | 12.59%/87.41% |
| Est1195 | 69 | HGSC | T3cN0M1 | 16.33%/83.67% |
| Est1177 | 34 | HGSC | T2bN0M1 | 17.44%/82.56% |
| Est1157 | 57 | HGSC | T3cN0M1 | 10.04%/89.96% |
| Est1187 | 61 | HGSC | T3cN0M1 | 21.92%/78.08% |
| Est1186 | 56 | HGSC | T3cN0M1 | 19.49%/80.51% |
| Est1167 | 70 | LGSC | T3cN0M0 | 23.32%/76.68% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, T.I.; Klabukov, I.D.; Krikunova, L.I.; Poluektova, M.V.; Sychenkova, N.I.; Khorokhorina, V.A.; Vorobyev, N.V.; Gaas, M.Y.; Baranovskii, D.S.; Goryainova, O.S.; et al. Prognostic Value of Serum Transferrin Analysis in Patients with Ovarian Cancer and Cancer-Related Functional Iron Deficiency: A Retrospective Case–Control Study. J. Clin. Med. 2022, 11, 7377. https://doi.org/10.3390/jcm11247377
Ivanova TI, Klabukov ID, Krikunova LI, Poluektova MV, Sychenkova NI, Khorokhorina VA, Vorobyev NV, Gaas MY, Baranovskii DS, Goryainova OS, et al. Prognostic Value of Serum Transferrin Analysis in Patients with Ovarian Cancer and Cancer-Related Functional Iron Deficiency: A Retrospective Case–Control Study. Journal of Clinical Medicine. 2022; 11(24):7377. https://doi.org/10.3390/jcm11247377
Chicago/Turabian StyleIvanova, Tatiana I., Ilya D. Klabukov, Ludmila I. Krikunova, Marina V. Poluektova, Natalia I. Sychenkova, Vera A. Khorokhorina, Nikolay V. Vorobyev, Margarita Ya. Gaas, Denis S. Baranovskii, Oksana S. Goryainova, and et al. 2022. "Prognostic Value of Serum Transferrin Analysis in Patients with Ovarian Cancer and Cancer-Related Functional Iron Deficiency: A Retrospective Case–Control Study" Journal of Clinical Medicine 11, no. 24: 7377. https://doi.org/10.3390/jcm11247377
APA StyleIvanova, T. I., Klabukov, I. D., Krikunova, L. I., Poluektova, M. V., Sychenkova, N. I., Khorokhorina, V. A., Vorobyev, N. V., Gaas, M. Y., Baranovskii, D. S., Goryainova, O. S., Sachko, A. M., Shegay, P. V., Kaprin, A. D., & Tillib, S. V. (2022). Prognostic Value of Serum Transferrin Analysis in Patients with Ovarian Cancer and Cancer-Related Functional Iron Deficiency: A Retrospective Case–Control Study. Journal of Clinical Medicine, 11(24), 7377. https://doi.org/10.3390/jcm11247377







