Development of a Novel Scoring Model to Estimate the Severity Grade of Mesenteric Artery Stenosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Definitions
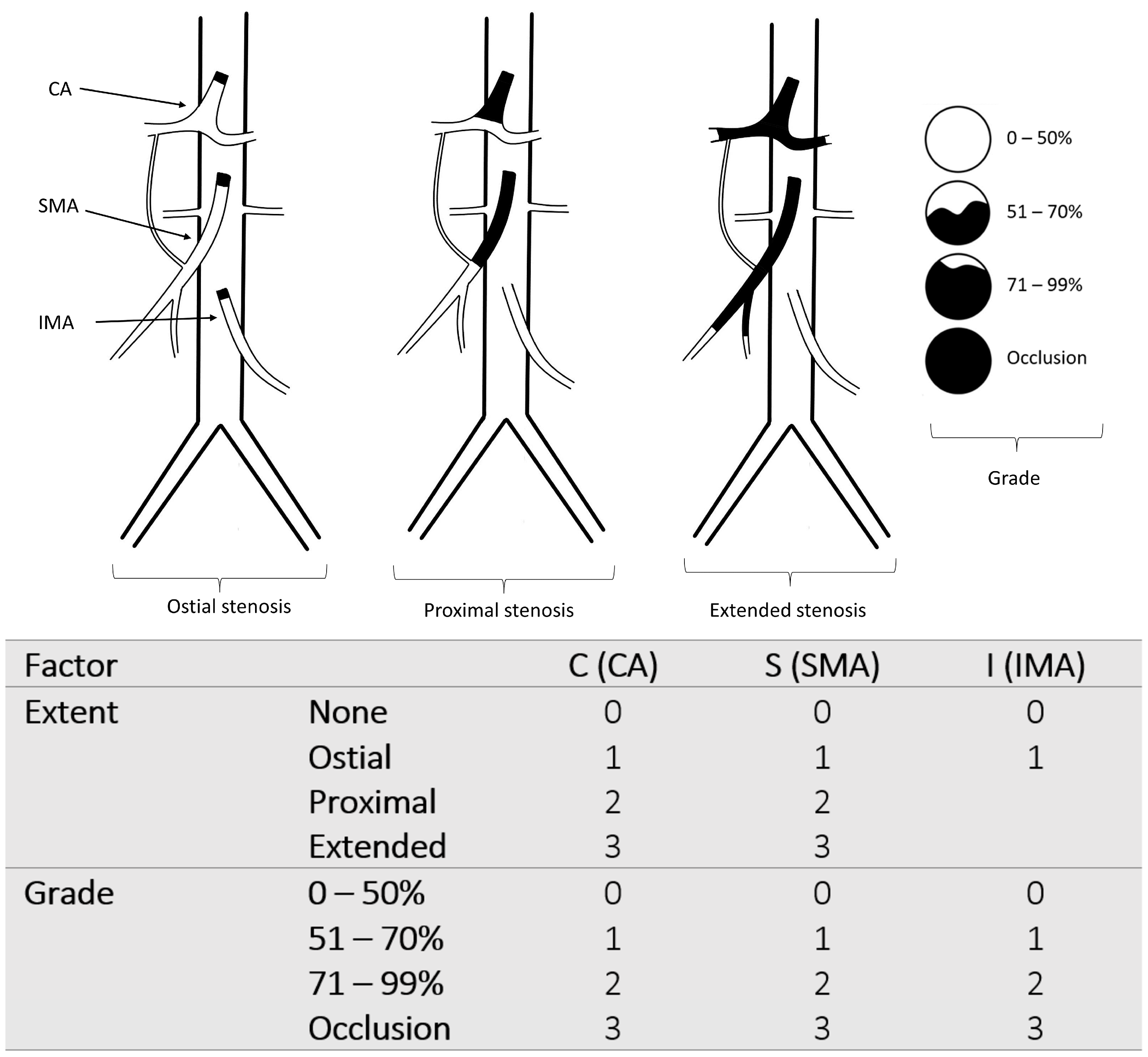
2.3. The New Classification
2.4. Development of the CSI-Score and Endpoints
2.5. Bootstrap Analysis and Score Validation
2.6. Statistical Methods
3. Results
3.1. Study Populations
3.2. Risk Factors
3.3. Clinical Presentation and Manifestations
3.4. Treatment and Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huber, T.S.; Björck, M.; Chandra, A.; Clouse, W.D.; Dalsing, M.C.; Oderich, G.S.; Smeds, M.R.; Murad, M.H. Chronic mesenteric ischemia: Clinical practice guidelines from the Society for Vascular Surgery. J. Vasc. Surg. 2021, 73, 87S–115S. [Google Scholar] [CrossRef] [PubMed]
- Bordet, M.; Tresson, P.; Huvelle, U.; Long, A.; Passot, G.; Bergoin, C.; Lermusiaux, P.; Millon, A.; Della Schiava, N. Natural History of Asymptomatic Superior Mesenteric Arterial Stenosis Depends on Coeliac and Inferior Mesenteric Artery Status. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 810–818. [Google Scholar] [CrossRef]
- Wilson, D.B.; Mostafavi, K.; Craven, T.E.; Ayerdi, J.; Edwards, M.S.; Hansen, K.J. Clinical course of mesenteric artery stenosis in elderly americans. Arch. Intern. Med. 2006, 166, 2095–2100. [Google Scholar] [CrossRef] [Green Version]
- Björck, M.; Koelemay, M.; Acosta, S.; Bastos Goncalves, F.; Kölbel, T.; Kolkman, J.J.; Lees, T.; Lefevre, J.H.; Menyhei, G.; Oderich, G.; et al. Editor’s Choice—Management of the Diseases of Mesenteric Arteries and Veins: Clinical Practice Guidelines of the European Society of Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 460–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, H.; Takase, K.; Rikimaru, H.; Tsuboi, M.; Yamada, T.; Sato, A.; Higano, S.; Ishibashi, T.; Takahashi, S. Quantitative vascular measurements in arterial occlusive disease. Radiographics 2005, 25, 1141–1158. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.V.D.; Barbosa, A.B.M.; Targino, V.A.; Silva, N.d.A.; Silva, Y.C.d.M.; Barbosa, F.; Oliveira, A.d.S.B.; Assis, T.d.O. Anatomical Variations of The Celiac Trunk: A Systematic Review. Arq. Bras. Cir. Dig. 2018, 31, e1403. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.G.O.; Barbosa, A.B.M.; Silva, N.d.A.; AraÚjo, D.N.; Assis, T.d.O. Anatomical Variations of the Superior Mesenteric Artery and Its Clinical and Surgical Implications in Humans. Arq. Bras. Cir. Dig. 2020, 33, e1508. [Google Scholar] [CrossRef]
- Harki, J.; Vergouwe, Y.; Spoor, J.A.; Mensink, P.B.; Bruno, M.J.; van Noord, D.; Kuipers, E.J.; Tjwa, E.T.T.L. Diagnostic Accuracy of the Combination of Clinical Symptoms and CT or MR Angiography in Patients with Chronic Gastrointestinal Ischemia. J. Clin. Gastroenterol. 2017, 51, e39–e47. [Google Scholar] [CrossRef]
