Hypothermic Oxygenated Machine Perfusion (HOPE) Prior to Liver Transplantation Mitigates Post-Reperfusion Syndrome and Perioperative Electrolyte Shifts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Hypothermic Oxygenated Machine Perfusion (HOPE)
2.3. Static Cold Storage (SCS)
2.4. Perioperative Data Sources
2.5. Outcomes
2.6. Donor-Specific Variables
2.7. Recipient-Specific Variables
2.8. Statistical Analysis
3. Results
3.1. Donor Characteristics
3.2. Recipient Characteristics
3.3. Incidence of Post-Reperfusion Syndrome
3.4. Hemodynamic Chracteristics
3.5. Serum Characteristics and Electrolyte Balance
3.6. Incidence of Early Allograft Dysfunction and Primary Non-Function: Thirty-Day Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aggarwal, S.; Kang, Y.; Freeman, J.A.; Fortunato, F.L.; Pinsky, M.R. Postreperfusion syndrome: Cardiovascular collapse following hepatic reperfusion during liver transplantation. Transpl. Proc. 1987, 19, 54–55. [Google Scholar]
- Siniscalchi, A.; Gamberini, L.; Laici, C.; Bardi, T.; Ercolani, G.; Lorenzini, L.; Faenza, S. Post reperfusion syndrome during liver transplantation: From pathophysiology to therapy and preventive strategies. World J. Gastroenterol. 2016, 22, 1551–1569. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, I.; Horton, C.N.; Planinsic, R.M.; Sakai, T.; Nicolau-Raducu, R.; Damian, D.; Gligor, S.; Marcos, A. The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver Transpl. 2008, 14, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.S.; Kim, H.Y.; Shin, Y.H.; Ko, J.S.; Gwak, M.S.; Sim, W.S.; Kim, G.S.; Lee, S.K. Incidence and predictors of post-reperfusion syndrome in living donor liver transplantation. Clin. Transpl. 2012, 26, 539–543. [Google Scholar] [CrossRef]
- Siniscalchi, A.; Dante, A.; Spedicato, S.; Riganello, L.; Zanoni, A.; Cimatti, M.; Pierucci, E.; Bernardi, E.; Miklosova, Z.; Moretti, C.; et al. Hyperdynamic circulation in acute liver failure: Reperfusion syndrome and outcome following liver transplantation. Transpl. Proc. 2010, 42, 1197–1199. [Google Scholar] [CrossRef]
- Chui, A.K.; Shi, L.; Tanaka, K.; Rao, A.R.; Wang, L.S.; Bookallil, M.; Mayr, M.; Chiu, E.; Verran, D.J.; Mears, D.; et al. Postreperfusion syndrome in orthotopic liver transplantation. Transpl. Proc. 2000, 32, 2116–2117. [Google Scholar] [CrossRef]
- Xu, Z.D.; Xu, H.T.; Yuan, H.B.; Zhang, H.; Ji, R.H.; Zou, Z.; Fu, Z.R.; Shi, X.Y. Postreperfusion syndrome during orthotopic liver transplantation: A single-center experience. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 34–39. [Google Scholar] [CrossRef]
- Fukazawa, K.; Yamada, Y.; Gologorsky, E.; Arheart, K.L.; Pretto, E.A., Jr. Hemodynamic recovery following postreperfusion syndrome in liver transplantation. J. Cardiothorac. Vasc. Anesth. 2014, 28, 994–1002. [Google Scholar] [CrossRef]