- Terlouw, L.G.; van Noord, D.; van Walsum, T.; van Dijk, L.J.D.; Moelker, A.; Bruno, M.J. Early risk stratification of patients with suspected chronic mesenteric ischaemia using a symptom and mesenteric artery calcium score based score chart. United Eur. Gastroenterol. J. 2021, 9, 626–634. [Google Scholar] [CrossRef]
- Thomas, J.H.; Blake, K.; Pierce, G.E.; Hermreck, A.S.; Seigel, E. The clinical course of asymptomatic mesenteric arterial stenosis. J. Vasc. Surg. 1998, 27, 840–844. [Google Scholar] [CrossRef]
- Barret, M.; Martineau, C.; Rahmi, G.; Pellerin, O.; Sapoval, M.; Alsac, J.-M.; Fabiani, J.-N.; Malamut, G.; Samaha, E.; Cellier, C. Chronic Mesenteric Ischemia: A Rare Cause of Chronic Abdominal Pain. Am. J. Med. 2015, 128, 1363.e1–1363.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dijk, L.J.D.; Moons, L.M.G.; van Noord, D.; Moelker, A.; Verhagen, H.J.M.; Bruno, M.J.; Rouwet, E.V. Persistent symptom relief after revascularization in patients with single-artery chronic mesenteric ischemia. J. Vasc. Surg. 2018, 68, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.C.; Jianu, A.M.; Manta, B.A.; Hostiuc, S. Aortic Origins of the Celiac Trunk and Superior Mesenteric Artery. Diagnostics 2021, 11, 1111. [Google Scholar] [CrossRef] [PubMed]
- Oderich, G.S.; Erdoes, L.S.; Lesar, C.; Mendes, B.C.; Gloviczki, P.; Cha, S.; Duncan, A.A.; Bower, T.C. Comparison of covered stents versus bare metal stents for treatment of chronic atherosclerotic mesenteric arterial disease. J. Vasc. Surg. 2013, 58, 1316–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogendoorn, W.; Hunink, M.G.M.; Schlösser, F.J.V.; Moll, F.L.; Muhs, B.E.; Sumpio, B.E. A comparison of open and endovascular revascularization for chronic mesenteric ischemia in a clinical decision model. J. Vasc. Surg. 2014, 60, 715–725.e2. [Google Scholar] [CrossRef] [Green Version]
- Saedon, M.; Saratzis, A.; Karim, A.; Goodyear, S. Endovascular Versus Surgical Revascularization for the Management of Chronic Mesenteric Ischemia. Vasc. Endovasc. Surg. 2015, 49, 37–44. [Google Scholar] [CrossRef]
- Ahanchi, S.S.; Stout, C.L.; Dahl, T.J.; Carty, R.L.; Messerschmidt, C.A.; Panneton, J.M. Comparative analysis of celiac versus mesenteric artery outcomes after angioplasty and stenting. J. Vasc. Surg. 2013, 57, 1062–1066. [Google Scholar] [CrossRef] [Green Version]
- Schermerhorn, M.L.; Giles, K.A.; Hamdan, A.D.; Wyers, M.C.; Pomposelli, F.B. Mesenteric revascularization: Management and outcomes in the United States, 1988-2006. J. Vasc. Surg. 2009, 50, 341–348.e1. [Google Scholar] [CrossRef] [Green Version]
- Tallarita, T.; Oderich, G.S.; Gloviczki, P.; Duncan, A.A.; Kalra, M.; Cha, S.; Misra, S.; Bower, T.C. Patient survival after open and endovascular mesenteric revascularization for chronic mesenteric ischemia. J. Vasc. Surg. 2013, 57, 747–755; discussion 754–755. [Google Scholar] [CrossRef] [Green Version]