- Bukowicka, B.; Akar, R.A.; Olszewska, A.; Smoter, P.; Krawczyk, M. The occurrence of postreperfusion syndrome in orthotopic liver transplantation and its significance in terms of complications and short-term survival. Ann. Transpl. 2011, 16, 26–30. [Google Scholar] [CrossRef]
- Ayanoglu, H.O.; Ulukaya, S.; Tokat, Y. Causes of postreperfusion syndrome in living or cadaveric donor liver transplantations. Transpl. Proc. 2003, 35, 1442–1444. [Google Scholar] [CrossRef]
- Paugam-Burtz, C.; Kavafyan, J.; Merckx, P.; Dahmani, S.; Sommacale, D.; Ramsay, M.; Belghiti, J.; Mantz, J. Postreperfusion syndrome during liver transplantation for cirrhosis: Outcome and predictors. Liver Transpl. 2009, 15, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, M.B.; Sattari, H.; Ghaffaripour, S.; Lahssaee, M.; Salahi, H.; Sahmeddini, M.A.; Bahador, A.; Nikeghbalian, S.; Parsa, S.; Shokrizadeh, S.; et al. Post-reperfusion Syndrome and Outcome Variables after Orthotopic Liver Transplantation. Int. J. Organ. Transpl. Med. 2010, 1, 115–120. [Google Scholar]
- Manning, M.W.; Kumar, P.A.; Maheshwari, K.; Arora, H. Post-Reperfusion Syndrome in Liver Transplantation-An Overview. J. Cardiothorac. Vasc. Anesth. 2020, 34, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Millis, J.M.; Melinek, J.; Csete, M.; Imagawa, D.K.; Olthoff, K.M.; Neelankanta, G.; Braunfeld, M.Y.; Sopher, M.J.; Chan, S.M.; Pregler, J.L.; et al. Randomized controlled trial to evaluate flush and reperfusion techniques in liver transplantation. Transplantation 1997, 63, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Ghafaripour, S.; Sahmeddini, M.A.; Lahsaee, S.M.; Khosravi, M.B.; Sattari, H.; Nikeghbalian, S.; Shokrizadeh, S.; Malekhosseine, S.A. Hypotension after reperfusion in liver transplantation: Histidine-tryptophan-ketoglutarate versus University of Wisconsin solution. Prog. Transpl. 2010, 20, 256–261. [Google Scholar] [CrossRef]
- Fukazawa, K.; Nishida, S.; Hibi, T.; Pretto, E.A., Jr. Crystalloid flush with backward unclamping may decrease post-reperfusion cardiac arrest and improve short-term graft function when compared to portal blood flush with forward unclamping during liver transplantation. Clin. Transpl. 2013, 27, 492–502. [Google Scholar] [CrossRef]
- Jaeschke, H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G15–G26. [Google Scholar] [CrossRef]
- Guan, L.Y.; Fu, P.Y.; Li, P.D.; Li, Z.N.; Liu, H.Y.; Xin, M.G.; Li, W. Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J. Gastrointest. Surg. 2014, 6, 122–128. [Google Scholar] [CrossRef]