- van Dijk, L.J.; van Noord, D.; Geelkerken, R.H.; Harki, J.; Berendsen, S.A.; Vries, A.C.D.; Moelker, A.; Vergouwe, Y.; Verhagen, H.J.; Kolkman, J.J.; et al. Validation of a score chart to predict the risk of chronic mesenteric ischemia and development of an updated score chart. United Eur. Gastroenterol. J. 2019, 7, 1261–1270. [Google Scholar] [CrossRef]
- Alam, W.; Kamareddine, M.H.; Geahchan, A.; Ghosn, Y.; Feghaly, M.; Chamseddine, A.; Bou Khalil, R.; Farhat, S. Celiacomesenteric trunk associated with superior mesenteric artery aneurysm: A case report and review of literature. SAGE Open Med. Case Rep. 2020, 8, 2050313X20938243. [Google Scholar] [CrossRef]
- van Petersen, A.S.; Kolkman, J.J.; Gerrits, D.G.; van der Palen, J.; Zeebregts, C.J.; Geelkerken, R.H. Clinical significance of mesenteric arterial collateral circulation in patients with celiac artery compression syndrome. J. Vasc. Surg. 2017, 65, 1366–1374. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, E.L. The Society for Vascular Surgery clinical practice guidelines define the optimal care of patients with chronic mesenteric ischemia. J. Vasc. Surg. 2021, 73, 84S–86S. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, R.M.; Fillinger, M.F.; Walsh, D.B.; LaBombard, F.A.; Musson, A.; Darling, C.E.; Cronenwett, J.L. Mesenteric and celiac duplex scanning: A validation study. J. Vasc. Surg. 1998, 27, 1078–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsburg, M.; Obara, P.; Lambert, D.L.; Hanley, M.; Steigner, M.L.; Camacho, M.A.; Chandra, A.; Chang, K.J.; Gage, K.L.; Peterson, C.M.; et al. ACR Appropriateness Criteria® Imaging of Mesenteric Ischemia. J. Am. Coll. Radiol. 2018, 15, S332–S340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aburahma, A.F.; Stone, P.A.; Srivastava, M.; Dean, L.S.; Keiffer, T.; Hass, S.M.; Mousa, A.Y. Mesenteric/celiac duplex ultrasound interpretation criteria revisited. J. Vasc. Surg. 2012, 55, 428–436.e6; discussion 435–436. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, I.D.C.; Kroeker, M.A.; Greenberg, H.M. Biphasic CT with mesenteric CT angiography in the evaluation of acute mesenteric ischemia: Initial experience. Radiology 2003, 229, 91–98. [Google Scholar] [CrossRef]
- Savastano, S.; Teso, S.; Corrà, S.; Fantozzi, O.; Miotto, D. Multislice CT angiography of the celiac and superior mesenteric arteries: Comparison with arteriographic findings. Radiol. Med. 2002, 103, 456–463. [Google Scholar]
- Shih, M.-C.P.; Hagspiel, K.D. CTA and MRA in mesenteric ischemia: Part 1, Role in diagnosis and differential diagnosis. Am. J. Roentgenol. 2007, 188, 452–461. [Google Scholar] [CrossRef]