- Guarrera, J.V.; Henry, S.D.; Samstein, B.; Odeh-Ramadan, R.; Kinkhabwala, M.; Goldstein, M.J.; Ratner, L.E.; Renz, J.F.; Lee, H.T.; Brown, R.S., Jr.; et al. Hypothermic machine preservation in human liver transplantation: The first clinical series. Am. J. Transpl. 2010, 10, 372–381. [Google Scholar] [CrossRef]
- Dutkowski, P.; Polak, W.G.; Muiesan, P.; Schlegel, A.; Verhoeven, C.J.; Scalera, I.; DeOliveira, M.L.; Kron, P.; Clavien, P.A. First Comparison of Hypothermic Oxygenated Perfusion Versus Static Cold Storage of Human Donation After Cardiac Death Liver Transplants: An International-matched Case Analysis. Ann. Surg. 2015, 262, 764–770; discussion 770–771. [Google Scholar] [CrossRef] [Green Version]
- van Rijn, R.; Karimian, N.; Matton, A.P.M.; Burlage, L.C.; Westerkamp, A.C.; van den Berg, A.P.; de Kleine, R.H.J.; de Boer, M.T.; Lisman, T.; Porte, R.J. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br. J. Surg. 2017, 104, 907–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olthoff, K.M.; Kulik, L.; Samstein, B.; Kaminski, M.; Abecassis, M.; Emond, J.; Shaked, A.; Christie, J.D. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010, 16, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Karangwa, S.; Panayotova, G.; Dutkowski, P.; Porte, R.J.; Guarrera, J.V.; Schlegel, A. Hypothermic machine perfusion in liver transplantation. Int. J. Surg. 2020, 82S, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; Garcia-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020, 11, 2939. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine 2020, 60, 103014. [Google Scholar] [CrossRef] [PubMed]
- Sousa Da Silva, R.X.; Weber, A.; Dutkowski, P.; Clavien, P.A. Machine perfusion in liver transplantation. Hepatology 2022, 76, 1531–1549. [Google Scholar] [CrossRef]
- Gaurav, R.; Atulugama, N.; Swift, L.; Butler, A.J.; Upponi, S.; Brais, R.; Allison, M.; Watson, C.J.E. Bile Biochemistry Following Liver Reperfusion in the Recipient and Its Association with Cholangiopathy. Liver Transpl. 2020, 26, 1000–1009. [Google Scholar] [CrossRef] [Green Version]
- Watson, C.J.E.; Jochmans, I. From “Gut Feeling” to Objectivity: Machine Preservation of the Liver as a Tool to Assess Organ Viability. Curr. Transpl. Rep. 2018, 5, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Dengu, F.; Abbas, S.H.; Ebeling, G.; Nasralla, D. Normothermic Machine Perfusion (NMP) of the Liver as a Platform for Therapeutic Interventions during Ex-Vivo Liver Preservation: A Review. J. Clin. Med. 2020, 9, 1046. [Google Scholar] [CrossRef] [Green Version]
- Guarrera, J.V.; Henry, S.D.; Chen, S.W.; Brown, T.; Nachber, E.; Arrington, B.; Boykin, J.; Samstein, B.; Brown, R.S., Jr.; Emond, J.C.; et al. Hypothermic machine preservation attenuates ischemia/reperfusion markers after liver transplantation: Preliminary results. J. Surg. Res. 2011, 167, e365–e373. [Google Scholar] [CrossRef] [PubMed]
- Muller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Wurdinger, M.; Meierhofer, D.; Clavien, P.A.; Dutkowski, P. Novel Real-time Prediction of Liver Graft Function During Hypothermic Oxygenated Machine Perfusion Before Liver Transplantation. Ann. Surg. 2019, 270, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Stoll, W.D.; Hand, W.R.; Chavin, K.D.; Felton, D.H.; Wolf, B.O.; Davis, G.P.; Harvey, N.R.; Whiteley, J.R.; Mester, R.A.; Bolin, E.D. Post-Reperfusion Syndrome in Liver Transplantation: Does a Caval Blood Flush Vent Help? Ann. Transpl. 2019, 24, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Gurusamy, K.S.; Naik, P.; Abu-Amara, M.; Fuller, B.; Davidson, B.R. Techniques of flushing and reperfusion for liver transplantation. Cochrane Database Syst. Rev. 2012, 3, Cd007512. [Google Scholar] [CrossRef]
- Brems, J.J.; Takiff, H.; McHutchison, J.; Collins, D.; Biermann, L.A.; Pockros, P. Systemic versus nonsystemic reperfusion of the transplanted liver. Transplantation 1993, 55, 527–529. [Google Scholar] [CrossRef]
- Rønholm, E.; Tomasdottir, H.; Runeborg, J.; Bengtsson, A.; Bengtson, J.P.; Stenqvist, O.; Friman, S. Complement system activation during orthotopic liver transplantation in man. Indications of peroperative complement system activation in the gut. Transplantation 1994, 57, 1594–1597. [Google Scholar] [CrossRef]
- Bellamy, M.C.; Galley, H.F.; Webster, N.R. Changes in inflammatory mediators during orthotopic liver transplantation. Br. J. Anaesth. 1997, 79, 338–341. [Google Scholar] [CrossRef] [Green Version]
- Olthof, P.B.; Reiniers, M.J.; Dirkes, M.C.; Gulik, T.M.V.; Golen, R.F.V. Protective Mechanisms of Hypothermia in Liver Surgery and Transplantation. Mol. Med. 2016, 21, 833–846. [Google Scholar] [CrossRef]
- Belzer, F.O.; Southard, J.H. Principles of solid-organ preservation by cold storage. Transplantation 1988, 45, 673–676. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Carini, R.; Bellomo, G.; Benedetti, A.; Fulceri, R.; Gamberucci, A.; Parola, M.; Dianzani, M.U.; Albano, E. Alteration of Na+ homeostasis as a critical step in the development of irreversible hepatocyte injury after adenosine triphosphate depletion. Hepatology 1995, 21, 1089–1098. [Google Scholar]
- Lozanovski, V.J.; Dohler, B.; Weiss, K.H.; Mehrabi, A.; Susal, C. The Differential Influence of Cold Ischemia Time on Outcome After Liver Transplantation for Different Indications-Who Is at Risk? A Collaborative Transplant Study Report. Front. Immunol. 2020, 11, 892. [Google Scholar] [CrossRef] [PubMed]