- Blachar, A.; Barnes, S.; Adam, S.Z.; Levy, G.; Weinstein, I.; Precel, R.; Federle, M.P.; Sosna, J. Radiologists’ performance in the diagnosis of acute intestinal ischemia, using MDCT and specific CT findings, using a variety of CT protocols. Emerg. Radiol. 2011, 18, 385–394. [Google Scholar] [CrossRef]
- Kärkkäinen, J.M.; Saari, P.; Kettunen, H.-P.; Lehtimäki, T.T.; Vanninen, R.; Paajanen, H.; Manninen, H. Interpretation of Abdominal CT Findings in Patients Who Develop Acute on Chronic Mesenteric Ischemia. J. Gastrointest. Surg. 2016, 20, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Ofer, A.; Abadi, S.; Nitecki, S.; Karram, T.; Kogan, I.; Leiderman, M.; Shmulevsky, P.; Israelit, S.; Engel, A. Multidetector CT angiography in the evaluation of acute mesenteric ischemia. Eur. Radiol. 2009, 19, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Oliva, I.B.; Davarpanah, A.H.; Rybicki, F.J.; Desjardins, B.; Flamm, S.D.; Francois, C.J.; Gerhard-Herman, M.D.; Kalva, S.P.; Ashraf Mansour, M.; Mohler, E.R.; et al. ACR Appropriateness Criteria ® imaging of mesenteric ischemia. Abdom. Imaging 2013, 38, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Moschetta, M.; Telegrafo, M.; Rella, L.; Stabile Ianora, A.A.; Angelelli, G. Multi-detector CT features of acute intestinal ischemia and their prognostic correlations. World J. Radiol. 2014, 6, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Tzortzakakis, A.; Kalarakis, G.; Huang, B.; Terezaki, E.; Koltsakis, E.; Kechagias, A.; Tsekrekos, A.; Rouvelas, I. Role of Radiology in the Preoperative Detection of Arterial Calcification and Celiac Trunk Stenosis and Its Association with Anastomotic Leakage Post Esophagectomy, an Up-to-Date Review of the Literature. Cancers 2022, 14, 1016. [Google Scholar] [CrossRef] [PubMed]
- Malviya, K.K.; Verma, A.; Nayak, A.K.; Mishra, A.; More, R.S. Unraveling Variations in Celiac Trunk and Hepatic Artery by CT Angiography to Aid in Surgeries of Upper Abdominal Region. Diagnostics 2021, 11, 2262. [Google Scholar] [CrossRef]
- Dumitrescu, C.I.; Ciobirca, C.; Popa, R.T.; Dumitrescu, D.; Tambura, C.G.; Ciobirca, D.M.; Stavaru, R.; Tiuca, M.F.; Maces, S.; Barbulescu, L.F.; et al. Preoperative Planning for Superior Mesenteric Artery Aneurysm. Appl. Sci. 2021, 11, 10311. [Google Scholar] [CrossRef]
- Cardia, P.P.; Penachim, T.J.; Prando, A.; Torres, U.S.; D’Ippólito, G. Non-contrast MR angiography using three-dimensional balanced steady-state free-precession imaging for evaluation of stenosis in the celiac trunk and superior mesenteric artery: A preliminary comparative study with computed tomography angiography. Br. J. Radiol. 2017, 90, 20170011. [Google Scholar] [CrossRef]
- Glockner, J.F. Three-dimensional gadolinium-enhanced MR angiography: Applications for abdominal imaging. Radiographics 2001, 21, 357–370. [Google Scholar] [CrossRef]