- Kron, P.; Schlegel, A.; Mancina, L.; Clavien, P.A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J. Hepatol. 2017, 68, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, F.S.; Tian, M.; Zhu, Z.J. Elevated effluent potassium concentrations predict the development of postreperfusion hyperkalemia in deceased liver transplantation: A retrospective cohort study. BMC Anesthesiol. 2022, 22, 161. [Google Scholar] [CrossRef] [PubMed]
- Acosta, F.; Sansano, T.; Contreras, R.F.; Reche, M.; Beltran, R.; Roques, V.; Rodriguez, M.A.; Robles, R.; Bueno, F.S.; Ramirez, P.; et al. Changes in serum potassium during reperfusion in liver transplantation. Transpl. Proc. 1999, 31, 2382–2383. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Del Val, A.; Guarrera, J.; Porte, R.J.; Selzner, M.; Spiro, M.; Raptis, D.A.; Friend, P.J. Does machine perfusion improve immediate and short-term outcomes by enhancing graft function and recipient recovery after liver transplantation?—A systematic review of the literature, meta-analysis and expert panel recommendations. Clin. Transpl. 2022, e14638. [Google Scholar] [CrossRef]
- Yang, L.S.; Shan, L.L.; Saxena, A.; Morris, D.L. Liver transplantation: A systematic review of long-term quality of life. Liver Int. 2014, 34, 1298–1313. [Google Scholar] [CrossRef]
- Czigany, Z.; Schoning, W.; Ulmer, T.F.; Bednarsch, J.; Amygdalos, I.; Cramer, T.; Rogiers, X.; Popescu, I.; Botea, F.; Fronek, J.; et al. Hypothermic oxygenated machine perfusion (HOPE) for orthotopic liver transplantation of human liver allografts from extended criteria donors (ECD) in donation after brain death (DBD): A prospective multicentre randomised controlled trial (HOPE ECD-DBD). BMJ Open 2017, 7, e017558. [Google Scholar] [CrossRef]
- Czigany, Z.; Pratschke, J.; Fronek, J.; Guba, M.; Schoning, W.; Raptis, D.A.; Andrassy, J.; Kramer, M.; Strnad, P.; Tolba, R.H.; et al. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-transplant Outcomes in Extended Criteria Donation Liver Transplantation From Donation After Brain Death: Results From a Multicenter Randomized Controlled Trial (HOPE ECD-DBD). Ann. Surg. 2021, 274, 705–712. [Google Scholar] [CrossRef]


| Donor Characteristics and Ischemic Times at Liver Transplantation | All, N (%)/Mean [95% CI] | Liver Graft Preservation Techniques | p-Value (HOPE vs. SCS) | |
|---|---|---|---|---|
| -SCS- N (%)/Mean [95% CI] | -HOPE- N (%)/Mean [95% CI] | |||
| Sex | 0.69 | |||
| Male | 51 (51.0%) | 24 (48.0%) | 27 (54.0%) | |
| Female | 49 (49.0%) | 26 (52.0%) | 23 (46.0%) | |
| Age (y) | ||||
| 52.1 [48.7–55.4] | 55.1 [50.5–59.6] | 49.2 [44.4–54.0] | 0.05 | |
| Donor Risk Index (DRI) | ||||
| 2.16 [2.08–2.24] | 2.21 [2.08–2.33] | 2.11 [2.00–2.33] | 0.38 | |
| Cold Ischemic Time (CIT) | ||||
| 603.9 [574.0–633.7] | 599.0 [554.3–643.7] | 608.0 [566.5–649.6] | 0.96 | |
| Warm Ischemic Time (WIT) | ||||
| 55.3 [51.1–59.6] | 52.4 [46.4–58.5] | 58.1 [52.0–64.2] | 0.25 | |
| Total | 100 (100%) | 50 (50.0%) | 50 (50.0%) | |
| Recipient Characteristics and Control Variables at Date of Liver Transplantation | All, N (%)/Mean [95% CI] | Liver Graft Preservation Techniques | p-Value (HOPE vs. SCS) | |
|---|---|---|---|---|
| -SCS- N (%)/Mean [95% CI] | -HOPE- N (%)/Mean [95% CI] | |||
| Sex | 0.086 | |||
| Male | 68 (68.0%) | 38 (76.0%) | 30 (60.0%) | |
| Female | 32 (32.0%) | 12 (24.0%) | 20 (40.0%) | |
| Age (y) | ||||
| 51.8 [49.7–54.0] | 50.6 [47.2–54.0] | 53.0 [50.3–55.8] | 0.37 | |
| BMI | ||||
| 25.4 [24.5–26.3] | 25.4 [24.1–26.8] | 25.3 [24.0–26.7] | 0.80 | |
| match-MELD Score | ||||
| 21.3 [19.3–23.3] | 20.4 [17.6–23.2] | 22.1 [19.2–25.1] | 0.44 | |
| ASA Score | ||||
| 3.6 [3.5–3.7] | 3.5 [3.3–3.6] | 3.7 [3.6–3.9] | 0.013 | |
| Healthcare setting prior to transplant | 0.16 | |||
| ICU/IMC | 24 (24.0%) | 9 (18.0%) | 15 (30.0%) | |
| Regular ward/outpatient | 76 (76.0%) | 41 (82.0%) | 35 (70.0%) | |
| CHILD-Pugh Score | 0.43 | |||
| No cirrhosis | 20 (20.0%) | 12 (24.0%) | 8 (16.0%) | |
| A | 20 (20.0%) | 12 (24.0%) | 8 (16.0%) | |
| B | 31 (31.0%) | 14 (28.0%) | 17 (34.0%) | |
| C | 29 (29.0%) | 12 (24.0%) | 17 (34.0%) | |
| Number of Comorbidities * | 0.32 | |||
| 0–1 | 55 (55.0%) | 30 (60.0%) | 25 (50.0%) | |
| 2–3 | 35 (35.0%) | 14 (28.0%) | 21 (42.0%) | |
| ≥4 | 10 (10.0%) | 6 (12.0%) | 4 (8.0%) | |
| Mean | 1.7 [1.4–1.9] | 1.6 [1.2–2.1] | 1.7 [1.3–2.0] | 0.52 |
| Indication for transplant | 0.42 | |||
| Acute Liver Failure | 14 (14.0%) | 9 (18.0%) | 5 (10.0%) | |
| Alcoholic Cirrhosis | 29 (29.0%) | 11 (22.0%) | 18 (36.0%) | |
| HCC | 25 (25.0%) | 16 (32.0%) | 9 (18.0%) | |
| NASH | 3 (3.0%) | 1 (2.0%) | 2 (4.0%) | |
| PSC | 19 (19.0%) | 10 (20.0%) | 9 (18.0%) | |
| Viral Hepatitis | 24 (24.0%) | 16 (32.0%) | 8 (16.0%) | |
| Autoimmune Hepatitis | 15 (15.0%) | 8 (8.0%) | 7 (14.0%) | |
| Other ** | 21 (21.0%) | 10 (20.0%) | 11 (22.0%) | |
| Early allograft dysfunction (EAD) | 0.04 | |||
| EAD | 39 (39.0%) | 25 (50.0%) | 14 (28.0%) | |
| No EAD | 61 (61.0%) | 25 (50.0%) | 36 (72.0%) | |
| Primary Non Function (PNF) | 0.24 | |||
| PNF | 3 (3.0%) | 3 (3.0%) | 0 (0.0%) | |
| No PNF | 97 (97.0%) | 47 (47.0%) | 50 (50.0%) | |
| 30-day Survival | 0.36 | |||
| Survival | 96 (96.0%) | 47 (94%) | 49 (98%) | |
| No Survival | 4 (4.0%) | 3 (6.0%) | 1 (2.0%) | |
| Total | 100 (100%) | 50 (50.0%) | 50 (50.0%) | |
| Perioperative Variables and Fluid/Transfusion Management during Liver Transplantation | All, N (%)/Mean [95% CI] | Liver Graft Preservation Techniques | p-Value (HOPE vs. SCS) | |
|---|---|---|---|---|
| -SCS- N (%)/Mean [95% CI] | -HOPE- N (%)/Mean [95% CI] | |||
| Packed RBC (mL) | ||||