- Simonini, R.; Bonaffini, P.A.; Porta, M.; Maino, C.; Carbone, F.S.; Dulcetta, L.; Brambilla, P.; Marra, P.; Sironi, S. Accuracy of Inflow Inversion Recovery (IFIR) for Upper Abdominal Arteries Evaluation: Comparison with Contrast-Enhanced MR and CTA. Diagnostics 2022, 12, 825. [Google Scholar] [CrossRef]
- Zeller, T.; Rastan, A.; Sixt, S. Chronic atherosclerotic mesenteric ischemia (CMI). Vasc. Med. 2010, 15, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.K.; Kalva, S.P.; Hsu, S.L.; Walker, T.G.; Silberzweig, J.E.; Annamalai, G.; Baerlocher, M.O.; Mitchell, J.W.; Midia, M.; Nikolic, B.; et al. Quality Improvement Guidelines for Mesenteric Angioplasty and Stent Placement for the Treatment of Chronic Mesenteric Ischemia. J. Vasc. Interv. Radiol. 2018, 29, 642–647. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Total | Low Score < 8 | High Score ≥ 8 | p |
|---|---|---|---|---|
| Number | 242 | 100 | 142 | |
| CMI | 109 (45) | 11 (11) | 98 (69) | <0.001 * |
| Demographics | ||||
| Age (median, range, y) | 71 (32–99) | 71 (32–90) | 71 (42–99) | 0.926 |
| Female | 128 (53) | 48 (48) | 80 (56) | 0.201 |
| Risk factors | ||||
| Coronary artery disease | 112 (46) | 36 (36) | 76 (54) | 0.007 * |
| Diabetes mellitus | 58 (24) | 18 (18) | 40 (28) | 0.068 |
| Hypertension | 180 (74) | 68 (68) | 112 (79) | 0.056 |
| Hyperlipoproteinemia | 64 (26) | 23 (23) | 41 (29) | 0.308 |
| Stroke | 50 (21) | 16 (16) | 34 (24) | 0.133 |
| COPD | 60 (25) | 24 (24) | 36 (25) | 0.810 |
| Chronic renal insufficiency | 101 (42) | 30 (30) | 71 (50) | 0.002 * |
| Smoking | 75 (31) | 28 (28) | 47 (33) | 0.398 |
| Alcoholism | 16 (7) | 6 (6) | 10 (7) | 748 |
| PAD | 95 (39) | 16 (16) | 79 (57) | <0.001 * |
| ASA class | 0.002 * | |||
| I | 3 (1) | 3 (3) | 0 | |
| II | 54 (22) | 31 (31) | 23 (16) | |
| III | 156 (66) | 59 (59) | 97 (68) | |
| IV | 29 (12) | 7 (7) | 22 (16) | |
| Number of affected vessels | <0.001 * | |||
| None | 46 (19) | 46 (46) | 0 | |
| One | 52 (22) | 48 (48) | 4 (3) | |
| Two | 53 (22) | 6 (6) | 47 (33) | |
| Three | 91 (38) | 0 | 91 (64) | |
| Stenotic arteries | <0.001 * | |||
| None | 46 (19) | 46 (46) | 0 | |
| CA | 22 (9) | 20 (20) | 2 (1) | |
| SMA | 15 (6) | 13 (13) | 2 (1) | |
| IMA | 15 (6) | 15 (15) | 0 | |
| CA and SMA | 34 (14) | 2 (2) | 32 (23) | |
| CA and IMA | 6 (3) | 2 (2) | 4 (3) | |
| SMA and IMA | 13 (5) | 2 (2) | 11 (8) | |
| CA and SMA and IMA | 91 (38) | 0 | 91 (64) |
| Characteristics | Total | Low Score < 8 | High Score ≥ 8 | p |
|---|---|---|---|---|
| Number | 242 | 100 | 142 | |
| Clinical Presentation | ||||
| Abdominal pain | 147 (61) | 27 (27) | 120 (85) | <0.001 * |
| Postprandial pain | 95 (39) | 17 (17) | 78 (55) | <0.001 * |
| Constant abdominal pain | 52 (22) | 10 (10) | 42 (30) | <0.001 * |
| BMI | 24 ± 5 | 25 ± 5 | 23 ± 5 | 0.009 * |
| Underweight BMI < 18.5 | 28 (12) | 4 (4) | 24 (17) | 0.002 * |