| 1315 [1049–1521] | 1235 [846–1625] | 1395 [1021–1769] | 0.40 | |
| FFP (mL) | ||||
| 2487 [2094–2880] | 2174 [1616–2732] | 2800 [2242–3358] | 0.045 | |
| Platelet Transfusion (mL) | ||||
| 366 [261–471] | 294 [159–429] | 438 [274–602] | 0.19 | |
| Machine Auto-Transfusion (mL) | ||||
| 663 [465–863] | 603 [377–829] | 724 [389–1059] | 0.60 | |
| Crystalloid Infusion (mL) | ||||
| 6006 [5470–6542] | 6161 [5517–6805] | 5851 [4973–6730] | 0.09 | |
| Colloid Infusion (mL) | ||||
| 529 [433–624] | 602 [458–746] | 455 [328–582] | 0.09 | |
| Tranexamic acid (mg) | ||||
| 601 [429–773] | 731 [447–1016] | 471 [273–669] | 0.26 | |
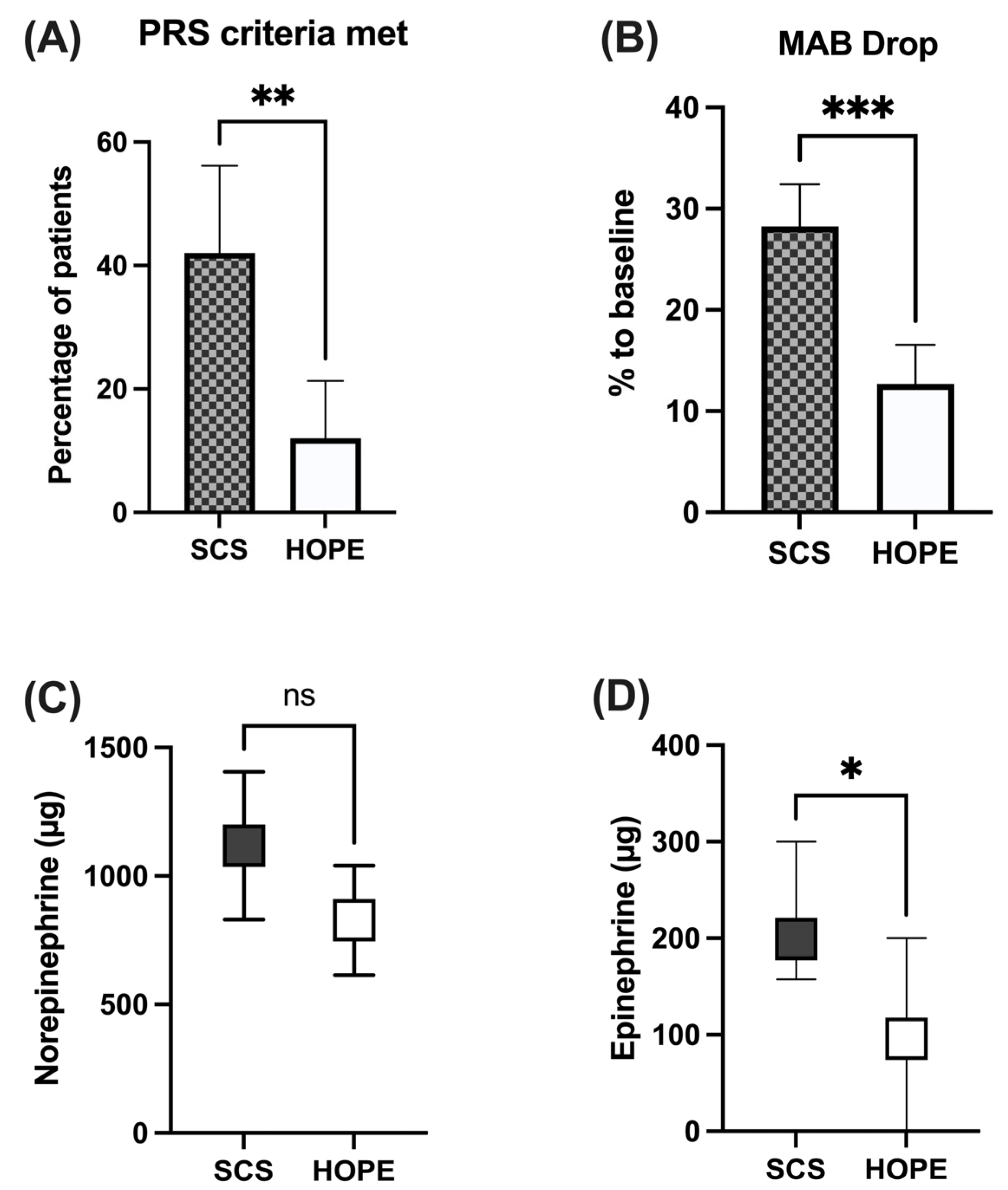
| MAB Drop (%) | ||||
| 20.5 [17.3–23.7] | 28.2 [24.1–32.4] | 12.7 [8.8–16.5] | <0.001 | |
| PRS Criteria | 0.0013 | |||
| PRS Criteria met | 27 (27.0%) | 21 (42.0%) | 6 (12.0%) | |
| PRS Criteria not met | 73 (73.0%) | 29 (58.0%) | 44 (88.0%) | |
| Increase in Norepinephrine (µg) | ||||
| 973 [795–1151] | 960 [830–1405] | 750 [614–1041] | 0.12 | |
| Increase in Epinephrine (µg) | ||||
| 153 [−34–340] | 199 [212–412] | 96 [−368–355] | 0.018 | |
| Increase in Vasopressin (IE) | ||||
| 0.31 [0.17–0.45] | 0.29 [0.10–0.49] | 0.33 [0.11–0.54] | 0.899 | |
| Potassium Drop (%) | ||||
| 7.6 [4.3–10.8] | −1 [−3.4–5.5] | 14.1 [10.0–18.1] | <0.001 | |
| Potassium Substitution | 0.0135 | |||
| Substitution | 28 (28.0%) | 8 (16.0%) | 20 (40.0%) | |
| No Substitution | 72 (72.0%) | 42 (84.0%) | 30 (60.0%) | |
| Total | 100 (100%) | 50 (50.0%) | 50 (50.0%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horné, F.; Drefs, M.; Schirren, M.J.; Koch, D.T.; Cepele, G.; Jacobi, S.J.; Payani, E.; Börner, N.; Werner, J.; Guba, M.O.; et al. Hypothermic Oxygenated Machine Perfusion (HOPE) Prior to Liver Transplantation Mitigates Post-Reperfusion Syndrome and Perioperative Electrolyte Shifts. J. Clin. Med. 2022, 11, 7381. https://doi.org/10.3390/jcm11247381
Horné F, Drefs M, Schirren MJ, Koch DT, Cepele G, Jacobi SJ, Payani E, Börner N, Werner J, Guba MO, et al. Hypothermic Oxygenated Machine Perfusion (HOPE) Prior to Liver Transplantation Mitigates Post-Reperfusion Syndrome and Perioperative Electrolyte Shifts. Journal of Clinical Medicine. 2022; 11(24):7381. https://doi.org/10.3390/jcm11247381
Chicago/Turabian StyleHorné, Fabian, Moritz Drefs, Malte Joachim Schirren, Dominik Thomas Koch, Ganildo Cepele, Severin Johannes Jacobi, Elnaz Payani, Nikolaus Börner, Jens Werner, Markus Otto Guba, and et al. 2022. "Hypothermic Oxygenated Machine Perfusion (HOPE) Prior to Liver Transplantation Mitigates Post-Reperfusion Syndrome and Perioperative Electrolyte Shifts" Journal of Clinical Medicine 11, no. 24: 7381. https://doi.org/10.3390/jcm11247381