| Weight loss | 68 (28) | 13 (13) | 55 (38) | <0.001 * |
| 5–10% of BM | 33 (14) | 5 (5) | 28 (20) | 0.001 * |
| >10% | 35 (15) | 8 (8) | 27 (19) | 0.016 * |
| Fear of food | 10 (4) | 1 (1) | 9 (6) | 0.050 * |
| Nausea | 39 (16) | 15 (15) | 24 (17) | 0.692 |
| Vomiting | 26 (11) | 8 (8) | 18 (13) | 0.247 |
| Loss of appetite | 80 (33) | 20 (20) | 60 (42) | <0.001 * |
| Diarrhea | 25 (10) | 10 (10) | 15 (11) | 0.887 |
| Exercise-induced abdominal pain | 3 (1) | 0 | 3 (2) | 0.270 |
| Gastrointestinal manifestation | ||||
| Ischemic gastritis | 25 (10) | 4 (4) | 21 (15) | 0.007 * |
| Colonic ischemia | 44 (18) | 19 (19) | 25 (18) | 0.782 |
| Ischemic hepatitis | 1(0.4) | 0 | 1 (0.7) | |
| Upper GI bleeding | 27 (11) | 12 (12) | 15 (11) | 0.727 |
| Lower GI bleeding | 15 (6) | 10 (10) | 5 (4) | 0.040 * |
| Gastrointestinal surgery (all reasons) | 31 (13) | 2 (2) | 29 (20) | 0.001 * |
| Colectomy | 17 (7) | 2 (2) | 15 (11) | 0.010 * |
| Small intestine resection | 19 (8) | 0 | 19 (13) | <0.001 * |
| Billroth II | 3 (1) | 0 | 3 (2) | 0.270 |
| Invasive treatment | 109 (45) | 11 (11) | 98 (69) | <0.001 * |
| Primary EVT | 80 (33) | 9 (9) | 71 (50) | <0.001 * |
| EVT primary success | 69 | 8 | 61 | |
| Failure of EVT | 12 | 1 | 11 | |
| Recurrent stenosis after EVT | 10 | 0 | 10 | |
| Failure or recurrence after EVT | 22 (9) | 1 (1) | 21 (15) | <0.001 * |
| Open conversion after EVT | 17 (7) | 1 (1) | 16 (11) | 0.002 * |
| Primary open surgical treatment | 44 (18) | 3 (3) | 41 (29) | <0.001 * |
| Bypass procedure | 32 (13) | 2 (2) | 30 (21) | <0.001 * |
| Outcome | ||||
| Overall deaths | 16 (7) | 1 (1) | 15 (11) | 0.003* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omran, S.; Konietschke, F.; Mueller, V.; de Bucourt, M.; Frese, J.P.; Greiner, A. Development of a Novel Scoring Model to Estimate the Severity Grade of Mesenteric Artery Stenosis. J. Clin. Med. 2022, 11, 7420. https://doi.org/10.3390/jcm11247420
Omran S, Konietschke F, Mueller V, de Bucourt M, Frese JP, Greiner A. Development of a Novel Scoring Model to Estimate the Severity Grade of Mesenteric Artery Stenosis. Journal of Clinical Medicine. 2022; 11(24):7420. https://doi.org/10.3390/jcm11247420
Chicago/Turabian StyleOmran, Safwan, Frank Konietschke, Verena Mueller, Maximilian de Bucourt, Jan Paul Frese, and Andreas Greiner. 2022. "Development of a Novel Scoring Model to Estimate the Severity Grade of Mesenteric Artery Stenosis" Journal of Clinical Medicine 11, no. 24: 7420. https://doi.org/10.3390/jcm11247420
APA StyleOmran, S., Konietschke, F., Mueller, V., de Bucourt, M., Frese, J. P., & Greiner, A. (2022). Development of a Novel Scoring Model to Estimate the Severity Grade of Mesenteric Artery Stenosis. Journal of Clinical Medicine, 11(24), 7420. https://doi.org/10.3390/jcm11247420






